Abstract
Recent research has demonstrated that mucocutaneous epithelial cells express functional nicotinic acetylcholine receptors (nAChRs) and that tobacco-derived carcinogenic nitrosamines, such as 4-(methylnitrosamino)-1-(3–pyridyl)-1-butanone (NNK), and SLURP (secreted mammalian Ly-6/urokinase plasminogen activator receptor-related protein)-1 and -2 can act as non-canonical ligands of these receptors. It was found that recombinant SLURP-1 and -2 can lessen tumorigenic activity of nitrosamines. The immortalized esophageal keratinocytes (Het-1A cells) exhibit low SLURP-1 and -2 mRNA levels that decrease further after treatment with NNK. Based on these observations, we hypothesized that overexpression of full length SLURP proteins may protect Het-1A cells from malignant transformation by NNK. The Het-1A cells transfected with either SLURP-1 or -2 vector produced the highest amounts of respective proteins between 24 and 48 h, at which point they were exposed to 1 μM NNK for 24 h and their tumorigenic activities were subsequently evaluated by plating in soft agar and injecting subcutaneously to Nu/Nu mice. Transfection with either SLURP-1 or -2 cDNA in both cases significantly (p<0.05) diminished the number of colonies produced by NNK exposed cells. SLURP-1 was more efficient than SLURP-2 in abolishing the tumorigenic effect in nude mice. Thus, the anti-tumorigenic activities of SLURP-1 and -2 were demonstrated both in vitro and in vivo. The obtained results suggest that SLURP-like proteins may become useful for developing novel anti-cancer therapies.
Keywords: nicotinic acetylcholine receptor, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, Het-1A cells, SLURP-1, SLURP-2
INTRODUCTION
Recent research has demonstrated that mucocutaneous epithelial cells express functional nicotinic acetylcholine receptors (nAChRs) and that tobacco-derided nitrosamines and SLURP (secreted mammalian Ly-6/urokinase plasminogen activator receptor-related protein)-1 and -2 are new non-canonical ligands of these receptors. Similarly to normal human oral keratinocytes [1], Het-1A cells—an established clonal population of SV40-immortalized human esophageal epithelial cells [2]—express α3, α5, α7, α9, β2, and β4 nAChR subunits [3]. They are anchorage-dependent cell and do not form tumors in immunosuppressed host animals. The nAChRs in Het-1A cells can be stimulated by the nitrosamine 4-(methylnitrosamino)-1-(3–pyridyl)-1-butanone (NNK), and their non-tumorigenic phenotype can be converted to progressively growing cells producing tumors in nude mice [3]. We have recently demonstrated that recombinant SLURP-1 and -2 can compete with the nicotinic radioligands [3H]nicotine and [3H]epibatidine for binding to the keratinocyte plasma membrane [4, 5]. Both SLURPs diminished tumorigenic effects of tobacco nitrosamines on Het-1A cells [6]. Compared to normal keratinocytes, the levels of gene expression of SLURP-1 and -2 in Het-1A cells as well as in the malignant keratinocytes SCC-25 and FaDu are significantly decreased [6]. We could not detected either SLURP protein in the 120-fold concentrated supernatants of Het-1A cell cultures, whereas the protein bands corresponding to SLURP-1 and -2 could be visualized in 80-fold concentrated supernatants of cultures of normal keratinocytes. The levels of SLURP-1 and -2 mRNA levels in Het-1A cells decreased further after treatment of cells with NNK [6]. These preliminary results suggested that SLURPs may protect keratinocytes from malignant transformation.
In this study, we determined the effect of overexpression of SLURP-1 and -2 on the ability of NNK to induce malignant transformation of Het-1A cells. We hypothesized that increased production of SLURP-1 or SLURP-2 may protect Het-1A cells from the carcinogenic action of NNK. The obtained results demonstrated the ability to diminish the tumorigenic activity of NNK due to overexpression of SLURP proteins, suggesting that SLURP-like proteins may become useful for developing novel approaches to anti-cancer therapy.
