Abstract
Background
Populations in South and East Asia and many other regions of the world are chronically exposed to arsenic-contaminated drinking water. To various degrees, ingested inorganic arsenic (InAs) is methylated to monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) via folate-dependent one-carbon metabolism; impaired methylation is associated with adverse health outcomes. Consequently, folate nutritional status may influence arsenic methylation and toxicity.
Objective
The objective of this study was to test the hypothesis that folic acid supplementation of arsenic-exposed adults would increase arsenic methylation.
Design
Two hundred adults in a rural region of Bangladesh, previously found to have low plasma concentrations of folate (≤9 nmol/L) were enrolled in a randomized, double-blind, placebo-controlled folic acid–supplementation trial. Plasma concentrations of folate and homocysteine and urinary concentrations of arsenic metabolites were analyzed at baseline and after 12 wk of supplementation with folic acid at a dose of 400 μg/d or placebo.
Results
The increase in the proportion of total urinary arsenic excreted as DMA in the folic acid group (72% before and 79% after supplementation) was significantly (P < 0.0001) greater than that in the placebo group, as was the reduction in the proportions of total urinary arsenic excreted as MMA (13% and 10%, respectively; P < 0.0001) and as InAs (15% and 11%, respectively; P < 0.001).
Conclusions
These data indicate that folic acid supplementation to participants with low plasma folate enhances arsenic methylation. Because persons whose urine contains low proportions of DMA and high proportions of MMA and InAs have been reported to be at greater risk of skin and bladder cancers and peripheral vascular disease, these results suggest that folic acid supplementation may reduce the risk of arsenic-related health outcomes.
Keywords: Folate, folic acid, folate deficiency, vitamin B-12, homocysteine, hyperhomocysteinemia, one-carbon metabolism, S-adenosylmethionine, creatine, creatinine, arsenic, Bangladesh, monomethylarsonic acid, dimethylarsinic acid
INTRODUCTION
Several regions scattered throughout South and East Asia, including China and Taiwan, as well as regions in Mexico, Chile, and the United States have naturally occurring arsenic in ground-water. In Asia alone, ≥60 million persons are at risk of chronic exposure, of whom roughly 35 million reside in Bangladesh (1). In Bangladesh, well water arsenic concentrations range from < 0.25 μg/L to 1670 μg/L (2), and many are far in excess both of the maximum contaminant concentration of 10 μg/L advocated by the US Environmental Protection Agency and the World Health Organization and of the Bangladeshi government’s standard of 50 μg/L.
Chronic exposure to arsenic in drinking water is associated with a greater risk of cancers of the skin, bladder, lung, and liver and of stroke (3), ischemic heart disease (4), and neurologic consequences (5) in adults and of neurologic consequences in children (6). Furthermore, inorganic arsenic (InAs) has long been considered to be a teratogen, causing neural tube defects in many mammalian species (7, 8). Clinical manifestations of arsenic toxicity vary widely between persons and populations. Several observational and biochemical studies have led to a prevalent supposition that nutritional status may account for a substantial portion of this variability. However, no controlled clinical studies have systematically addressed this matter.
Arsenic occurs in drinking water as InAs, ie, arsenite (AsIII) and arsenate (AsV). In Southeast Asia, AsIII is the predominant form to which people are exposed. In vivo, hepatic methylation of InAsIII, which is highly variable in humans (9), first generates monomethylarsonic acid, MMAV. After reduction to MMAIII, a second methylation can occur to generate dimethylarsinic acid, DMAV (10, 11; Figure 1). One-carbon metabolism, the biochemical pathway responsible for methylation of arsenic, is a folate-dependent pathway. Whereas rodent studies suggest that folate nutritional status may influence the metabolism of arsenic (10, 15, 16), little evidence of this is currently available in humans.
FIGURE 1.
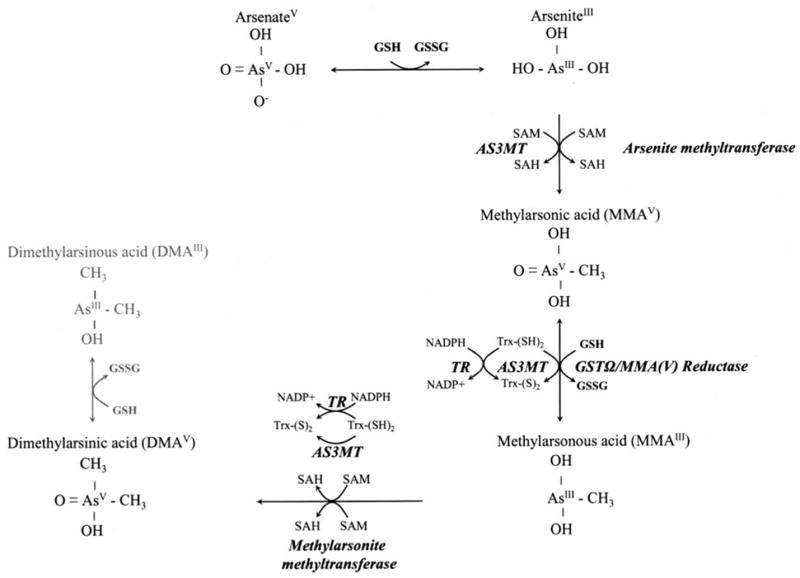
The arsenic metabolic pathway. Arsenate is reduced to arsenite in a reaction thought to be dependent on glutathione (GSH) or other endogenous reductants. Arsenite then undergoes an oxidative methylation, with S-adenosylmethionine (SAM) as the methyl donor, forming monomethylarsonic acid (MMAV) and S-adenosylhomocysteine (SAH). MMAV is reduced to MMAIII before a subsequent oxidative methylation step yielding dimethylarsinic acid (DMAV) and SAH. Little is known about the in vivo reduction of DMAV to DMAIII. Enzymes capable of catalyzing the illustrated reactions include arsenic-3-methyltransferase (AS3MT, formerly known as Cyt19) (12), arsenite methyltransferase and methylarsonite methyltransferase (13), and MMAV reductase (also known as GST omega) (14). GSSG, glutathione disulfide; GST, glutathione-S-transferase; TR, thioredoxin; Trx, thioredoxin reductase.
Methylation facilitates the urinary excretion of arsenic (17), and the pentavalent methylated metabolites are less reactive than are the InAs (18). Several studies in Taiwan found that persons with relatively lower proportions of urinary DMA(III+V) are at greater risk of skin and bladder cancers (19–22) and peripheral vascular disease (23). For these reasons, methylation of InAs has traditionally been considered to be a detoxification pathway. However, this concept is currently being challenged as a growing number of reports of studies in experimental model systems indicate that the trivalent methylated arsenic intermediates (MMAIII and DMAIII) are more toxic than is InAs or than are any of the pentavalent intermediates (24–27).
