Abstract
Transgenic tobacco deficient in the H2O2-removing enzyme catalase (Cat1AS) was used as an inducible and noninvasive system to study the role of H2O2 as an activator of pathogenesis-related (PR) proteins in plants. Excess H2O2 in Cat1AS plants was generated by simply increasing light intensities. Sustained exposure of Cat1AS plants to excess H2O2 provoked tissue damage, stimulated salicylic acid and ethylene production, and induced the expression of acidic and basic PR proteins with a timing and magnitude similar to the hypersensitive response against pathogens. Salicylic acid production was biphasic, and the first peak of salicylic acid as well as the peak of ethylene occurred within the first hours of high light, which is long before the development of tissue necrosis. Under these conditions, accumulation of acidic PR proteins was also seen in upper leaves that were not exposed to high light, indicating systemic induction of expression. Short exposure of Cat1AS plants to excess H2O2 did not cause damage, induced local expression of acidic and basic PR proteins, and enhanced pathogen tolerance. However, the timing and magnitude of PR protein induction was in this case more similar to that in upper uninfected leaves than to that in hypersensitive-response leaves of pathogen-infected plants. Together, these data demonstrate that sublethal levels of H2O2 activate expression of acidic and basic PR proteins and lead to enhanced pathogen tolerance. However, rapid and strong activation of PR protein expression, as seen during the hypersensitive response, occurs only when excess H2O2 is accompanied by leaf necrosis.
Keywords: catalase, ethylene, pathogenesis-related proteins, salicylic acid, systemic acquired resistance
Plants contain a whole array of cellular mechanisms to defend themselves against invading pathogens. In many cases, pathogen recognition by a host activates the so-called hypersensitive response (HR), which is a resistance response characterized by localized cell death, production of superoxide and hydrogen peroxide, deposition of callose, strengthening of cell walls, and the synthesis of secondary metabolites and proteins with antimicrobial activity (1). All these responses are deployed between minutes and days after infection. Besides this acute defense at the infection site, some necrotizing pathogens also induce defense responses in distal parts of host plants. Activation of these distal defenses lasts for weeks to months and enhances the resistance against secondary infection by a broad range of unrelated pathogens. In certain plant species, this induced “immunity” develops only in nonchallenged parts of infected leaves (local acquired resistance or LAR), whereas in others such as tobacco, Arabidopsis, and cucumber, it is also seen in upper noninfected leaves (systemic acquired resistance or SAR) (2). Induction of these distal responses is slow compared with local responses; e.g., it usually takes 1–2 weeks in the case of SAR in tobacco.
Development of SAR is associated with the expression of antimicrobial proteins, but it does not seem to involve any of the other local defense mechanisms against pathogens (3). SAR proteins are a subset of antimicrobial proteins that are also induced during HR, including primarily different classes of acidic pathogenesis-related (PR) proteins (4). The level of expression of these proteins is, however, often lower during SAR than during HR (5). The fact that low but constitutive expression of PR and other defense proteins effectively confers protection against pathogens underscores the importance of a timely expression of such proteins for the development of resistance. Consistent with this view is the observation that PR proteins are also induced during a sensitive response but with a delayed timing (ref. 6 and unpublished data). Consequently, understanding the molecular mechanisms that control the timing of expression of PR proteins holds considerable promise for the design of disease control strategies.
Salicylic acid (SA) has been identified as a key signal for the expression of PR proteins during LAR and SAR in tobacco, cucumber, and Arabidopsis. After pathogen infection, levels of SA increase in infected and uninfected leaves, and this SA accumulation is essential for the expression of PR proteins and for resistance during LAR and SAR (7–10). Also, H2O2 has been implicated as a signal in PR protein expression, but its role and position in the cascade are a matter of debate (11). Initially, H2O2 was proposed to function downstream of SA on the basis of evidence that SA can bind and inhibit the H2O2-removing enzymes catalase (Cat) and ascorbate peroxidase (12, 13). Nevertheless, subsequent studies with SA-degrading tobacco have indicated that local induction of the acidic PR-1 protein, a commonly used marker for SA-mediated expression, by exogenous H2O2 or H2O2-inducing chemicals requires SA (14, 15). Likewise, local induction of PR-1 in transgenic tobacco deficient in the H2O2-removing enzyme Cat was recently shown to depend on SA (16). These results do not exclude that binding of H2O2-removing enzymes by SA may increase H2O2 levels or have other effects, but they demonstrate that local induction of PR-1 by H2O2 involves an SA-dependent pathway.
