Abstract
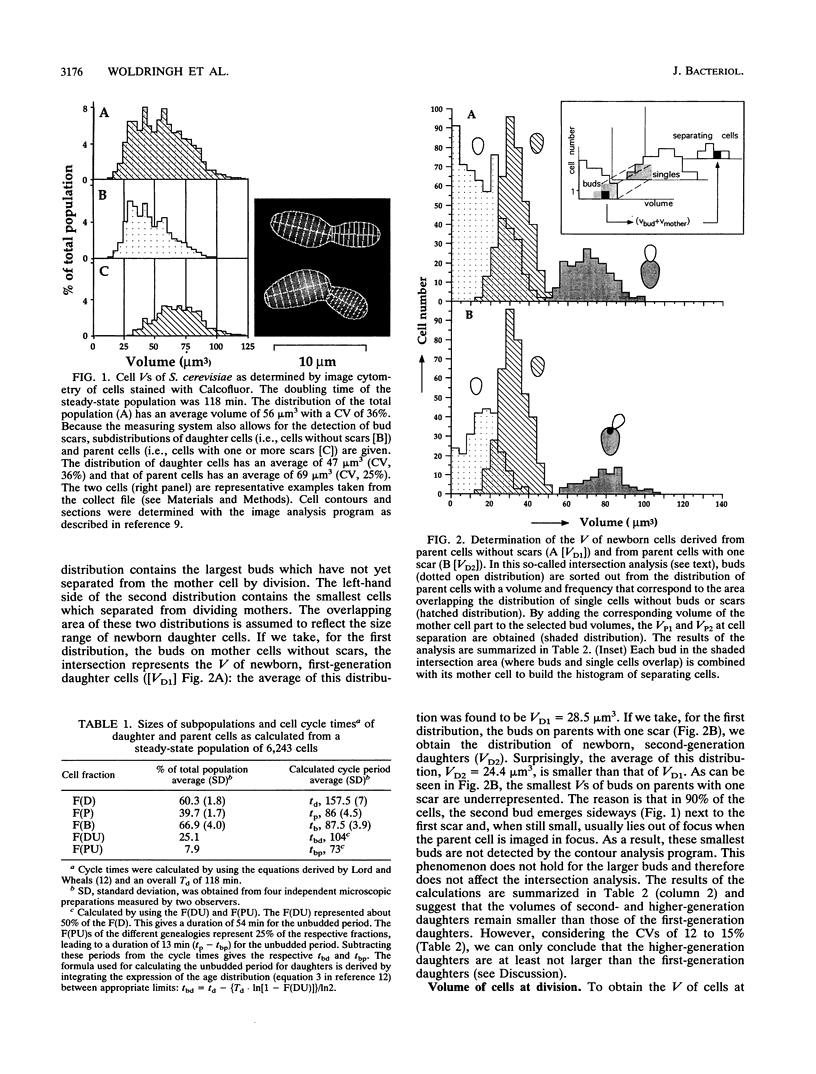
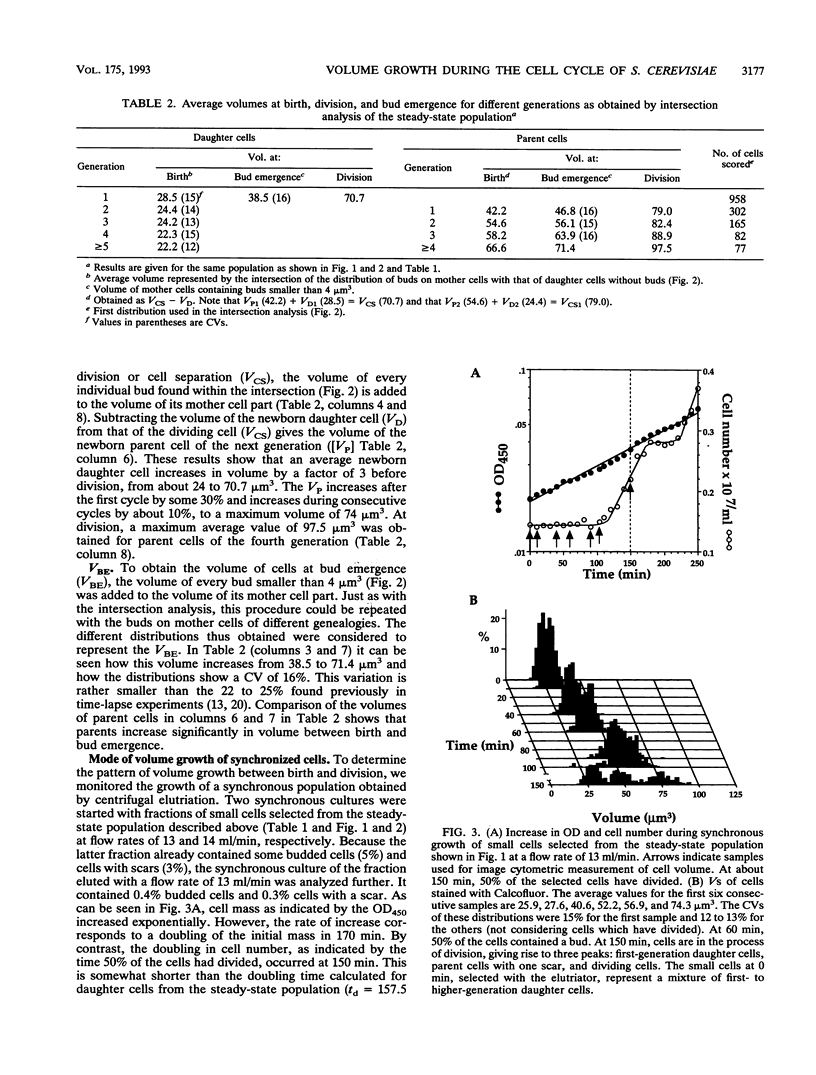
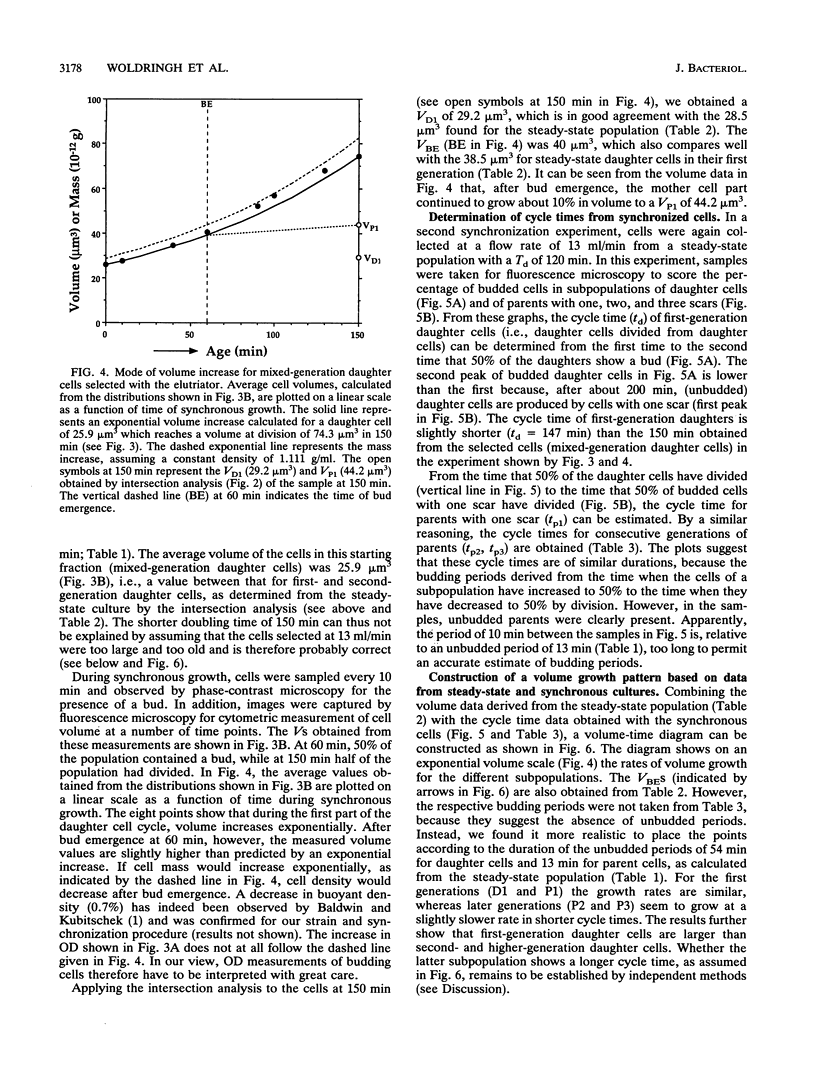
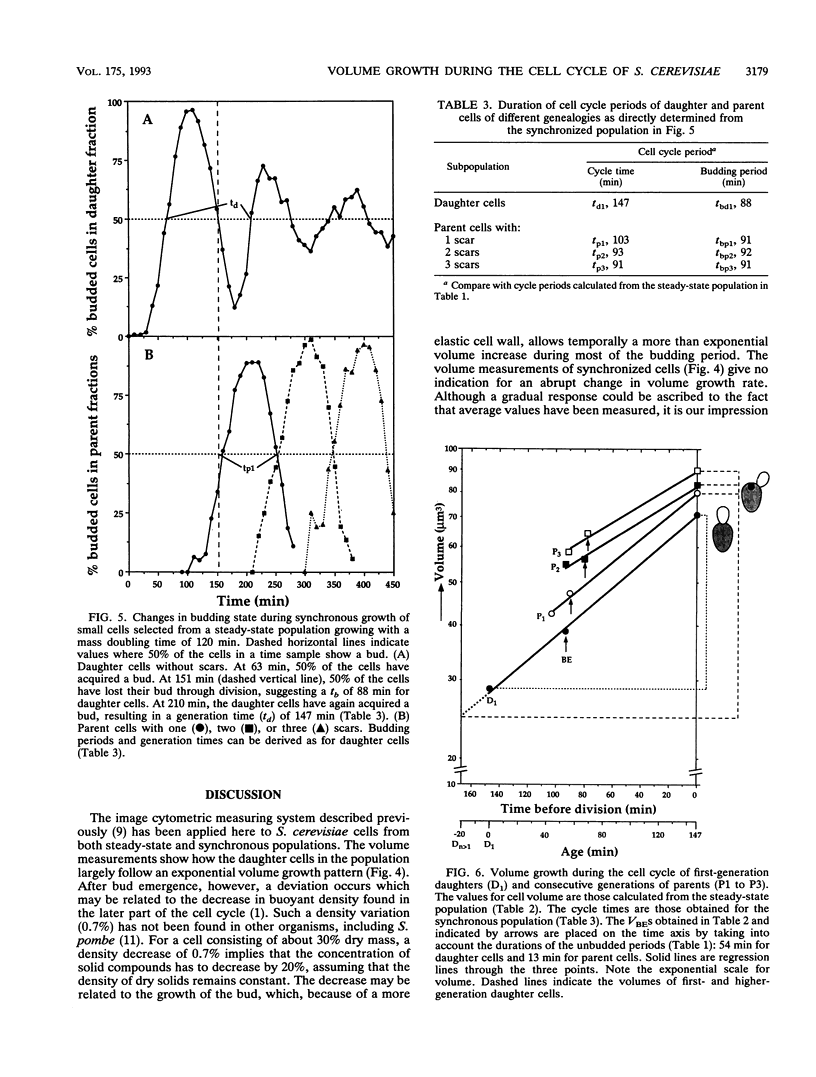
The pattern of volume growth of Saccharomyces cerevisiae a/alpha was determined by image cytometry for daughter cells and consecutive cycles of parent cells. An image analysis program was specially developed to measure separately the volume of bud and mother cell parts and to quantify the number of bud scars on each parent cell. All volumetric data and cell attributes (budding state, number of scars) were stored in such a way that separate volume distributions of cells or cell parts with any combination of properties--for instance, buds present on mothers with two scars or cells without scars (i.e., daughter cells) and without buds--could be obtained. By a new method called intersection analysis, the average volumes of daughter and parent cells at birth and at division could be determined for a steady-state population. These volumes compared well with those directly measured from cells synchronized by centrifugal elutriation. During synchronous growth of daughter cells, the pattern of volume increase appeared to be largely exponential. However, after bud emergence, larger volumes than those predicted by a continuous exponential increase were obtained, which confirms the reported decrease in buoyant density. The cycle times calculated from the steady-state population by applying the age distribution equation deviated from those directly obtained from