Although folate is relatively ubiquitous in the food supply, folate deficiency is not uncommon, largely because naturally occurring folates are highly susceptible to oxidative degradation, as can occur during cooking (28). We previously reported a high prevalence of folate deficiency and hyperhomocysteinemia in a rural area of Bangladesh (29). In addition, in a cross-sectional study, we ascertained that these conditions are associated with lower arsenic methylation (11). The current study aimed to test the hypothesis that folic acid supplementation to Bangladeshi adults with low plasma folate would increase arsenic methylation.
SUBJECTS AND METHODS
The Nutritional Influences on Arsenic Toxicity Study has worked in collaboration with a large multidisciplinary team of earth, health, and social scientists from Columbia University (New York, NY) and, in Bangladesh, from the National Institute of Preventive and Social Medicine and Dhaka University. This program includes the Health Effects of Arsenic Longitudinal Study, a prospective cohort study of 11 746 adults exposed to a wide range of water arsenic concentrations, from which the current sample is derived (30).
The region
The study site is a 25-km2 region within the thana of Araihazar (a thana is an administrative unit, or subdivision, of one of the 64 districts of Bangladesh), located ≈30 km east of Dhaka (26). This site was chosen because it has a wide range of arsenic concentrations in the drinking water, which permits dose-response analyses. Our data on socioeconomic status indicate that this region is not particularly poor by Bangladeshi standards.
Participants
The Health Effects of Arsenic Longitudinal Study cohort includes 11 746 men and women between the ages of 18 and 65 y who were recruited between October 2000 and May 2002 and who continue to be followed at 2-y intervals. A cross-sectional study of 1650 of these participants, reported elsewhere (29), was first conducted to ascertain the prevalence of folate and cobalamin deficiencies and of hyperhomocysteinemia and to identify a pool of participants with low plasma concentrations of folate for recruitment into the folic acid–intervention study. The 200 participants enrolled in the folic acid–supplementation trial were a random selection from the 550 participants who fell into the lowest tertile for plasma folate in the cross-sectional study. Participants were excluded if they were pregnant or cobalamin deficient (vitamin B-12 ≤185 pmol/L) or were taking vitamin supplements.
Oral informed consent was obtained by our Bangladeshi field staff physicians, who read an approved assent form to the study participants. Ethical approval was obtained from the Institutional Review Board of Columbia Presbyterian Medical Center and the Bangladesh Medical Research Council.
Study design and field work
Field staff teams, each consisting of 1 physician and 2 interviewers, visited the homes of potential subjects to assess eligibility and to invite those eligible to enroll in the folic acid–intervention study. Eligible persons who consented to participate were randomly assigned to receive folic acid (400 μg/d) or placebo. One bottle containing 100 tablets of folic acid or placebo was assigned to each subject. After blood and urine samples were collected, the field staff observed the participant take the folic acid or placebo tablet. Field staff retained the bottles of folic acid or placebo and returned to each subject’s home daily to witness compliance.
Of the 200 study participants enrolled, 6 were dropped because they were unavailable to meet with our field staff to receive the folic acid or placebo tablet on a daily basis. Of these 6 dropped participants, 2 were women and 4 were men; 3 had been randomly assigned to the folic acid group and 3 to the placebo group. No adverse events were reported. All participants were provided with a supply of multivitamins on completion of the study.
Procedures and analytic techniques
Sample collection and handling
Plasma samples for total homocysteine (tHcy), folate, and total cobalamin were obtained by venipuncture at the time of recruitment and after the 12-wk intervention. Blood was collected into heparin-containing evacuated tubes, which were placed in IsoRack cool packs (Brinkmann Instruments, West-bury, NY) that were designed to maintain a sample temperature of 0 °C for 6 h. Within 4 h, samples were transported in hand-carried coolers to the local laboratory, which is situated in our field clinic in Araihazar. Samples were spun at 3000 × g for 10 min at 4 °C, and plasma was separated from cells. Aliquots of plasma were stored at −80 °C and shipped in a frozen state on dry ice to Columbia University (New York, NY) for analysis. Urine samples were collected in 50-mL acid-washed polypropylene tubes. These tubes were kept in portable coolers, frozen at −20 °C within 4 h, and similarly shipped on dry ice.
Water arsenic
The water arsenic concentrations in the tube wells at each participant’s home were obtained during a survey of all wells in the study region carried out between January and May 2000 (31). Samples were analyzed at Columbia University’s Lamont Doherty Earth Observatory by using graphite furnace atomic absorption in a Hitachi instrument (Z8200; Hitachi, Tokyo, Japan), which has a detection limit of 5 μg/L. Those samples found to have nondetectable arsenic by graphite furnace atomic absorption were subsequently analyzed by using inductively coupled mass spectrometry [(ICP-MS) Axiom Single-Collector HR ICP-MS; Thermo Elemental, Erlangen, Germany], which has a detection limit of 0.1 μg/L (32).
Total urinary arsenic
Total urinary arsenic concentrations were measured by using graphite furnace atomic absorption spectrometry in a Perkin-Elmer graphite furnace system (AAnalyst 600; Perkin-Elmer, Shelton, CT) in the Columbia University Trace Metals Core Laboratory, as described previously (33). Our laboratory participates in a quality-control program for total urinary arsenic that is coordinated by Philippe Weber at the Quebec Toxicology Center (Quebec, Canada). During the course of this study, intra-class correlation coefficients between our laboratory’s values and the samples calibrated at Weber’s laboratory were 0.99. Urinary creatinine was analyzed by using a method based on the Jaffe reaction (34) and those values were included in multiple regression models to adjust total urinary arsenic concentrations for concentration of urine.
Urinary arsenic metabolites
Urinary arsenic metabolites were speciated by using a method described by Reuter et al (35). This method uses HPLC separation of arsenobetaine, arsenocholine, AsV, AsIII, total MMA (MMAIII and MMAV co-elute in a single peak), and total DMA, which is followed by detection by ICP-MS with dynamic reaction cell (ICP-MS-DRC). Because AsIII in urine can oxidize to AsV during sample transport and preparation, we report total InAs. The proportions of total urinary arsenic excreted as InAs (%InAs), MMA (%MMA), and DMA (%DMA) were calculated after subtraction of arsenocholine and arsenobetaine (ie, non-toxic dietary sources of arsenic) from the total.