H2O2 and other active oxygen species are rapidly produced after pathogen infection during the so-called oxidative burst (17, 18). The oxidative burst inhibitor diphenyleneiodonium (DPI) has been used to investigate the role of active oxygen species in defense responses against pathogens. Elicitor-induced expression of glutathione transferase in soybean (19) and of phytoalexins in parsley (20) was affected by inhibition of the oxidative burst with diphenyleneiodonium, whereas chalcone synthase expression in soybean (19) and phytoalexin production in tobacco (21) were unaltered. Whether H2O2 is also an integral part of the signaling cascade leading from pathogen recognition to SA production and PR protein expression remains controversial, in part because of the lack of a good inducible system for studying H2O2 effects in intact plants. We made use of transgenic tobacco deficient in Cat (Cat1AS) to compare responses induced by pathogens and H2O2 stress. Because of their impaired buffering capacity for H2O2, induction of H2O2 stress is facilitated in Cat1AS compared with wild-type plants and is achieved by simply increasing light intensities (22, 23). We report here that H2O2 modulation induces local expression of acidic and basic PR proteins in the absence of necrosis, but that strong local induction and systemic induction of PR proteins, as seen during HR and SAR, respectively, is achieved only when H2O2 stress is accompanied by necrosis.
MATERIALS AND METHODS
Plant Growth Conditions and Pathogen Treatment.
Cat1AS and control plants were precultivated under low light (LL) (80 μmol⋅m−2⋅s−1 photosynthetic photon fluence rates (PPFR) (400–700 nm), 14 h light/10 h dark) at 25°C and 70% relative humidity. Mature preflowering plants were used for all experiments. High light (HL) treatment consisted of 250 μmol⋅m−2⋅s−1 PPFR, 14 h light/10 h dark, in all experiments except for those described in Figs. 1 and 4, where 1,000 μmol⋅m−2⋅s−1 PPFR, 14 h light/10 h dark, was applied. LL post-treatments were always at 80 μmol⋅m−2⋅s−1 PPFR, 14 h light/10 h dark. Pathogen treatments and determination of bacterial titers were performed as described (22).
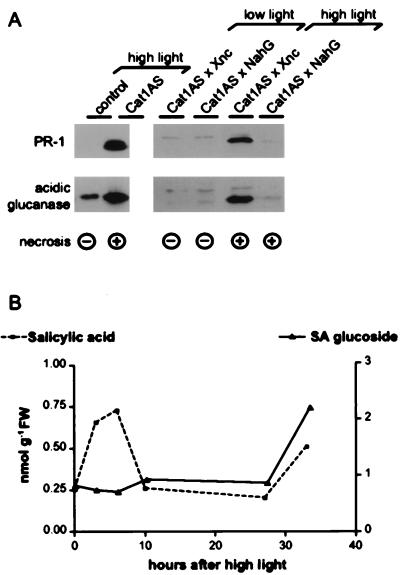
Figure 1.
Expression of acidic PR proteins in Cat-deficient tobacco and SA requirement for induction. Cat1AS and control plants precultivated at LL were exposed to HL for 2 days. (A) Western blot analysis showing high expression of acidic PR proteins in Cat1AS plants exposed to HL. Cat1AS × Xnc is a cross of homozygous Cat1AS and Xanthi nc lines, whereas Cat1AS × NahG is a cross of homozygous Cat1AS and a transgenic Xanthi nc line containing the NahG gene of Pseudomonas putida, which codes for an SA hydroxylase (9). (B) Time course analysis of SA and SA β-glucoside (SAG) accumulation in Cat1AS plants after HL induction. FW, fresh weight.
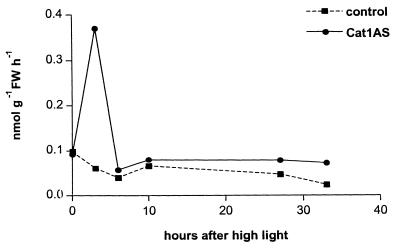
Figure 4.
Time course analysis of ethylene formation in Cat1AS plants after exposure to HL. Cat1AS and control plants precultivated at LL were exposed to HL for 2 days and leaf samples were taken for ethylene quantitation at the times indicated.
Protein Extraction and Immunodetection.
Proteins were extracted from leaves from which primary and secondary veins were removed as described (22). Fifty micrograms of total proteins was separated on SDS/PAGE, blotted, and immunodetected as described (23). Primary antibodies used were 1:4000-diluted polyclonal anti-acidic PR-1 antibody (24), 1:2000-diluted polyclonal anti-acidic glucanase antibody (5), 1:2000-diluted polyclonal anti-basic glucanase antibody (5), and 1:5000-diluted anti-GPx antibody (unpublished results). All primary antibodies were incubated for 3 h at room temperature.
SA and Ethylene Determination.