METHODS
Cells and reagents
The Het-1A cell line was purchased from ATCC (Manassas, VA) and grown in the Cambrex brand bronchial cell medium without retinoic acid (Cambrex Bio Sciences, Walkersville, MD). NNK was purchased from Toronto Research Chemicals (North York, ON, Canada). The anti-SLURP-1 monoclonal antibody 336H12-1A3 and the anti-SLURP-2 monoclonal antibody 341F10-1F12 characterized in a previous study [4, 5] are commercially available from Research and Diagnostic Antibodies (North Las Vegas, NV). The SLURP-1 and -2 cDNAs were cloned into the pCMV TrueClone Vectors and purified by OriGene Technologies (Rockville, MD). The SLURP-1 had a full-length 400 bp cDNA, and SLURP-2 had a 600 bp insert size. Both fragments were directionally inserted downstream from a eukaryotic transcriptional promoter. The control plasmid vector pMEV was purchased from Biomyx Technology (San Diego, CA). The TransIT-TKO transfection reagent was from Mirus (Madison, WI). To amplify and purify the SLURP-1 and -2 cDNA plasmids, we used One Shot TOP10 cells (Invitrogen, Carlsbad, CA) and the Mega Plasmid Purification kit (Qiagen, Valencia, CA), respectively.
Overexpression of SLURP-1 and -2
Het-1A cells were seeded at a density of 5 × 104 cells per well of a 24-well plate and incubated overnight to allow cells to adhere to the dish bottom. To each well, increasing concentrations of SLURP-1 or -2 cDNA in the transfection solution with the TransIT-TKO transfection reagent were added, and the transfection was continued for 24 h at 37 °C in a humid, 5% CO2 incubator. On the next day, the transfection medium was replaced by the culture medium, and the cells were incubated for additional periods of time to determine the point of maximum overexpression. The overexpression of SLURP proteins started approximately 24 h after transfection.
Detection of SLURP-1 and -2
For immunoblotting experiments, the monolayers of experimental and control Het-1A cells or 20-fold concentrated supernatants of cell monolayers were dissolved in a sample buffer, separated via 4–10% SDS-PAGE, and electroblotted onto a 0.2 μm nitrocellulose membrane (Bio-Rad, Hercules, CA). The membranes were developed using the ECL + Plus chemiluminescent detection system (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) and scanned with Storm™/FluorImager (Molecular Dynamics, Mountain View, CA). For indirect immunofluorescence, we used monolayers of experimental and control Het-1A cells grown on coverslips. The cells were air dried for 15 min, fixed with 3% paraformaldehyde with 7% sucrose for 7 min, washed, permeabilized with 0.1% Triton X-100 and incubated overnight at 4 °C with anti-SLURP-1 or -2 monoclonal antibody diluted 1:10 in PBS. After washing, the monolayers were exposed for 1 h at room temperature to the secondary, fluorescein-isothiocyanate conjugated rabbit anti-mouse IgG antibody (Sigma-Aldrich Corporation, St. Louis, MO).
Analysis of NNK-induced transformation of Het-1A cells
Approximately 80% confluent, intact or transfected Het-1A cell monolayers were exposed for 24 h to 1 μM of NNK. The stock solution of NNK (100 mM dimethyl sulfoxide) was diluted in the culture medium. After incubation, the cells were washed thrice with prewarmed PBS and either replated into soft agar plates for the studies of anchorage-independent growth or inoculated into athymic nude mice. Anchorage independent growth was studied using a soft agar assay as described in detail elsewhere [3]. After 6 weeks, colonies with more than 50 cells were scored with the aid of an inverted microscope. The Nu/Nu mice were purchased from the Jackson Laboratory (Bar Harbor, ME), housed four to a cage in polypropylene boxes with Teklad bedding (Harlan Teklad, Madison, WI), and allowed free access to food and water during the entire experiment. The experimental and control Het-1A cells (1.5 × 107 cells in 0.2 ml of 0.9% saline) were injected subcutaneously (s.c.) into 2 different sites (interscapular area and lower back) on each side of the body with Het-1A cells. The skin at the inoculation sites was marked with a permanent pen. Six weeks after injection, the mice were euthanized and areas of injections were excised, stained with hematoxylin and eosin and examined microscopically. The results were expressed as percentage of tumor nodules >0.5 cm in diameter at the injection site per each mouse, as follows: 1 nodule equals 25%, 2 = 50%, 3 = 75% and 4 =100%.