Plasma folate and vitamin B-12
Plasma folate and total cobalamin (vitamin B-12) were analyzed by using a radioimmunoassay (Quantaphase II; Bio-Rad Laboratories, Richmond, CA) as described previously (29). The within- and between-day CVs for folate were 3% and 11%, respectively, and those for cobalamin were 4% and 8%, respectively.
Plasma total homocysteine concentrations
Plasma tHcy concentrations were measured by using HPLC with fluorescence detection according to the method described by Pfeiffer et al (36). The within- and between-day CVs for tHcy were 5% and 8%, respectively.
Statistical analysis
The distributions of water arsenic; plasma concentrations of tHcy, folate, and cobalamin; and urinary concentrations of arsenic, creatinine and %InAs were skewed. For these variables, logarithmic transformation was used. Treatment group differences were first tested by using the chi-square test for categorical variables and Wilcoxon’s rank-sum test for continuous variables.
Our primary outcome variables were %InAs, %MMA, and %DMA in urine. Urinary arsenic metabolites were measured at 3 time points: baseline, day 7, and day 84 (the last day of treatment). Repeated-measures linear regression was used to examine treatment effects on the primary outcome variables, as well as the ratios of MMA to InAs (MMA:InAs) and of DMA to MMA (DMA:MMA). We explored these secondary outcome variables, known as the primary methylation index (PMI) and secondary methylation index (SMI), respectively, for the purpose of consistency with various other studies (19, 20, 22). Our regression models also included indicators of time (in wk) from baseline and treatment × time interactions. The regression coefficients of the interaction terms indicate the extent to which treatment varied by time (ie, from baseline to week 1 or from baseline to week 12). Analyses were conducted with and without control for covariates, including sex, age, body mass index, betel nut use, water arsenic, and urinary creatinine. All regression parameters were estimated by using generalized estimation equations, which accounts for within-subject correlations in the repeated measures.
RESULTS
The characteristics of the study population are presented in Table 1. The differences between treatment groups in baseline plasma concentrations of folate, cobalamin, or tHcy; sex distribution; age, height, weight, and body mass index; total urinary arsenic; urinary arsenic metabolites; water arsenic; or sociodemographic variables such as education or housing type were not significant. Water arsenic concentrations for the study participants ranged from 0.1 to 435 μg/L: 81% of the participants had concentrations >10 μg/L, and 62% had concentrations >50 μg/L.
TABLE 1.
Characteristics of the study population1
| Baseline variables | Folate group (n = 96) | Placebo group (n = 98) |
|---|---|---|
| Men [n (%)] | 49 (51) | 47 (48) |
| As-induced skin lesions [n (%)] | 4 (4) | 6 (6) |
| Cigarette smoking [n (%)] | 41 (43) | 40 (41) |
| Use of betel nut [n (%)] | 38 (40) | 39 (40) |
| House type | ||
| Thatched or other [n (%)] | 5 (5) | 7 (7) |
| Corrugate [n (%)] | 86 (90) | 83 (85) |
| Semi-pakka [n (%)] | 5 (5) | 8 (8) |
| Number of children [n (%)] | ||
| 0 | 50 (52) | 53 (54) |
| 1–2 | 22 (23) | 14 (14) |
| ≥3 | 24 (25) | 31 (32) |
| Education | ||
| None [n (%)] | 41 (42.7) | 35 (35.7) |
| 1–5 y [n (%)] | 34 (35.4) | 40 (40.8) |
| >5 y [n (%)] | 21 (21.9) | 23 (23.5) |
| Age (y) | 40 ± 112 | 38 ± 10 |
| Education (y) | 3.3 ± 3.5 | 3.6 ± 3.5 |
| Weight (kg) | 49 ± 10 | 48 ± 8 |
| Height (cm) | 157 ± 8 | 155 ± 8.6 |
| BMI (kg/m2) | 20 ± 3.4 | 20 ± 2.7 |
| Children (n) | 1.4 ± 1.9 | 1.6 ± 2.1 |
| Urinary arsenic | ||
| (μg/L) | 147 ± 130 | 136 ± 129 |
| (μg/g Cr) | 296 ± 262 | 256 ± 217 |
| Urinary creatinine (mg/dL) | 58.4 ± 44.2 | 60.6 ± 42.1 |
| Urinary arsenic metabolites | ||
| %DMA | 71.9 ± 7.8 | 71.8 ± 8.7 |
| %MMA | 12.6 ± 3.9 | 13.2 ± 4.6 |
| %InAs | 15.5 ± 7.4 | 15.0 ± 6.1 |
| Plasma folate (nmol/L) | 8.3 ± 4.7 | 7.8 ± 3.0 |
| Plasma vitamin B-12 (pmol/L) | 289 ± 128 | 288 ± 126 |
| Plasma homocysteine (μmol/L) | 11.1 ± 4.0 | 11.4 ± 8.2 |
| Water arsenic (μg/L) | 106 ± 91 | 104 ± 113 |
%DMA, %bMMA, and %InAs, the proportion of total urinary arsenic excreted as dimethylarsinic acid, monomethylarsonic acid, and inorganic arsenic, respectively. Chi-square and Wilcoxon’s rank-sum tests were used for treatment group differences in categorical and quantitative variables, respectively. No significant group differences were found in any of the variables.
x̄ ± SD (all such values).
Nutritional variables measured before and after 12 wk of folic acid supplementation are shown in Table 2. Folic acid supplementation significantly increased plasma concentrations of folate, significantly decreased tHcy concentrations, and did not influence plasma cobalamin. These effects on folate and tHcy were not observed in the placebo group. We did not find a time × sex interaction within the supplemented group for either plasma folate or vitamin B-12. We did, however, find a significant time × sex interaction for tHcy in the group that received supplementation. Before supplementation, tHcy concentrations in men were higher than those in women (13.2 ± 4.2 and 9.1 ± 2.6 μmol/L, respectively). Although supplementation significantly (P < 0.0001) reduced tHcy in both men and women, the reduction was greater in men than in women (x̄ ± SD within-person change: −4.19 ± 4.29 μmol/L in men and −1.91 ± 1.94 μmol/L in women; P = 0.002). No interactions were found among those receiving placebo. Urinary creatinine increased significantly after 12 wk of folic acid supplementation in men (63.1 ± 55.4 mg/dL before supplementation and 83.0 ± 44.4 mg/dL after supplementation; P = 0.004), whereas the change in women was not significant (53.4 ± 27.9 mg/dL before supplementation and 60.7 ± 36.6 mg/dL after supplementation; P = 0.28). Small, nonsignificant increases in urinary creatinine were found in the placebo group (60.6 ± 42.1 mg/dL before supplementation and 67.4 ± 46.0 mg/dL after supplementation; P = 0.32). After adjustment for covariates (ie, age, sex, betel nut use, cigarette smoking, body mass index, water arsenic, and urinary arsenic), the treatment × time interaction was significant (P = 0.03), which suggested that folic acid supplementation resulted in significant increases in urinary creatinine, whereas placebo did not. The time × treatment × sex interaction for urinary creatinine was not significant, with or without adjustment for covariates.