Extraction and quantitation of free and conjugated SA were performed basically according to Meuwly and Métraux (25). SA was detected by using a Shimazu RF 535 fluorescence detector at excitation and emission wavelengths of 305 and 407 nm, respectively.
Ethylene accumulation and accumulation of free 1-aminocyclopropane-1-carboxylate (ACC) were measured as described by Tuomainen et al. (26) with the use of a Perkin—Elmer Autosystem XL gas chromatograph equipped with a Porapak Q column (80 × 100 mesh, 1.22 m long, 2 mm i.d.; Supelco) and a flame ionization detector.
Detection of Cellular Deterioration by Confocal Scanning Laser Microscopy.
YOYO-1 iodide (Molecular Probes) is a nuclear dye that is membrane-impermeant and therefore stains only nuclei of damaged cells. YOYO-1 iodide (1 mM in phosphate-buffered saline) was infiltrated in the leaf through the stomata with a syringe without a needle, and leaf pieces were cut after 1 h. Excess YOYO-1 iodide that had not intercalated in the DNA was subsequently washed out by incubation of the leaf pieces in 1 ml of phosphate-buffered saline. Double-scanned optical sections were generated with a confocal laser microscope (CSLM 510, Zeiss) with argon laser excitation at 488 nm and a band-pass emission filter of 505–530 nm and with helium–neon laser excitation at 543 nm and a long-pass emission filter of 560 nm.
RESULTS
H2O2 is removed very rapidly in plants (19), implying that exposure to exogenously applied H2O2 is very brief and requires very high doses to exert any effects (14, 15). In contrast, generation of H2O2 during HR is a sustained response (17). To understand which defense responses are activated by sustained exposure to excess H2O2, we used transgenic tobacco plants that express only 10% of wild-type Cat activity (Cat1AS) because of antisense expression of one of the Cat genes (22). This severe reduction in Cat activity had no apparent consequences under LL. Yet exposure to moderate or HL intensities caused necrosis on the leaves of Cat1AS but not of wild-type plants, indicating that H2O2 production at elevated light exceeds the impaired scavenging capacity of Cat1AS plants. Increased H2O2 production at HL was attributed to photorespiration, because inhibition of photorespiration prevented necrosis (23). Hence, H2O2 stress in Cat1AS plants follows photorespiration and can be induced by modulation of light conditions without the need of invasive techniques.
SA Accumulation and Local Expression of Acidic PR Proteins.
Previously, we demonstrated that Cat1AS plants do not express PR-1 constitutively, but induce PR-1 accumulation after exposure to HL (22) (Fig. 1A, lane 2). Induction of PR-1 expression coincided with the development of macroscopic lesions on the leaves (22) (see also Fig. 2 Right) and was not observed in HL-treated control plants (22) (Fig. 1A, lane 1). HL also induced the expression of acidic glucanase (Fig. 1A, lane 2) and acidic chitinase (data not shown) in Cat1AS plants, indicating that the same set of acidic PR proteins are induced during Cat deficiency and after pathogen infection. Expression of acidic PR proteins is induced by SA, and systemic induction of acidic PR proteins by pathogens was shown to depend on SA accumulation (27).
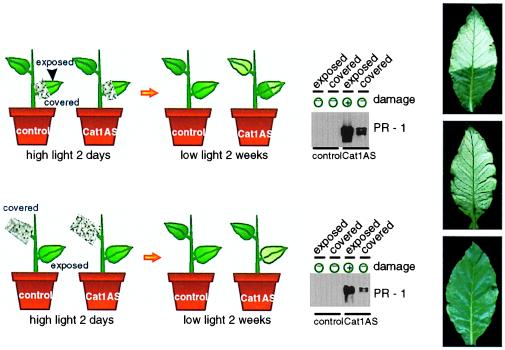
Figure 2.
Systemic expression of PR-1 in local and distal leaves of Cat1AS plants after HL induction. Cat1AS and control plants precultivated at LL were exposed to HL for 2 days and then returned for 2 weeks at LL before harvest. (Upper Left) Half of a leaf was covered with foil during the HL treatment for analysis of LAR gene expression. Parts of the leaf that had been covered during the HL exposure showed no visible necrosis, whereas the exposed parts of the same leaf developed necrotic lesions within 2 days after the HL treatment. The immunoblot shows PR-1 accumulation in HL-exposed and covered parts of the same leaf. The presence of visible leaf damage at the time of harvest is indicated. (Lower Left) A top leaf was entirely covered to shield it from the light during the HL treatment to assess SAR gene expression. Samples were taken from a lower HL-exposed leaf that showed severe necrosis and from the HL-protected upper leaf, which was undamaged. Western blot analysis is as in the panel above. The presence of leaf damage at the time of harvest is indicated. (Right) Photographs showing representative leaves of Cat1AS plants harvested after HL treatment for analysis of LAR (Top) and SAR (damaged lower leaf and intact upper leaf in Middle and Bottom, respectively) gene expression.