Statistical Analysis
All experiments were performed in triplicate and the results were expressed as mean ± SD. Statistical significance was determined using Student’s t-test. Differences were deemed significant if the calculated p value was <0.05.
RESULTS
Overexpression of SLURP-1 and -2 in Het-1A cells
Cells transfected with either SLURP-1 or -2 cDNA produced the highest amounts of respective proteins at 48 h after transfection (Figure 1). SLURP-1 and -2 had an apparent molecular weigh of 9 kDa. Both SLURP proteins were also detected in culture supernatants. Neither intact (Figure 1) nor pMEV-transfected (not shown) Het-A1 cells contained SLURP-1 or -2 proteins detectable by immunoblotting. SLURP-1 and -2 were also visualized in the cytoplasm of Het-1A cells by immunofluorescence (Figure 2). Both monoclonal antibodies produced diffuse homogenous cytoplasmic staining. In contrast to intact control Het-1A cells that exhibited a faint staining, the cells overexpressing SLURP-1 or -2 stained very brightly (Figure 2). Such cells were used in the NNK exposure experiments.
Figure 1. Visualization of SLURP-1 and -2 in SDS-PAGE gels.
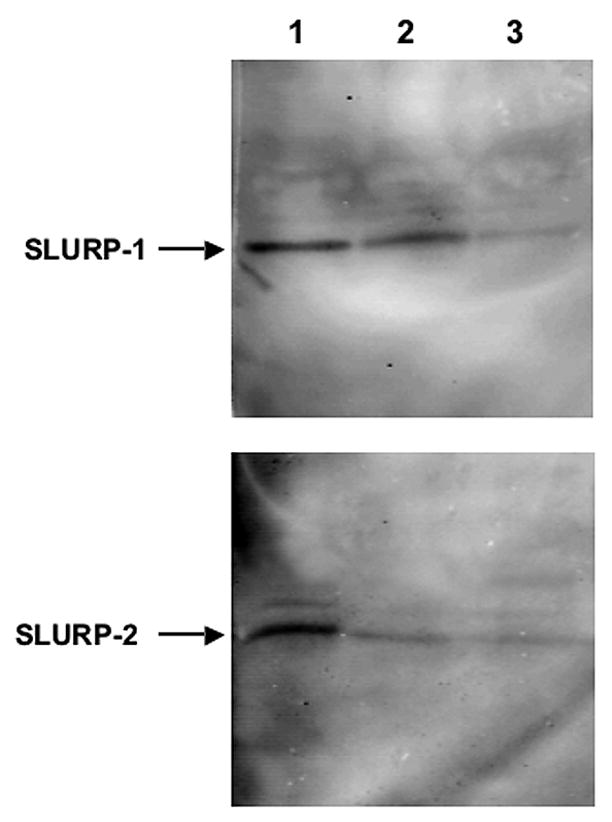
Visualization of the 9 kDa SLURP-1 and -2 proteins in 10% SDS-PAGE gel of Het-1A cells transfected with the respective cDNA vectors 48 after transfection (lane 1), culture supernatants of transfected Het-1A cells (lane 2) and homogenates of intact Het-1A cells (lane 3) using the mouse monoclonal antibodies 336H12-1A3 and 341F10-1F12, respectively, diluted 1:100.
Figure 2. Visualization of SLURP-1 and -2 in cultured Het-1A cells.

Using the anti-SLURP-1 and -2 monoclonal antibodies diluted 1:10, the respective proteins were visualized in the cytoplasm of intact Het-1 A cells (control) and Het-1 A cell transfected with SLURP-1 or -2 cDNA vectors (experiment), using the protocol detailed in Methods. The Het-1A cells overexpressing either SLURP protein demonstrated bright homogenous cytoplasmic staining. Bar = 50 μm.