TABLE 2.
Plasma concentrations of folate, total homocysteine (tHcy), and vitamin B-12 before and after 12 wk of folate supplementation1
| Folate group (n = 96) | Placebo group (n = 98) | P for treatment × time interaction2 | |
|---|---|---|---|
| Folate (nmol/L) | <0.0001 | ||
| Before supplementation | 8.28 ± 4.743 | 7.81 ± 2.96 | |
| After supplementation | 61.57 ± 27.67 | 8.86 ± 9.14 | |
| Within-person change | 53.29 ± 27.844 | 1.05 ± 9.32 | |
| tHcy (μmol/L) | <0.0001 | ||
| Before supplementation | 11.14 ± 4.03 | 11.41 ± 8.21 | |
| After supplementation | 8.08 ± 2.29 | 11.37 ± 9.29 | |
| Within person change | −3.06 ± 3.514 | −0.05 ± 4.31 | |
| Vitamin B-12 (pmol/L) | 0.09 | ||
| Before supplementation | 289.52 ± 127.22 | 288.12 ± 126.27 | |
| After supplementation | 282.37 ± 105.34 | 304.31 ± 143.36 | |
| Within-person change | −7.15 ± 95.04 | 16.20 ± 80.135 |
There were no significant differences between the groups before supplementation.
Based on a generalized score test. The time × treatment × sex interaction for tHcy was not significant.
x̄ ± SD (all such values).
P < 0.0001.
P < 0.05.
The unadjusted urinary arsenic metabolite data, plotted in Figure 2, illustrate the increase in %DMA and decreases in %MMA and %InAs over time in the folic acid and placebo groups. In unadjusted analyses, the increase in %DMA in the folic acid group resulting from the 12-wk intervention (72 ± 7.8% before intervention and 79 ± 7.3% at 12 wk) was significantly (P < 0.0001) greater than that in the placebo group, as were the reductions in %MMA (13 ± 3.9% before intervention and 10 ± 3.9% after intervention; P < 0.0001) and %InAs (15 ± 7.4% and 11 ± 6.2%, respectively; P < 0.0001). There were small but significant changes in %InAs and %DMA in the placebo group after 12 wk, possibly as a result of “placebo effects” associated with participation in this clinical trial, to secular trends, or both. The effects of folic acid supplementation were significantly greater than those of placebo. The difference in the frequency distributions of %DMA between the folic acid group and the placebo group after the 12-wk intervention are shown in Figure 3.
FIGURE 2.
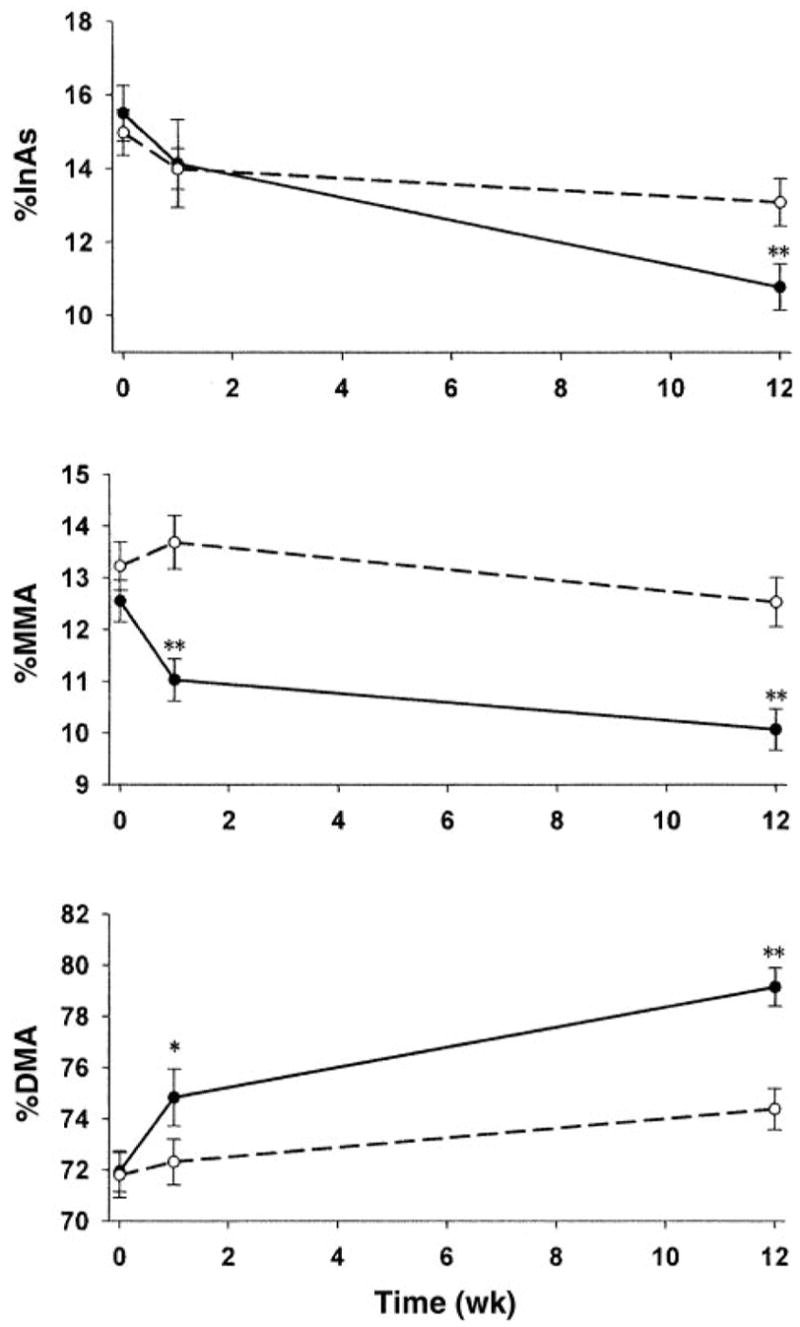
Comparison of the effects of supplementation with folic acid (—) and with placebo (- - -) on arsenic metabolites in urine by using one-sample t tests for within-person changes from baseline to week 1 or to week 12 in each treatment group. The proportions of total urinary arsenic excreted as inorganic arsenic (InAs), dimethylarsinic acid (DMA), and monomethylarsonic acid (MMA) are indicated as %InAs, %DMA, and %MMA. *,**Significant within-person changes: *P < 0.01, **P < 0.0001. In the placebo group, small but significant (P ≤ 0.05) changes were found between baseline and week 12 for each variable.