To determine whether induction of PR-1 by H2O2 follows a SA-dependent pathway, the involvement of SA in PR-1 and acidic glucanase induction during Cat deficiency was investigated. Crosses were made between Cat1AS and transgenic tobacco that does not accumulate SA after pathogen infection because of ectopic expression of a bacterial SA-hydroxylase (NahG) (9). Progeny of this cross did not induce PR-1 and acidic glucanase expression after exposure to HL (Fig. 1A, lane 6), showing that SA is essential for acidic PR protein accumulation during Cat deficiency. This result is in accord with earlier reports on SA requirement for PR-1 induction by exogenous H2O2 (15) or Cat deficiency (16). However, previous measurements of SA levels in Cat-deficient tobacco had revealed a substantial rise in SAG, but only a slight increase in free SA (28), in contrast to pathogen-infected tissues, where both forms generally accumulate to high levels (e.g., ref. 29). This discrepancy may reflect differences in SA responses in tissues infected with pathogens or exposed to H2O2, or, alternatively, it could be due to a different timing of SA determination because conjugated and free SA are interconvertible, the ratio between the two possibly being altered with time (30, 31).
To address the validity of these explanations, time course measurements of SA and SAG in Cat1AS and wild-type plants were performed after exposure of Cat1AS and wild-type plants to HL. No increases in free SA or SAG were observed in wild-type plants exposed to HL (data not shown). In Cat1AS, free SA showed a first transient peak between 3 and 6 h after HL, which was before any signs of leaf injury were discernible (Fig. 1B). Free SA levels showed a second rise after 33 h of HL, and this increase was paralleled by changes in the conjugated form. The total levels of SA were highest in the second phase, because the conjugated form constituted the major pool of SA (see ordinates for free and conjugated SA in Fig. 1B), and maximal levels of the second phase were not reached within the shown time course (data not shown). Together, these results indicate that sustained exposure to H2O2 stress induces a biphasic increase in SA production, and this SA accumulation signals the expression of acidic PR proteins in leaves exposed to H2O2 stress.
Systemic Expression of Acidic PR Proteins.
Treatment of plants with SA (32) or SA-inducing chemicals (33) does not induce SAR or systemic PR gene expression, indicating that signals upstream of SA are required for the development of SAR. The nature of these signals, however, has thus far not been elucidated. To investigate whether localized production of H2O2 induces systemic PR responses, half parts of lower leaves and entire upper leaves were wrapped in foil during 2 days of HL exposure, which is the time required to obtain maximal induction of PR-1 in the HL-exposed leaves. Plants were subsequently returned to LL, and covered leaf parts were unwrapped. After 2 more weeks, PR-1 expression was analyzed in the covered leaf parts of Cat-deficient and wild-type plants (Fig. 2). Whereas wild-type plants did not show PR-1 expression, Cat-deficient plants accumulated PR-1 both in covered parts of HL-exposed leaves and in upper leaves that had been entirely covered, indicating that H2O2 functions as a trigger for both local and distal systemic PR responses.
Induction of Other Defense Responses.
To assess whether defense responses other than the acidic PR proteins are inducible by H2O2, expression of glutathione peroxidase (GPx) and basic glucanase (bPR-2) was analyzed in Cat1AS plants. GPx is part of the antioxidant defense and has previously been shown to be induced by H2O2 and by elicitors in soybean cell cultures (19). bPR-2 is used to index expression of PR proteins that are mainly activated through ethylene and not through SA (34). HL exposure of Cat1AS plants induced expression of both GPx and bPR-2 (Fig. 3). Interestingly, induction of GPx in Cat-deficient plants was observed not only in HL-exposed leaves but also during local and distal SAR (Fig. 3). bPR-2 mRNA is almost undetectable in uninfected leaves (4), but the protein has been shown to accumulate during LAR (5). Consistent with these data, bPR-2 induction in Cat1AS plants was observed in parts of the leaves that had been directly exposed to HL, as well as in leaves that developed LAR (Fig. 3). The enhanced expression of bPR-2 in these plants suggests that H2O2 induces stress ethylene biosynthesis. Indeed, analysis of ethylene levels in Cat1AS plants revealed a dramatic but transient increase in ethylene production, which peaked 3 h after exposure to HL (Fig. 4). This peak in ethylene emission correlated with elevated levels of its precursor, ACC. Levels of free ACC were approximately 4-fold higher in Cat1AS plants than in controls after 3 h at HL (0.86 and 0.24 nmol per g fresh weight, respectively). A more refined time-course analysis showed a sharp increment in ACC and ethylene production already after 2 h of HL (data not shown), which was earlier than the induction of SA production (Fig. 1B).