the synchronized culture, probably because of inadequate scoring of bud scars. Therefore, for the construction of a volume-time diagram, we used volume measurements obtained from the steady-state population and cycle times obtained from the synchronized population. The diagram shows that after bud emergence, mother cell parts continue to grow at a smaller rate, increasing about 10% in volume during the budding period. Second-generation daughter cells, ie., cells born from parents left with two scars, were significantly smaller than first-generation daughter cells. Second- and third-generation parent cells showed a decreased volume growth rate and a shorter budding period than that of daughter cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENTHAL L. K., ZAHLER S. A. Index for measurement of synchronization of cell populations. Science. 1962 Mar 2;135(3505):724–724. doi: 10.1126/science.135.3505.724. [DOI] [PubMed] [Google Scholar]
- Baldwin W. W., Kubitschek H. E. Buoyant density variation during the cell cycle of Saccharomyces cerevisiae. J Bacteriol. 1984 May;158(2):701–704. doi: 10.1128/jb.158.2.701-704.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanor J., Elliott S. G., Bisset Y. C., Mitchison J. M. Absence of step changes in activity of certain enzymes during the cell cycle of budding and fission yeasts in synchronous cultures. J Cell Sci. 1983 May;61:339–349. doi: 10.1242/jcs.61.1.339. [DOI] [PubMed] [Google Scholar]