FIGURE 3.
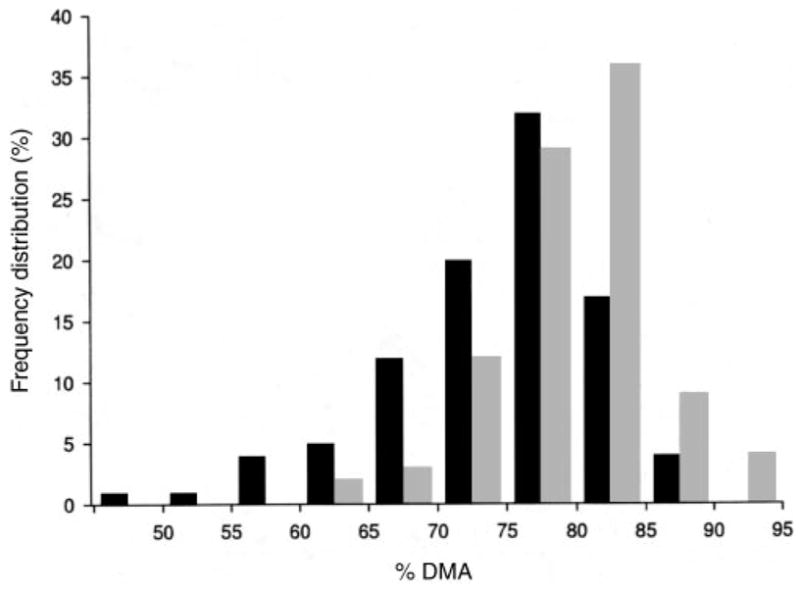
Frequency distribution of the proportion of total urinary arsenic excreted as dimethylarsinic acid (%DMA) after 12 wk of intervention with folic acid (
 ) or placebo (
) or placebo (
 ).
).
The effects of folic acid supplementation on the changes in %InAs, %MMA, and %DMA in urine over time, after adjustment for covariates, are shown in Table 3. The average increase in %DMA was 5.9%. The findings were essentially the same when the arsenic metabolite data were further corrected for total urinary arsenic (data not shown).
TABLE 3.
Baseline values and estimated percentage within-person changes from baseline in adjusted urinary arsenic metabolites over time1
| Folate group (n = 96) | Placebo group (n = 98) | P for treatment × time interaction2 | |
|---|---|---|---|
| log (%InAs) | 0.0068 | ||
| Baseline | 2.66 ± 0.033 | 2.63 ± 0.03 | |
| Change up to week 1 (%) | −0.15 ± 0.044 | −0.06 ± 0.03 | |
| Change up to week 12 (%) | −0.31 ± 0.045 | −0.13 ± 0.044 | |
| % MMA | <0.0001 | ||
| Baseline | 12.55 ± 0.34 | 13.27 ± 0.41 | |
| Change up to week 1 (%) | −1.6 ± 0.265 | 0.43 ± 0.31 | |
| Change up to week 12 (%) | −2.6 ± 0.375 | −0.71 ± 0.43 | |
| % DMA | 0.0029 | ||
| Baseline | 72.02 ± 0.67 | 71.74 ± 0.75 | |
| Change up to week 1 (%) | 2.8 ± 1.036 | 0.48 ± 0.57 | |
| Change up to week 12 (%) | 5.9 ± 0.825 | 2.14 ± 0.716 | |
| PMI7 | 0.4639 | ||
| Baseline | 0.94 ± 0.04 | 0.97 ± 0.03 | |
| Change up to week 1 (%) | 0.04 ± 0.03 | 0.08 ± 0.048 | |
| Change up to week 12 (%) | 0.11 ± 0.058 | 0.13 ± 0.056 | |
| SMI9 | <0.0001 | ||
| Baseline | 6.46 ± 0.21 | 6.42 ± 0.31 | |
| Change up to week 1 (%) | 1.25 ± 0.185 | 0.04 ± 0.26 | |
| Change up to week 12 (%) | 2.88 ± 0.435 | 0.40 ± 0.32 |
Values were derived from fitted linear models with repeated measures after adjustment for sex, age, betel nut use, water arsenic, and urinary creatinine.
InAs, %MMA, and %DMA, the proportion of total urinary arsenic excreted as inorganic arsenic, monomethylarsonic acid, and dimethylarsinic acid, respectively; PMI, primary methylation index; SMI, secondary methylation index. The groups did not differ significantly before supplementation.
Based on a generalized score test.
x̄ ± SE (all such values).
Significant within-person change over time (Wald test on relevant model coefficients): 4P < 0.001, 5P < 0.0001, 6P < 0.01, 8P < 0.05.
Significant effect of time, P = 0.0029. Measured as the ratio of MMA to InAs.
Measured as the ratio of DMA to MMA.
An alternative method for describing arsenic metabolite data uses PMI and SMI (19). The PMI is defined as MMA:InAs, and the SMI is defined as DMA:MMA. Presented for purposes of comparison to other studies, the PMI (adjusted for covariates) did not significantly increase after 7 d of folic acid supplementation, although, by 12 wk, the estimated within-person change of 0.11 was significant (P ≤ 0.05). The smallness of the effect on the PMI is likely due to the fact that both %InAs and %MMA decreased in response to folic acid supplementation; thus, these effects cancel each other out when expressed as a ratio. However, folic acid supplementation significantly increased the SMI at day 7 by 1.25 and at 12 wk by 2.88 (P <0.0001 for both). None of the treatment effects differed by sex. The influence of folic acid supplementation on arsenic methylation also did not differ according to the degree of water arsenic exposure or by baseline folate or tHcy concentration (data not shown).
Study participants did not change their source of drinking water over the course of the study. Nevertheless, after adjustment for urinary creatinine, we observed a small (3%), nonsignificant increase in urinary arsenic at 1 wk and a significant (P = 0.02) 9% decrease in total urinary arsenic from baseline at 12 wk; this effect was not observed in the placebo group.