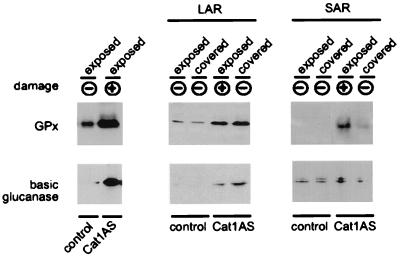
Figure 3.
Western analysis showing the effect of HL on the expression of GPx and bPR-2 in Cat1AS plants. Cat1AS and control plants precultivated at LL were exposed to HL for 2 days. Samples were harvested 2 days after HL initiation for analysis of protein expression in HL-exposed tissue (Left), or after 2 additional weeks at LL for analysis of LAR (Center) and SAR gene expression (Right). The presence of visible leaf damage at the time of harvest is indicated. The experimental design for analysis of LAR and SAR gene expression was as described in Fig. 2.
Induction of PR Protein Expression and Enhanced Pathogen Tolerance by Sublethal Levels of H2O2.
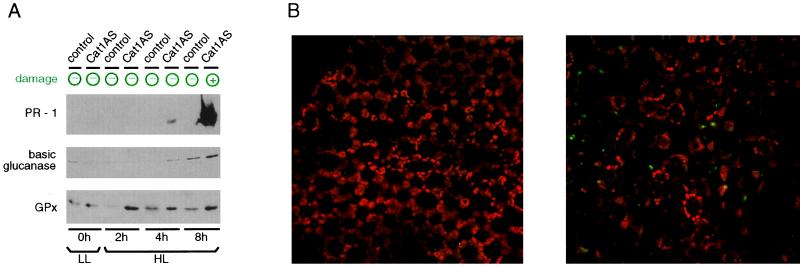
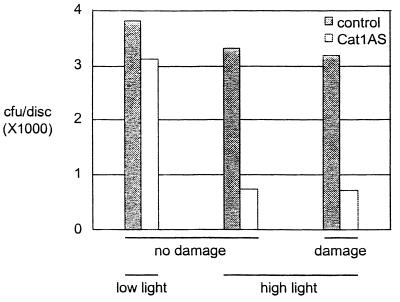
Induction of SA biosynthesis and PR-1 expression by pathogens, H2O2, or inappropriate expression of specific proteins correlates with the development of leaf necrosis (11). On the other hand, chemical or UV-B induction of SA responses occurs in the absence of any discernible tissue damage (33, 35). To investigate whether PR-1 induction in Cat1AS plants requires necrosis, experiments were designed to uncouple PR-1 induction from necrosis during Cat deficiency. Cat1AS plants were exposed to HL for various times, returned to LL, and analyzed for PR-1 expression. Whereas PR-1 expression in heavily necrotic leaves of Cat-deficient plants was similar in level and kinetics to that during HR, PR-1 accumulation after short treatments with HL was a relatively weak and late response, much like that during SAR (Fig. 5A). Lesions on Cat1AS plants appeared after 1–2 days and required 8 h of HL treatment to develop. Yet, PR-1 accumulation was already induced by 4 h of HL (Fig. 5A). Close inspection of Cat1AS plants treated with 4 h of HL did not reveal discernible lesions at any stage after the treatment and no premature senescence was seen in these plants (data not shown). Furthermore, no evidence was found for deterioration of single cells, as indexed by nuclear staining with the membrane-impermeant dye YOYO-1 iodide; this observation is in contrast to bleached leaves of Cat1AS or wounded controls (Fig. 5B; data not shown). Besides PR-1, GPx and bPR-2 were induced by short HL treatment in Cat1AS plants (Fig. 5A), indicating that general induction of defense proteins by H2O2 is not dependent on necrosis. Cat1AS plants in which defense activation was uncoupled from damage manifested also enhanced resistance against the bacterial pathogen Pseudomonas syringae pv. syringae (Fig. 6). This enhanced resistance correlated with the accumulation of defense proteins because wild-type plants exposed to HL and Cat-deficient plants kept at LL showed similar sensitivity to wild-type plants at LL. However, a linear relationship between the level of defense protein expression and tolerance was not observed, because Cat1AS plants expressing low or high levels of defense proteins showed similar degrees of protection.
Figure 5.