Overexpression of SLURP proteins alleviates the tumorigenic effects of NNK
At 24 h after transfection, the Het-1A cells transfected with either control or SLURP-1 or -2 vector were exposed to NNK for 24 h, after which their tumorigenic activities were evaluated by plating the cells in soft agar and injecting them s.c. to Nu/Nu mice. As expected, treatment with NNK resulted in acquisition of tumorigenic phenotype. The cells formed expanding colonies containing cell clumps, or plaques in soft agar and produced squamous cell carcinoma-like tumor nodules of >0.5 cm in diameter in mouse skin (Figure 3). Transfection with either SLURP-1 or -2 cDNA in both cases significantly (p<0.05) diminished the number of colonies produced by NNK exposed cells. SLURP-1 was more efficient than SLURP-2 in abolishing the tumorigenic effect in nude mice (Figure 3). There were no differences in the morphology or localization of tumors produced by intact Het-1A cells or transfected Het-1A cells (not shown). Thus, the anti-tumorigenic effects of SLURP-1 and -2 were demonstrated both in vitro and in vivo.
Figure 3. Effects of overexpression of SLURP-1 and -2 on the tumorigenic activity of NNK in Het-1A cells.
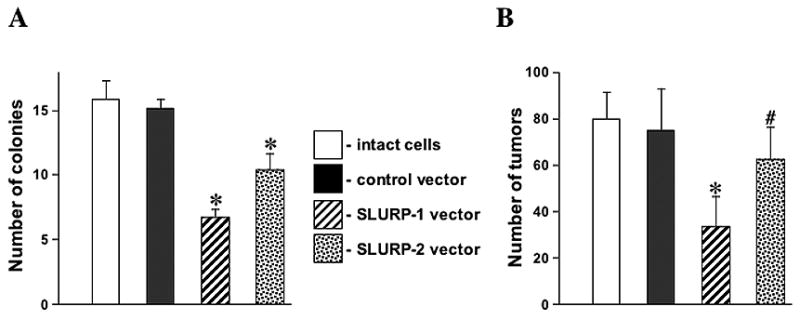
Intact Het-1A cells or the cells transfected with either control or SLURP-1 or -2 vectors were preincubated for 24 h with 1 μM of NNK, after which their tumorigenic activities were measured in vitro and in vivo as detailed in Methods. Asterisks indicate significant (p<0.05) differences compared to results obtained using intact Het-1A cells exposed to NNK. Pound sign indicates p=0.05.
A, Overexpression of SLURP-1 or -2 in each case reduces numbers of colonies in soft agar produced by nitrosamine-treated Het-1A cells.
B, Overexpression of SLURP-1 or -2 in each case reduces number of tumor nodules
DISCUSSION
This study was designed to evaluate the abilities of endogenously produced and secreted SLURP-1 and -2 to protect immortalized esophageal keratinocytes from tumorigenic transformation induced by the tobacco-derived nitrosamine NNK. It was found that overexpression of SLURPs reduced, albeit incompletely, the number of colonies in soft agar and the number of tumor nodules >0.5 cm in diameter in mouse skin formed by the NNK-treated Het-1A cells, with SLURP-1 being more efficient than SLURP-2.. Since the nAChR subtypes expressed in mucocutaneous epithelial cells can be ligated by both tobacco nitrosamines and SLURPs [3–5], SLURP-1 and -2 might exhibit their anti-nitrosamine activity by competing with NNK at the nAChR ligand-binding site.
Previous studies have demonstrated that nAChRs expressed by non-neuronal cells can be involved in malignant transformation caused by tobacco-derived carcinogenic nitrosamines [3, 7]. Het-1A cells express both the heteromeric and homomeric nAChR channels [3]. It has been shown in experiments with Het-1A cells and some other epithelial cells that NNK preferentially binds to the α7 nAChR subtype [3, 8–10]. Cumulative results suggested that SLURP-1 preferentially binds to and activates homomeric acetylcholine channels, such as α7 nAChRs, evoking intracellular Ca2+ signaling, whereas SLURP-2 seems to act through heteromeric receptors, such as α3 nAChRs [4, 5, 11, 12].