For both the placebo and folic acid groups, urinary creatinine was a significant predictor of %DMA and %InAs at all visits (P < 0.001). When linear models with repeat measures (including covariates) were used, the estimated parameter associated with urinary creatinine (in log scale) was b = −0.30 for outcome of log(%InAs) and b = 5.4 for outcome of %DMA (P <0.0001 for both). These effects remained highly significant after further adjustment for total urinary arsenic (data not shown). Urinary creatinine was not significantly associated with %MMA.
DISCUSSION
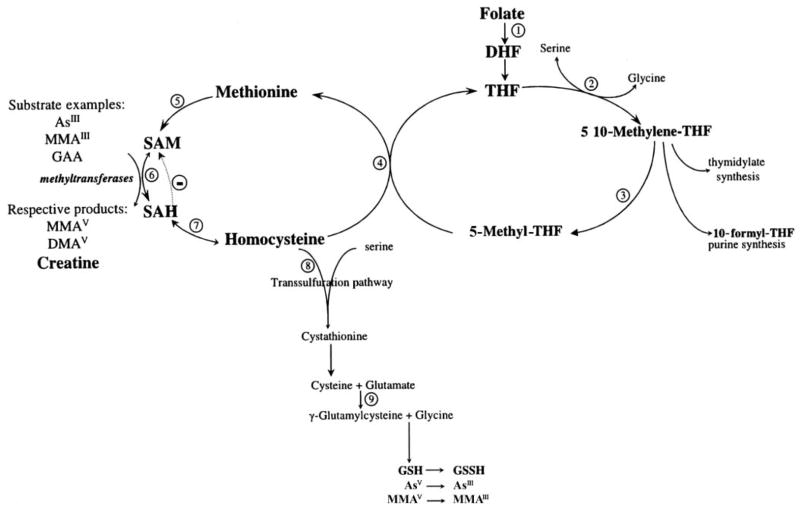
This study provides compelling evidence in support of the hypothesis that folic acid supplementation to adults with low plasma concentrations of folate would result in increased arsenic methylation. This hypothesis is based on the premise that arsenic is methylated by folate-dependent one-carbon metabolism with the use of S-adenosylmethionine (SAM) as the universal methyl donor (11; Figure 4). Methionine biosynthesis in the methionine synthase reaction utilizes 5-methyl-tetrahydrofolate as a cosubstrate and cobalamin as a cofactor in the remethylation of homocysteine. Methionine is subsequently activated by ATP to generate SAM. SAM-dependent methylation reactions yield the methylated product and S-adenosylhomocysteine (SAH) (Figure 4, reaction 6). Hydrolysis of SAH generates adenosine and homocysteine, but this reaction is readily reversible. As a consequence, plasma SAH concentrations increase linearly with even mild elevations in homocysteine concentrations (37). This is of particular importance because SAH is a potent inhibitor of most transmethylation reactions (37), including those of arsenic (38). SAH binds tightly to methyltransferases and is removed only if the pathway is pulled forward by downstream removal of tHcy, as may be achieved with folic acid supplementation (Figure 4, reaction 4).
FIGURE 4.
Overview of one-carbon metabolism. Numbers inside circles identify the different reactions. Reaction 1: Dietary folates are reduced to dihydrofolate (DHF) and tetrahydrofolate (THF) by dihydrofolate reductase. Reaction 2: The β-carbon of serine is transferred to THF by serine hydroxy-methyltransferase, forming 5,10-methylene-THF and glycine. Reaction 3: At a major branch point between transmethylation reactions and nucleotide biosynthesis, 5,10-methylene-THF can be reduced to 5-methyl-THF by 5,10-methylene-THF reductase. Reaction 4: In a reaction catalyzed by the vitamin B-12–containing enzyme methionine synthetase, the methyl group of 5-methyl-THF is transferred to homocysteine, generating methionine and regenerating THF. Reaction 5: Methionine adenosyltransferase activates methionine to form S-adenosylmethionine (SAM). Reaction 6: SAM serves as a universal methyl donor for numerous acceptors, predominantly guanidinoacetate (GAA) but also DNA, arsenic, and others, in reactions that involve numerous methyltransferases. Reaction 7: The byproduct of these methylation reactions, S-adenosylhomocysteine (SAH), is hydrolyzed to generate homocysteine. SAH is a potent inhibitor of most SAM-dependent methylations. Reaction 8: Homocysteine either is used to regenerate methionine or is directed to the transsulfuration pathway through which it is ultimately catabolized. Reaction 9: The transsulfuration pathway is also responsible for glutathione (GSH) biosynthesis. AsV, arsenate; AsIII, arsenite; MMAV, monomethylarsonic acid; MMAIII, monomethylarsonous acid; DMAV, dimethylarsinic acid.
Evidence from animal studies supports the notion that folate deficiency influences arsenic methylation. For example, methyl donor deficiency induced by methyl-deficient diets has been shown to significantly decrease total urinary arsenic excretion in mice (15) and rabbits (10), mainly because of less DMA excretion. These diets also gave rise to greater retention of arsenic in liver and lung, tissues that are prone to develop arsenic-related cancers in humans (10). More recently, an elegant series of studies on arsenic-induced neural tube defects used mice who were nullizygous for folate-binding protein 1 and 2 and reduced-folate carrier 1 (8, 16, 39, 40). These studies showed that, for all genotypes studied (including wild-type), dietary folate deficiency caused a reduction in the total amount of arsenic excreted in the urine, primarily because of a reduction in DMA. Furthermore, folate-binding protein-2−/− mice were more susceptible to arsenic-induced neural tube defects than were wild-type mice, and this phenotype was further exacerbated by a folate-deficient diet (8). Whereas these studies provide strong experimental evidence that nutritional regulation of one-carbon metabolism influences arsenic methylation, excretion, and toxicity, the arsenic doses were quite high, and the dietary deficiencies were severe. Moreover, marked species variations are found in the efficiency of arsenic methylation (41).
Earlier nutrition studies in human populations exposed to arsenic focused on antioxidants such as β-carotene, according to their association with cancer outcomes. A recent cross-sectional study assessed dietary intake by using food-frequency questionnaires completed by 87 subjects from 2 arsenic-exposed regions in the western United States and compared the dietary intake of 30 nutrients to urinary arsenic metabolites. Subjects in the lowest quartile for protein intake were found to have a higher %MMA (14.6 and 11.6%, respectively; P = 0.01) and a significantly lower %DMA (72.3 and 77.0%, respectively; P = 0.01) than did subjects in the highest quartile for protein intake (42). No association between dietary folate intake and arsenic methylation was found, likely because the study was conducted after the United States mandated folic acid fortification, and all of the participants in that study had already benefited from this countrywide intervention. A case-control study in West Bengal, India, found a modest increase in the risk of arsenic-induced skin lesions in persons who fell within the lowest quintiles for dietary intake of animal protein, folate, calcium, and fiber (43).
