Abstract
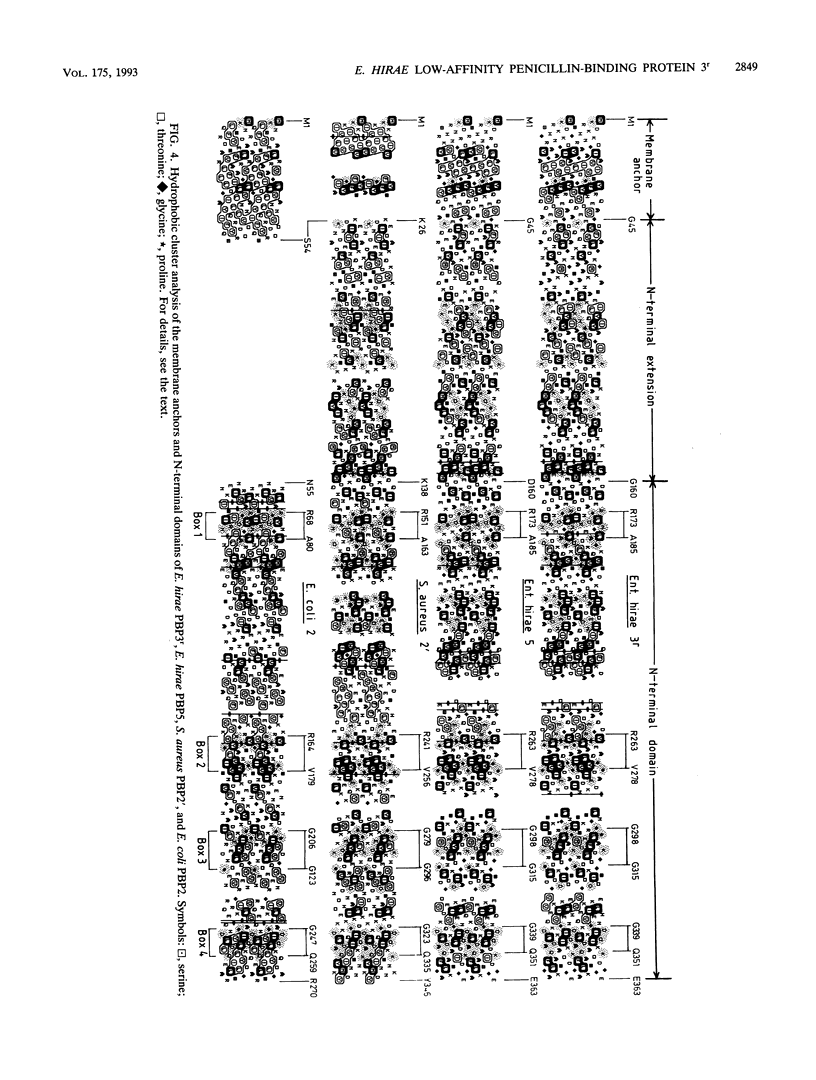
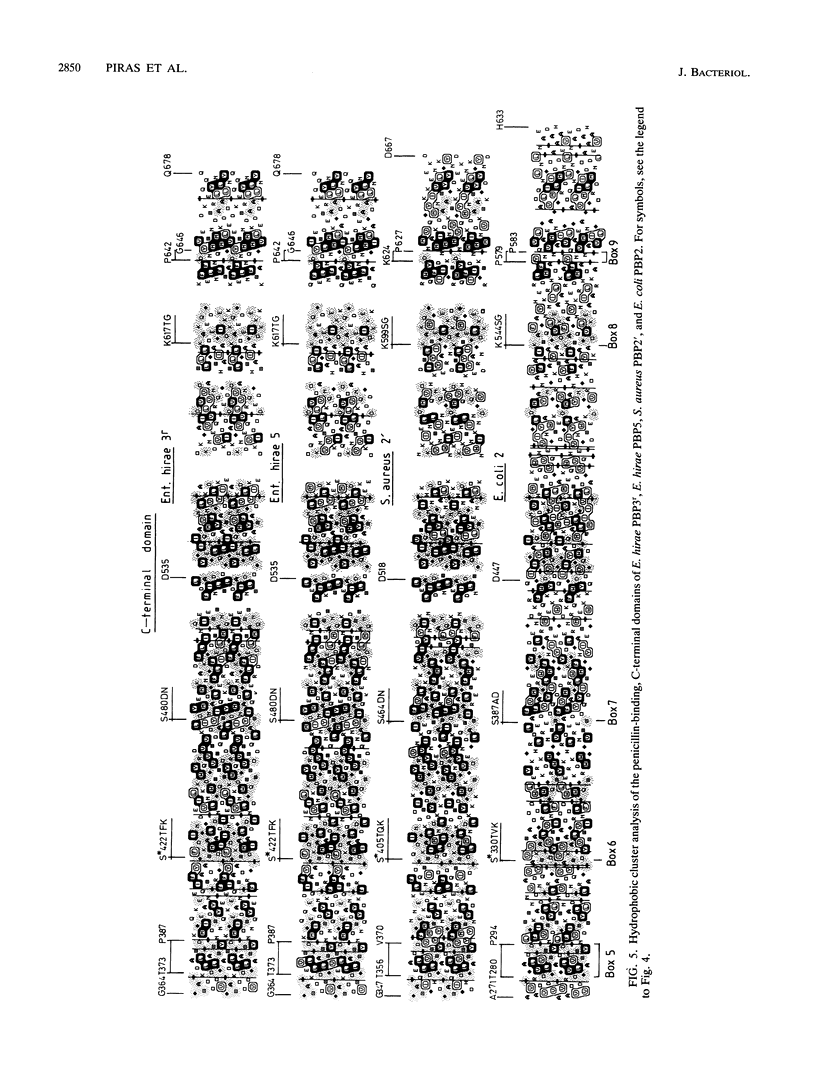
The clinical isolate Enterococcus hirae S185 has a peculiar mode of resistance to penicillin in that it possesses two low-affinity penicillin-binding proteins (PBPs): the 71-kDa PBP5, also found in other enterococci, and the 77-kDa PBP3r. The two PBPs have the same low affinity for the drug and are immunochemically related to each other. The PBP3r-encoding gene has been cloned and sequenced, and the derived amino acid sequence has been compared by computer-assisted hydrophobic cluster analysis with that of the low-affinity PBP5 of E. hirae R40, the low-affinity PBP2' of Staphylococcus aureus, and the PBP2 of Escherichia coli used as the standard of reference of the high-M(r) PBPs of class B. On the basis of the shapes, sizes, and distributions of the hydrophobic and nonhydrophobic clusters along the sequences and the linear amino acid alignments derived from this analysis, the dyad PBP3r-PBP5 has an identity index of 78.5%, the triad PBP3r-PBP5-PBP2' has an identity index of 29%, and the tetrad PBP3r-PBP5-PBP2'-PBP2 (of E. coli) has an identity index of 13%. In spite of this divergence, the low-affinity PBPs are of identical modular design and possess the nine amino acid groupings (boxes) typical of the N-terminal and C-terminal domains of the high-M(r) PBPs of class B. At variance with the latter PBPs, however, the low-affinity PBPs have an additional approximately 110-amino-acid polypeptide stretch that is inserted between the amino end of the N-terminal domain and the carboxy end of the membrane anchor. While the enterococcal PBP5 gene is chromosome borne, the PBP3r gene appears to be physically linked to the erm gene, which confers resistance to erythromycin and is known to be plasmid borne in almost all the Streptococcus spp. examined.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Ishiguro M., Imajoh S., Ohta T., Matsuzawa H. Active-site residues of the transpeptidase domain of penicillin-binding protein 2 from Escherichia coli: similarity in catalytic mechanism to class A beta-lactamases. Biochemistry. 1992 Jan 21;31(2):430–437. doi: 10.1021/bi00117a018. [DOI] [PubMed] [Google Scholar]