- Gomes de Mesquita D. S., ten Hoopen R., Woldringh C. L. Vacuolar segregation to the bud of Saccharomyces cerevisiae: an analysis of morphology and timing in the cell cycle. J Gen Microbiol. 1991 Oct;137(10):2447–2454. doi: 10.1099/00221287-137-10-2447. [DOI] [PubMed] [Google Scholar]
- Gordon C. N., Elliott S. C. Fractionation of Saccharomyces cerevisiae cell populations by centrifugal elutriation. J Bacteriol. 1977 Jan;129(1):97–100. doi: 10.1128/jb.129.1.97-100.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Unger M. W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977 Nov;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Ehrhardt C. W., Lorincz A., Carter B. L. Regulation of cell size in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):1–5. doi: 10.1128/jb.137.1.1-5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Buoyant density variation during the cell cycle in microorganisms. Crit Rev Microbiol. 1987;14(1):73–97. doi: 10.3109/10408418709104436. [DOI] [PubMed] [Google Scholar]
- Lord P. G., Wheals A. E. Asymmetrical division of Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):808–818. doi: 10.1128/jb.142.3.808-818.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord P. G., Wheals A. E. Rate of cell cycle initiation of yeast cells when cell size is not a rate-determining factor. J Cell Sci. 1983 Jan;59:183–201. doi: 10.1242/jcs.59.1.183. [DOI] [PubMed] [Google Scholar]