We previously conducted a cross-sectional study of 300 participants and found that plasma folate concentrations were positively correlated with %DMA and negatively correlated with %MMA and %InAs (11). The results of the current folic acid–intervention study confirm these findings and, moreover, indicate a causal relation. Although the average effect sizes were moderate, individual responses were variable, and it is not possible to ascertain from the current study whether methylation of arsenic achieved maximal capacity or whether it would have continued to increase for some persons, given a longer period of folic acid supplementation. In a study of folate-depleted elderly women, genomic DNA hypomethylation began to respond to a similar regimen of folic acid repletion only after 7 wk (44), which is consistent with kinetic estimates of a very slow turnover of whole-body folate pools (42). In the current study, the slight increase at week 1 and the decrease at week 12 in total urinary arsenic, despite continued consumption of arsenic-contaminated drinking water, suggest that folic acid supplementation may help to reduce the overall body burden of arsenic. We speculate that our schedule for urine collections missed a more substantial early peak of arsenic excretion in response to folic acid.
Other nutritional factors, such as protein, may further contribute to the variability in arsenic methylation. The association between urinary creatinine and %InAs and %DMA confirms a similar, unanticipated observation from our cross-sectional study (11). Although the mechanistic basis for that finding remains obscure (11, 46), one of several possible explanations has to do with the fact that urinary creatinine is influenced by recent dietary intake of animal protein. Animal protein contains creatine, a substance that down-regulates SAM-dependent endogenous creatine synthesis—ie, creatine biosynthesis is a major consumer of methyl groups (47)—and that may thereby lower homocysteine (48, 49) and facilitate the methylation of other substrates, including arsenic.
Although it was not a focus of this study, the finding that urinary creatinine increased after 12 wk of folic acid supplementation was somewhat surprising, but it is consistent with the requirement of folate for creatine biosynthesis. Earlier studies reported that SAM may be limiting for creatine biosynthesis in the presence of a deficiency of folate or vitamin B-12, although that should not be case when nutritional status is adequate (50–53).
What effect may we expect folic acid supplementation to have on arsenic-related health outcomes? Studies in Taiwan reported that persons who have a low SMI (ie, SMI ≤5) and who are exposed to high concentrations of arsenic are at greater risk of skin and bladder cancers than are persons with an SMI >5 (19–23). The odds ratio associated with an SMI of < 5 for arsenic-related skin lesions is estimated to be 1.55 (95% CI: 1.23, 1.96) after adjustment for age and sex in a separate case-control study (594 cases and 1042 controls) conducted in the same Bangladeshi population (H Ahsan et al, unpublished observations, 2006). In the current study, the proportion of subjects in the folic acid group who had an SMI ≤ 5 decreased from 39% before supplementation to 11% after supplementation. This reduction would be related to a decrease from 17.7% to 5.7% in the proportion of arsenic-related skin lesions attributable to an SMI of <5 (population-attributable risk adjusted for age and sex). This estimate of the population’s attributable risk, however, is based on one study and should therefore be interpreted with caution.
In conclusion, the results of this study indicate that folic acid supplementation to persons with marginal folate nutritional status enhances arsenic methylation. Future research should aim to determine whether such therapy is associated with reductions in arsenic-related morbidity and mortality.
Acknowledgments
We thank Mominul Islam for directing the field staff, Shafiul Alam for overseeing laboratory operations in Araihazar, and our staff, the fieldworkers, and the study participants in Bangladesh, without whom this work would not have been possible. We also thank Jennie Kline for her contributions to the interpretation of these findings.
MVG was responsible for the study design; HA, FP, YC, PF-L, and JHG also contributed to the study design; MVG was responsible for the interpretation of results; HA, FP, YC, PF-L, and JHG also contributed to this interpretation; XL carried out the statistical analyses; JRP, VI, JHG, and VS performed laboratory analyses; MVG was responsible for writing the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Supported by grants no. RO1 ES011601, 5P30ES09089, and 1 P42 ES10349 from the National Institutes of Health.
References
- 1.The World Bank. Towards a more effective operational response. Washington, DC: The World Bank; 2005. [Google Scholar]
- 2.Kinniburgh DG, Smedley PL. Arsenic contamination of groundwater in Bangladesh. Final report, BGS Technical Report WC/00/19; Keyworth, United Kingdom: British Geological Survey. 2001. [Google Scholar]
- 3.Chiou HY, Huang WI, Su CL, Chang SF, Hsu YH, Chen CJ. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997;28:1717–23. doi: 10.1161/01.str.28.9.1717. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH, Chong CK, Tseng CP, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 5.Feldman RG, Niles CA, Kelly-Hayes M, et al. Peripheral neuropathy in arsenic smelter workers. Neurology. 1979;29:939–44. doi: 10.1212/wnl.29.7.939. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman GA, Liu X, Parvez F, et al. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112:1329–33. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalat SL, Walker DB, Finnell RH. Role of arsenic as a reproductive toxin with particular attention to neural tube defects. J Toxicol Environ Health. 1996;48:253–72. doi: 10.1080/009841096161320. [DOI] [PubMed] [Google Scholar]
- 8.Wlodarczyk B, Spiegelstein O, Gelineauvan Waes J, et al. Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicol Appl Pharmacol. 2001;177:238–46. doi: 10.1006/taap.2001.9303. [DOI] [PubMed] [Google Scholar]
- 9.Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104:620–8. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahter M, Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987;37:41–6. doi: 10.1016/0378-4274(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 11.Gamble MV, Liu X, Ahsan H, et al. Folate, homocysteine and arsenic metabolism in Bangladesh. Environ Health Perspect. 2005;113:1683–8. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S, Shi Q, Nix FB, et al. A novel S-adenosyl-L-methionine: arseni-c(III) methyltransferase from rat liver cytosol. J Biol Chem. 2002;277:10795–803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- 13.Zakharyan R, Wu Y, Bogdan GM, Aposhian HV. Enzymatic methylation of arsenic compounds: assay, partial purification, and properties of arsenite methyltransferase and monomethylarsonic acid methyltransferase of rabbit liver. Chem Res Toxicol. 1995;8:1029–38. doi: 10.1021/tx00050a006. [DOI] [PubMed] [Google Scholar]