Necrosis-independent expression of defense proteins in Cat1AS plants. (A) Western blot analysis with PR-1, bPR-2, and GPx antibodies. Cat1AS and control plants were exposed to HL for various times (2–8 h) and then returned to LL for 2 weeks before leaf sampling. Expression of PR-1, bPR-2, and GPx was induced by 4 h of HL in Cat1AS, whereas necrosis required 8 h of exposure. PR-1 expression in the control line was not increased by HL. bPR-2 and GPx showed HL induction in the control line but not to the same level as in Cat1AS. Leaf damage was assessed at the time of harvest: no damage indicates that none of the leaves of that plant had any visible sign of injury. (B) Optical sections of a YOYO-1 iodide-stained leaf from a Cat1AS plant treated with HL for 4 h (Left) or 8 h (Right). YOYO-1 iodide is a nuclear dye that is membrane-impermeant and therefore stains only nuclei of damaged cells. (×700.)
Figure 6.
HL-induced protection of Cat1AS plants against Pseudomonas syringae pv. syringae. Cat1AS and control plants were either maintained at LL or exposed to HL for different times as described in the legend of Fig. 5 and returned to LL for 2 weeks. Leaf damage was assessed as described in Fig. 5. Then, Pseudomonas syringae pv. syringae at 106 per ml were inoculated by infiltration in the leaf and bacterial growth was determined after 24 h by plating serial dilutions of homogenate from inoculated tissue on solid medium (22). cfu, colony-forming units. The figure displays a representative result of three repetitions.
DISCUSSION
Transgenic plants in which H2O2 homeostasis is perturbed because of decreased scavenging capacity (22, 28) or increased production of H2O2 (36) show enhanced expression of acidic PR proteins in the absence of pathogen challenge. It was therefore suggested that H2O2 may be part of the signaling cascade leading from pathogen infection to acidic PR protein expression. Here, we have further explored this hypothesis by comparing the kinetics of acidic PR protein accumulation in response to H2O2 and to pathogens, and this for both toxic and sublethal doses of H2O2. Although sublethal doses of H2O2 induced acidic PR protein accumulation, strong and rapid activation of PR protein synthesis, as seen during HR, was obtained only with toxic doses of H2O2. This finding indicates either that high doses of H2O2 are required for full induction or that signals that agonize the induction of acidic PR proteins by H2O2 are produced during necrosis (37). Yet, the observation that H2O2 stress triggers the expression of several classes of acidic PR proteins with a timing and magnitude similar to HR strongly suggests that H2O2 and pathogens induce acidic PR protein accumulation through the same signaling pathway. Because H2O2 is produced during the oxidative burst early during pathogen infection, it is most probable that H2O2 is an integral part of the signaling cascade leading from pathogen infection to local expression of acidic PR proteins.
In accordance with Du and Klessig (16), we found that Cat deficiency did not induce local accumulation of acidic PR proteins in a NahG background. This result is at variance with local induction of acidic PR proteins by pathogens, which is not prevented in NahG transgenics (38). The reason for this discrepancy is not clear, but it may indicate some subtle differences between local induction of acidic PR proteins by pathogens and by Cat deficiency, possibly related to the cellular distribution, kinetics, and magnitude of SA accumulation. Interestingly, we found a biphasic induction of SA in Cat1AS plants, with a first peak after 6 h and a second increase after 1 day. Only the second rise in SA was accompanied by increases in SAG. Whether a similar biphasic induction of SA occurs after pathogen infection is not known, because early SA responses have in general not been analyzed. Yet, Dorey et al. (37) reported a transient increase in SA in the leaf zone infiltrated with elicitor, which peaked after 14 h, whereas SA levels in the surrounding zone rose only after 1 day. This may yield a biphasic response when infiltrated and surrounding tissues are not discriminated. The first peak of SA in Cat1AS clearly preceded tissue injury and necrosis, indicating that its induction was not caused by damage per se. Rather, this early production of SA may potentiate the activation of various defense mechanisms, including the oxidative burst and host cell death (39, 40).
Besides increases in SA and acidic PR proteins, Cat1AS plants also showed enhanced expression of GPx and basic glucanase and manifested a transient peak in ethylene formation, responses that are also induced by pathogens (19, 24, 34). A strong increment in ACC and ethylene was observed in Cat1AS within 2 h of HL exposure, which is before the rise in SA production. Such a rapid but transient rise in ethylene is consistent with the ethylene response during pathogen infection (41). These results indicate that during plant–pathogen interactions H2O2 functions as an intermediate signal upstream of both SA and ethylene-dependent signaling pathways.