- Oehlen L. J., van Doorn J., Scholte M. E., Postma P. W., van Dam K. Changes in the incorporation of carbon derived from glucose into cellular pools during the cell cycle of Saccharomyces cerevisiae. J Gen Microbiol. 1990 Mar;136(3):413–418. doi: 10.1099/00221287-136-3-413. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Sharrow S. O., Gart J. J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3850–3854. doi: 10.1073/pnas.74.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P. E., Goodey A. R., Carter B. L. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature. 1980 Nov 27;288(5789):401–404. doi: 10.1038/288401a0. [DOI] [PubMed] [Google Scholar]
- Van Doorn J., Valkenburg J. A., Scholte M. E., Oehlen L. J., Van Driel R., Postma P. W., Nanninga N., Van Dam K. Changes in activities of several enzymes involved in carbohydrate metabolism during the cell cycle of Saccharomyces cerevisiae. J Bacteriol. 1988 Oct;170(10):4808–4815. doi: 10.1128/jb.170.10.4808-4815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoni M., Vai M., Popolo L., Alberghina L. Structural heterogeneity in populations of the budding yeast Saccharomyces cerevisiae. J Bacteriol. 1983 Dec;156(3):1282–1291. doi: 10.1128/jb.156.3.1282-1291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheals A. E. Size control models of Saccharomyces cerevisiae cell proliferation. Mol Cell Biol. 1982 Apr;2(4):361–368. doi: 10.1128/mcb.2.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning W., Groeneveld K., Oehlen L. J., Berden J. A., van Dam K. Changes in the activities of key enzymes of glycolysis during the cell cycle in yeast: a rectification. J Gen Microbiol. 1991 Apr;137(4):971–976. doi: 10.1099/00221287-137-4-971. [DOI] [PubMed] [Google Scholar]