- 14.Zakharyan RA, Sampayo-Reyes A, Healy SM, et al. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem Res Toxicol. 2001;14:1051–7. doi: 10.1021/tx010052h. [DOI] [PubMed] [Google Scholar]
- 15.Tice RR, Yager JW, Andrews P, Crecelius E. Effect of hepatic methyl donor status on urinary excretion and DNA damage in B6C3F1 mice treated with sodium arsenite. Mutat Res. 1997;386:315–34. doi: 10.1016/s1383-5742(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 16.Spiegelstein O, Lu X, Le CX, et al. Effects of dietary folate intake and folate binding protein-2 (Folbp2) on urinary speciation of sodium arsenate in mice. Environ Toxicol Pharmacol. 2005;19:1–7. doi: 10.1016/j.etap.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Buchet JP, Lauwerys R, Roels H. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health. 1981;48:71–9. doi: 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- 18.Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89:1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, Guo YL, Su HJ, et al. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–8. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- 20.Chen YC, Su HJ, Guo YL, et al. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003;14:303–10. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- 21.Hsueh YM, Chiou HY, Huang YL, et al. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:589–96. [PubMed] [Google Scholar]
- 22.Yu RC, Hsu KH, Chen CJ, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1259–62. [PubMed] [Google Scholar]
- 23.Tseng CH, Huang YK, Huang YL, et al. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206:299–308. doi: 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Anderson WL, Kitchin KT. Dimethylarsinic acid effects on DNA damage and oxidative stress related biochemical parameters in B6C3F1 mice. Cancer Lett. 1999;139:129–35. doi: 10.1016/s0304-3835(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee TC, Tanaka N, Lamb PW, Gilmer TM, Barrett JC. Induction of gene amplification by arsenic. Science. 1988;241:79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- 26.Nesnow S, Roop BC, Lambert G, et al. DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol. 2002;15:1627–34. doi: 10.1021/tx025598y. [DOI] [PubMed] [Google Scholar]
- 27.Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of bio-methylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Perspect. 2002;110(suppl):767–71. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbert V, Colman N. Folic acid and vitamin B12. In: Shils M, Young VR, editors. Modern nutrition in health and disease. 7. Philadelphia, PA: Lea & Febiger; 1988. pp. 388–416. [Google Scholar]
- 29.Gamble MV, Ahsan H, Liu X, et al. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. Am J Clin Nutr. 2005;81:1372–7. doi: 10.1093/ajcn/81.6.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): a description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 31.van Geen A, Zheng Y, Versteeg R, et al. Spatial variability of arsenic in 6000 tube wells in a 25 km2 area of Bangladesh. Water Resources Res. 2003;39:1140–50. [Google Scholar]
- 32.Cheng Z, Zheng Y, Mortlock R, van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–8. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 33.Nixon D, Mussmann G, Eckdahl S, Moyer T. Total arsenic in urine: palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin Chem. 1991;37:1575–9. [PubMed] [Google Scholar]
- 34.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–7. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 35.Reuter W, Davidowski L, Neubauer K. Speciation of five arsenic compounds in urine by HPLC/ICP-MS. [accessed on 1 August 2003];Perkin Elmer Application Notes. 2003 Internet: http://las.perkinelmer.com/content/ApplicationNotes/d_6736_screen.pdf.
- 36.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2. [PubMed] [Google Scholar]
- 37.Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–23. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]
- 38.De Kimpe J, Cornelis R, Vanholder R. In vitro methylation of arsenite by rabbit liver cytosol: effect of metal ions, metal chelating agents, methyltransferase inhibitors and uremic toxins. Drug Chem Toxicol. 1999;22:613–28. doi: 10.3109/01480549908993171. [DOI] [PubMed] [Google Scholar]
- 39.Spiegelstein O, Lu X, Le XC, et al. Effects of dietary folate intake and folate binding protein-1 (Folbp1) on urinary speciation of sodium arsenate in mice. Toxicol Lett. 2003;145:167–74. doi: 10.1016/s0378-4274(03)00307-2. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelstein O, Gould A, Wlodarczyk B, et al. Developmental consequences of in utero sodium arsenate exposure in mice with folate transport deficiencies. Toxicol Appl Pharmacol. 2005;203:18–26. doi: 10.1016/j.taap.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005;113:1153–9. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitra SR, Mazumder DN, Basu A, et al. Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environ Health Perspect. 2004;112:1104–9. doi: 10.1289/ehp.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr. 2000;72:998–1003. doi: 10.1093/ajcn/72.4.998. [DOI] [PubMed] [Google Scholar]
- 45.Gregory JF, III, Williamson J, Liao JF, Bailey LB, Toth JP. Kinetic model of folate metabolism in nonpregnant women consuming [2H2]folic acid: isotopic labeling of urinary folate and the catabolite para-acetamidobenzoylglutamate indicates slow, intake-dependent, turnover of folate pools. J Nutr. 1998;128:1896–906. doi: 10.1093/jn/128.11.1896. [DOI] [PubMed] [Google Scholar]
- 46.Gamble MV, Liu X. Urinary creatinine and arsenic metabolism. Environ Health Perspect. 2005;113:A442. doi: 10.1289/ehp.113-a442a. (letter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–35. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- 48.Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab. 2001;281:E1095–100. doi: 10.1152/ajpendo.2001.281.5.E1095. [DOI] [PubMed] [Google Scholar]
- 49.Korzun WJ. Oral creatine supplements lower plasma homocysteine concentrations in humans. Clin Lab Sci. 2004;17:102–6. [PubMed] [Google Scholar]
- 50.Fatterpaker P, Marfatia U, Sreenivasan A. Influence of folic acid and vitamin B12 on formation of creatine in vitro and in vivo. Nature. 1951;167:1067–8. doi: 10.1038/1671067a0. [DOI] [PubMed] [Google Scholar]
- 51.Stekol JA, Weiss S, Smith P, Weiss K. The synthesis of choline and creatine in rats under various dietary conditions. J Biol Chem. 1953;201:299–316. [PubMed] [Google Scholar]
- 52.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 53.Rauh M, Verwied S, Knerr I, Dorr HG, Sonnichsen A, Koletzko B. Homocysteine concentrations in a German cohort of 500 individuals: reference ranges and determinants of plasma levels in healthy children and their parents. Amino Acids. 2001;20:409–18. doi: 10.1007/s007260170037. [DOI] [PubMed] [Google Scholar]