SA not only is involved in local responses but also is required for the systemic expression of acidic PR proteins (9). Although evidence has been presented that SA is transported in tobacco from the infection site to upper leaves (42), other studies have indicated that SA is not the mobile signal for SAR, but rather is newly synthesized in the uninfected leaf. Exogenous application of SA in tobacco does not induce SAR (32). Furthermore, SAR could be established when wild-type tobacco scions are grafted on NahG rootstocks (43), and, likewise, SAR could be established in cucumber even when infected leaves were removed from the plant prior to detectable SA accumulation (44). We have shown now that toxic doses of H2O2 induce the systemic expression of acidic PR proteins. Because necrosis per se is not a trigger of systemic defenses (2, 3), this result suggests that severe H2O2 stress, possibly in combination with necrosis, is the inducing agent of SAR. The observation that prooxidant chemicals are inducers of SAR is consistent with this model (45), although a systemic movement of the xenobiotics was not ruled out. Interestingly, the latter study showed that leaves that manifested SAR were also more resistant to oxidative stress, which is in accordance with the identification of an antioxidant enzyme (GPx) as a SAR protein (Fig. 3). Together, these data are consistent with a signaling function of H2O2 during plant–pathogen interactions and position H2O2 upstream of SA, ethylene, and the mobile signal responsible for SAR.
Our data also demonstrate that sublethal doses of H2O2 induce a set of defense proteins similar to the set induced by toxic doses, but with a delayed timing. Deterioration of single cells was not observed in leaves exposed to sublethal H2O2 doses, indicating that cell death is not essential for defense activation, although it may have a potentiating effect. The uncoupling of defense activation and necrosis not only shows that lower doses of H2O2 are required for the activation of defense responses than for the induction of necrosis [in line with earlier H2O2 dose-response experiments in soybean cell cultures (19)] but also provides experimental proof for the concept that H2O2 modulation can lead to enhanced disease resistance without apparent negative effects. Of particular interest in this respect is the observation that H2O2 modulation also activates basic PR proteins, which mostly have stronger antifungal activity than their acidic counterparts (46). For this reason, H2O2 modulation in transgenic plants may constitute a strategy for disease control that is superior to chemical activators of the SAR response such as SA, 2,6-dichloroisonicotinic acid (INA) (47), and benzothiadiazole (BTH) (48).
Acknowledgments
We thank Dr. H. D. Payer and the staff of the closed-chamber group for help in high-light exposure techniques, Dr. J. Ryals for NahG tobacco seeds, Dr. R. Fluhr for the acidic PR-1 and acidic chitinase antibodies, Dr. B. Fritig for the acidic and basic glucanase antibodies, Dr. G. Engler and S. Bauwens for confocal laser microscopy imaging, F. De Winter and R. Ludwig for technical assistance, and Dr. M. De Cock, C. Germonprez, K. Spruyt, and R. Verbanck for help in preparing the manuscript. This work was supported by grants from the Vlaams Actieprogramma Biotechnologie (no. 067), the European Commission Biotech Programme (ERBBIO4CT960101), the International Atomic Energy Agency (no. 5285), the International Human Frontier Science Program (IHFSP RG-434/94M), the Belgian Administration of Development Cooperation, and the Deutsche Forschungsgemeinschaft (La 985/1-1). H.W. and W.V.C. are Postdoctoral Fellows of the Fund for Scientific Research–Flanders (Belgium), and D.I. is a Research Director of the Institut National de la Recherche Agronomique (France).
ABBREVIATIONS
- ACC
1-aminocyclopropane-1-carboxylate
- bPR-2
basic glucanase
- Cat
catalase
- GPx
glutathione peroxidase
- HL
high light
- HR
hypersensitive response
- LAR
local acquired resistance
- LL
low light
- PPFR
photosynthetic photon fluence rate
- PR
pathogenesis-related
- SA
salicylic acid
- SAG
SA β-glucoside
- SAR
systemic acquired resistance
References
- 1.Hammond-Kosack K E, Jones J D G. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sticher L, Mauch-Mani B, Métraux J P. Ann Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- 3.Hunt M D, Ryals J A. Crit Rev Plant Sci. 1996;15:583–606. [Google Scholar]
- 4.Ward E R, Uknes S J, Williams S C, Dincher S S, Wiederhold D L, Alexander D C, Ahl-Goy P, Métraux J-P, Ryals J A. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitz T, Fritig B, Legrand M. Mol Plant–Microbe Interact. 1994;7:776–779. [Google Scholar]
- 6.Dixon R A, Lamb C J. Ann Rev Plant Physiol Plant Mol Biol. 1990;41:339–367. [Google Scholar]