- Amábile-Cuevas C. F., Chicurel M. E. Bacterial plasmids and gene flux. Cell. 1992 Jul 24;70(2):189–199. doi: 10.1016/0092-8674(92)90095-t. [DOI] [PubMed] [Google Scholar]
- Asoh S., Matsuzawa H., Ishino F., Strominger J. L., Matsuhashi M., Ohta T. Nucleotide sequence of the pbpA gene and characteristics of the deduced amino acid sequence of penicillin-binding protein 2 of Escherichia coli K12. Eur J Biochem. 1986 Oct 15;160(2):231–238. doi: 10.1111/j.1432-1033.1986.tb09961.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. The penicillin-binding proteins in Streptococcus faecalis ATCC 9790. Eur J Biochem. 1980 Sep;110(2):445–456. doi: 10.1111/j.1432-1033.1980.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Lleò M. M., Satta G. Mechanisms of resistance of enterococci to beta-lactam antibiotics. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):103–105. doi: 10.1007/BF01963633. [DOI] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriaud C., Bissery V., Benchetrit T., Mornon J. P. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 1987 Nov 16;224(1):149–155. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- Horaud T., Le Bouguenec C., Pepper K. Molecular genetics of resistance to macrolides, lincosamides and streptogramin B (MLS) in streptococci. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):111–135. doi: 10.1093/jac/16.suppl_a.111. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Sato T., Wachi M., Jung H. K., Ishino F., Kobayashi Y., Matsuhashi M. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J Bacteriol. 1989 Nov;171(11):6375–6378. doi: 10.1128/jb.171.11.6375-6378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis B., Matthews P. R., Stewart P. R. Induced deletions within a cluster of resistance genes in the mec region of the chromosome of Staphylococcus aureus. J Gen Microbiol. 1990 Nov;136(11):2231–2239. doi: 10.1099/00221287-136-11-2231. [DOI] [PubMed] [Google Scholar]