Both nitrosamines and SLURPs cause profound effects on vital cell functions and gene expression [3–5, 7]. In a previous study, we found that recombinant SLURP-1 and -2 can lessen tumorigenic activities of tobacco-derived nitrosamines [6]. The intact Het-1A cells had decreased SLURP-1 and -2 mRNA levels that further decreased after treatment of cells with carcinogenic nitrosamines. Based on these findings, we hypothesized that overexpression of SLURP proteins may protect Het-1A cells from malignant transformation. The results of this study demonstrated feasibility of this approach. Lack of complete inhibition of the tumorigenic effects of nitrosamines on the Het-1A cells overexpressing SLURP-1 or -2 may be due to an inability to completely prevent binding of NNK to cellular nAChRs and/or a non-receptor mechanism of the carcinogenic activity of NNK. Future studies should determine if a mixture of two SLURP proteins will have a higher efficacy.
It is well known that the methylating and pyridyloxobutylating agents derived from tissue metabolism of NNK can attack DNA leading to mutations [13]. Therefore, it can be postulated that the tumorigenic action of nitrosamines includes at least two “hits,” an extracellular one—activation of nAChRs, and an intracellular one—DNA damage. W hile pharmacologic agonists of nAChRs activate receptors at the cell membrane they are not carcinogenic because they lack the intracellular tumorigenic activity characteristic of nitrosamines.
SLURP-1 and -2 are abundantly expressed in various types of human cells and tissues [4–6, 11, 12, 14–18]. Both SLURP proteins can regulate proliferation, apoptosis and differentiation of keratinocytes, and also may be involved in regulation of inflammation [4, 5, 11, 17]. The biologic effects of SLURP-1 and -2, however, may differ due to their differential binding to the nAChR subtypes expressed in each cell type. Since patients with autosomal dominant palmoplantar keratodermas, such as Mal de Meleda that features mutations of the gene encoding SLURP-1 [19], have an increased risk for developing esophageal carcinoma and other types of mucocutaneous and internal malignancies [20, 21], and since the SLURP-1 and -2 levels are decreased in malignant and immortalized cells [6], mutations affecting the integrity of SLURP proteins may play a role in an increased susceptibility to cancer.
The transformed epithelial cells have a functional non-neuronal cholinergic system for signal transduction with acetylcholine as a single extracellular messenger, or a cytotransmitter (reviewed in [22]). The existence of a cholinergic autocrine loop in epithelial cells explains certain aspects of the carcinogenic effects of tobacco products, and provides a basis for identification of new targets for potential therapeutic intervention. Although our observations of nAChR involvement in the pro-oncogenic effects of nitrosamines and anti-oncogenic effects of SLURPs are rather limited, the obtained results clearly demonstrate that the oncogenic potential of nitrosamines can be diminished. Future studies should establish contribution of specific nAChR subtypes to mediating pro-oncogenic action of tobacco-derived nitrosamines and protective effects of SLURP proteins. In nature, the biological role of SLURPs maybe to protect a cell from malignant transformation, and downregulation or aberrant expression of SLURPs may cause a loss of the protective function and lead to cancer. Hence, SLURP-like peptides may serve as a prototype of anti-cancer drugs to prevent, reverse, or retard oncogenic transformation of epithelial cells.