- 7.Malamy J, Carr J P, Klessig D F, Raskin I. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- 8.Métraux J P, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- 9.Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 10.Lawton K, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J. Mol Plant–Microbe Interact. 1995;8:863–870. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- 11.Durner J, Shah J, Klessig D F. Trends Plant Sci. 1997;2:266–274. [Google Scholar]
- 12.Chen Z, Silva H, Klessig D F. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- 13.Durner J, Klessig D F. Proc Natl Acad Sci USA. 1995;92:11312–11316. doi: 10.1073/pnas.92.24.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi Y-M, Kenton P, Mur L, Darby R, Draper J. Plant J. 1995;8:235–245. doi: 10.1046/j.1365-313x.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- 15.Neuenschwander U, Vernooij B, Friedrich L, Uknes S, Kessmann H, Ryals J. Plant J. 1995;8:227–233. doi: 10.1073/pnas.92.10.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du H, Klessig D F. Mol Plant–Microbe Interact. 1997;10:922–925. doi: 10.1094/MPMI.1997.10.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Draper J. Trends Plant Sci. 1997;2:162–165. [Google Scholar]
- 18.Lamb C, Dixon R A. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 19.Levine A, Tenhaken R, Dixon R, Lamb C. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 20.Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rustérucci C, Stallaert V, Milat M-L, Pugin A, Ricci P, Blein J-P. Plant Physiol. 1996;111:885–891. doi: 10.1104/pp.111.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Plant J. 1996;10:491–503. [Google Scholar]
- 23.Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotan T, Fluhr R. Plant Physiol. 1990;93:811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meuwly P, Métraux J-P. Anal Biochem. 1993;214:500–505. doi: 10.1006/abio.1993.1529. [DOI] [PubMed] [Google Scholar]
- 26.Tuomainen J, Betz C, Kangasjärvi J, Ernst D, Yin Z-H, Langebartels C, Sandermann H., Jr Plant J. 1997;12:1151–1162. [Google Scholar]
- 27.Ryals J A, Neuenschwander U H, Willits M G, Molina A, Steiner H-Y, Hunt M D. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi H, Chen Z, Du H, Liu Y, Klessig D F. Plant J. 1997;11:993–1005. doi: 10.1046/j.1365-313x.1997.11050993.x. [DOI] [PubMed] [Google Scholar]
- 29.Malamy J, Hennig J, Klessig D F. Plant Cell. 1992;4:359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennig J, Malamy J, Grynkiewicz G, Indulski J, Klessig D F. Plant J. 1993;4:593–600. doi: 10.1046/j.1365-313x.1993.04040593.x. [DOI] [PubMed] [Google Scholar]
- 31.Yalpani N, León J, Lawton M A, Raskin I. Plant Physiol. 1993;103:315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Loon L C, Antoniw J F. Neth J Plant Pathol. 1982;88:237–256. [Google Scholar]
- 33.Malamy J, Sánchez-Casas P, Hennig J, Guo A, Klessig D F. Mol Plant–Microbe Interact. 1996;9:474–482. [Google Scholar]
- 34.Brederode F T, Linthorst H J M, Bol J F. Plant Mol Biol. 1991;17:1117–1125. doi: 10.1007/BF00028729. [DOI] [PubMed] [Google Scholar]
- 35.Green R, Fluhr R. Plant Cell. 1995;7:203–212. doi: 10.1105/tpc.7.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, Shortt B J, Lawrence E B, León J, Fitzsimmons K C, Levine E B, Raskin I, Shah D M. Plant Physiol. 1997;115:427–435. doi: 10.1104/pp.115.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dorey S, Baillieul F, Pierrel M-A, Saindrenan P, Fritig B, Kauffmann S. Mol Plant–Microbe Interact. 1997;10:646–655. [Google Scholar]
- 38.Friedrich L, Vernooij B, Gaffney T, Morse A, Ryals J. Plant Mol Biol. 1995;29:959–968. doi: 10.1007/BF00014969. [DOI] [PubMed] [Google Scholar]
- 39.Mur L A J, Bi Y-M, Darby R M, Firek S, Draper J. Plant J. 1997;12:1113–1126. doi: 10.1046/j.1365-313x.1997.12051113.x. [DOI] [PubMed] [Google Scholar]
- 40.Shirasu K, Nakajima H, Rajasekhar V K, Dixon R A, Lamb C. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauch F, Hadwiger L A, Boller T. Plant Physiol. 1984;76:607–611. doi: 10.1104/pp.76.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shulaev V, León J, Raskin I. Plant Cell. 1995;7:1691–1701. doi: 10.1105/tpc.7.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J. Plant Cell. 1994;6:959–965. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasmussen J B, Hammerschmidt R, Zook M N. Plant Physiol. 1991;97:1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strobel N E, Kuć J A. Phytopathology. 1995;85:1306–1310. [Google Scholar]
- 46.Sela-Buurlage M B, Ponstein A S, Bres-Vloemans S A, Melchers L S, van den Elzen P J M, Cornelissen B J C. Plant Physiol. 1993;101:857–863. doi: 10.1104/pp.101.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernooij B, Friedrich L, Ahl Goy P, Staub T, Kessmann H, Ryals J. Mol Plant–Microbe Interact. 1995;8:228–234. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- 48.Lawton K A, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]