- Lamotte-Brasseur J., Dive G., Dideberg O., Charlier P., Frère J. M., Ghuysen J. M. Mechanism of acyl transfer by the class A serine beta-lactamase of Streptomyces albus G. Biochem J. 1991 Oct 1;279(Pt 1):213–221. doi: 10.1042/bj2790213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991 Jul;35(7):1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Asoh S., Kunai K., Muraiso K., Takasuga A., Ohta T. Nucleotide sequence of the rodA gene, responsible for the rod shape of Escherichia coli: rodA and the pbpA gene, encoding penicillin-binding protein 2, constitute the rodA operon. J Bacteriol. 1989 Jan;171(1):558–560. doi: 10.1128/jb.171.1.558-560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr The enterococci: an enigma and a continuing therapeutic challenge. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):73–74. doi: 10.1007/BF01963629. [DOI] [PubMed] [Google Scholar]
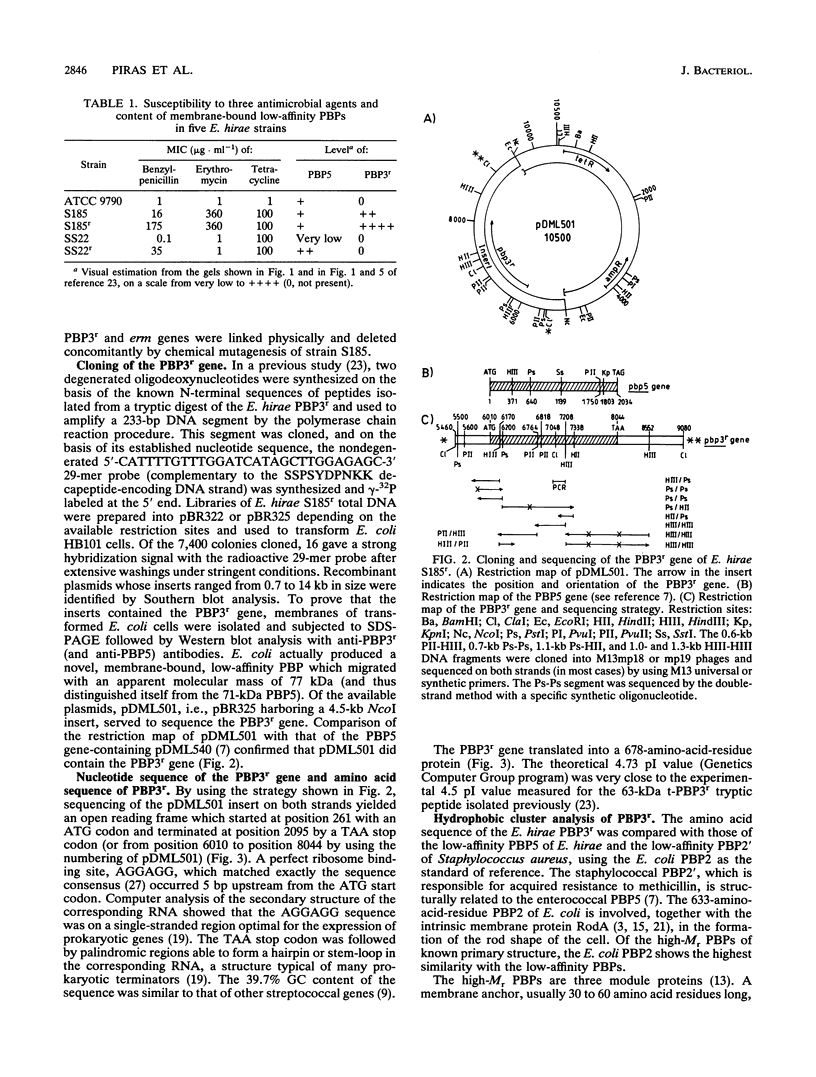
- Piras G., el Kharroubi A., van Beeumen J., Coeme E., Coyette J., Ghuysen J. M. Characterization of an Enterococcus hirae penicillin-binding protein 3 with low penicillin affinity. J Bacteriol. 1990 Dec;172(12):6856–6862. doi: 10.1128/jb.172.12.6856-6862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Matsuhashi M., Song M. D., Konno M. Restriction maps of the regions coding for methicillin and tobramycin resistances on chromosomal DNA in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1989 Sep;33(9):1624–1626. doi: 10.1128/aac.33.9.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Kharroubi A., Jacques P., Piras G., Van Beeumen J., Coyette J., Ghuysen J. M. The Enterococcus hirae R40 penicillin-binding protein 5 and the methicillin-resistant Staphylococcus aureus penicillin-binding protein 2' are similar. Biochem J. 1991 Dec 1;280(Pt 2):463–469. [PMC free article] [PubMed] [Google Scholar]