Acknowledgments
This work was supported by the NIH grants CA117327, ES014384 and DE14173, and a research grant from Flight Attendant Medical Research Institute to S.A.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nguyen VT, Hall LL, Gallacher G, Ndoye A, Jolkovsky DL, Webber RJ, Buchli R, Grando SA. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939–49. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- 2.Stoner GD, Kaighn ME, Reddel RR, Resau JH, Bowman D, Naito Z, Matsukura N, You M, Galati AJ, Harris CC. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 1991;51:365–71. [PubMed] [Google Scholar]
- 3.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–7. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 4.Arredondo J, Chernyavsky AI, Jolkovsky DL, Webber RJ, Grando SA. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol. 2006;208:238–45. doi: 10.1002/jcp.20661. [DOI] [PubMed] [Google Scholar]
- 5.Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125:1236–41. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- 6.Arredondo J, Chernyavsky AI, Grando SA. SLURP-1 and -2 in normal, immortalized and malignant oral keratinocytes. Life Sci. 2007;80:2243–7. doi: 10.1016/j.lfs.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arredondo J, Chernyavsky AI, Grando SA. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J Cancer Res Clin Oncol. 2006;132:653–63. doi: 10.1007/s00432-006-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jull BA, Plummer HK, 3rd, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. J Cancer Res Clin Oncol. 2001;127:707–17. doi: 10.1007/s004320100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plummer HK, 3rd, Dhar M, Schuller HM. Expression of the α7 nicotinic acetylcholine receptor in human lung cells. Respir Res. 2005;6:29. doi: 10.1186/1465-9921-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheppard BJ, Williams M, Plummer HK, Schuller HM. Activation of voltage-operated Ca2+-channels in human small cell lung carcinoma by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Oncol. 2000;16:513–8. doi: 10.3892/ijo.16.3.513. [DOI] [PubMed] [Google Scholar]
- 11.Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–24. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- 12.Moriwaki Y, Yoshikawa K, Fukuda H, Fujii YX, Misawa H, Kawashima K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 2007 doi: 10.1016/j.lfs.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Nettesheim P. Cells of origin of primary pulmonary neoplasms in mice. Exp Lung Res. 1991;17:215–7. doi: 10.3109/01902149109064412. [DOI] [PubMed] [Google Scholar]
- 14.Adermann K, Wattler F, Wattler S, Heine G, Meyer M, Forssmann WG, Nehls M. Structural and phylogenetic characterization of human SLURP-1, the first secreted mammalian member of the Ly-6/uPAR protein superfamily. Protein Sci. 1999;8:810–9. doi: 10.1110/ps.8.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mastrangeli R, Donini S, Kelton CA, He C, Bressan A, Milazzo F, Ciolli V, Borrelli F, Martelli F, Biffoni M, Serlupi-Crescenzi O, Serani S, Micangeli E, El Tayar N, Vaccaro R, Renda T, Lisciani R, Rossi M, Papoian R. ARS Component B: structural characterization, tissue expression and regulation of the gene and protein (SLURP-1) associated with Mal de Meleda. Eur J Dermatol. 2003;13:560–70. [PubMed] [Google Scholar]
- 16.Favre B, Plantard L, Aeschbach L, Brakch N, Christen-Zaech S, de Viragh PA, Sergeant A, Huber M, Hohl D. SLURP1 is a late marker of epidermal differentiation and is absent in Mal de Meleda. J Invest Dermatol. 2007;127:301–8. doi: 10.1038/sj.jid.5700551. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji H, Okamoto K, Matsuzaka Y, Iizuka H, Tamiya G, Inoko H. SLURP-2, a novel member of the human Ly-6 superfamily that is up-regulated in psoriasis vulgaris. Genomics. 2003;81:26–33. doi: 10.1016/s0888-7543(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007 doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 19.Fischer J, Bouadjar B, Heilig R, Huber M, Lefevre C, Jobard F, Macari F, Bakija-Konsuo A, Ait-Belkacem F, Weissenbach J, Lathrop M, Hohl D, Prud’homme JF. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Hum Mol Genet. 2001;10:875–80. doi: 10.1093/hmg/10.8.875. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro U, Jr, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174–85. [PubMed] [Google Scholar]
- 21.Mozzillo N, Nunziata CA, Caraco C, Fazioli F, Botti G. Malignant melanoma developing in an area of hereditary palmoplantar keratoderma (Mal de Meleda) J Surg Oncol. 2003;84:229–33. doi: 10.1002/jso.10317. [DOI] [PubMed] [Google Scholar]
- 22.Grando SA, Kawashima K, Kirkpatrick CJ, Wessler I. Recent progress in understanding the non-neuronal cholinergic system in humans. Life Sci. 2007;80:2181–5. doi: 10.1016/j.lfs.2007.03.015. [DOI] [PubMed] [Google Scholar]


