Abstract
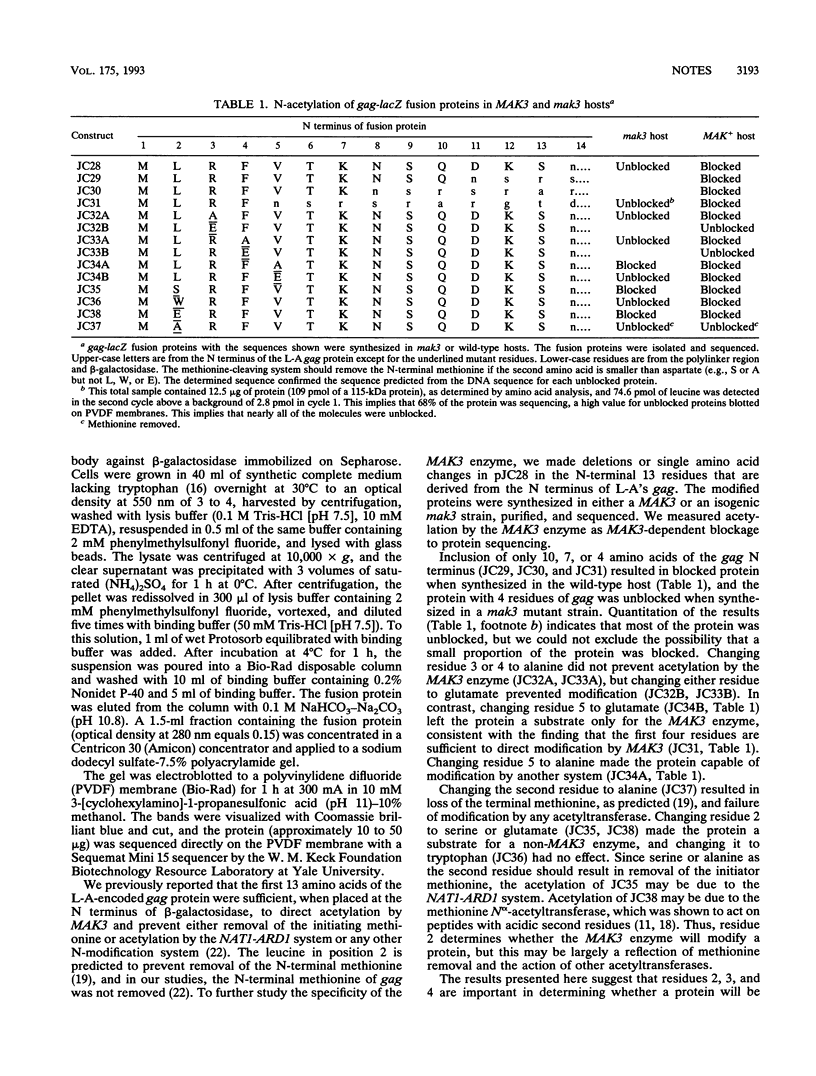
The MAK3 gene of Saccharomyces cerevisiae encodes an N-acetyltransferase whose acetylation of the N terminus of the L-A double-stranded RNA virus major coat protein (gag) is necessary for viral assembly. We show that the first 4 amino acids of the L-A gag protein sequence, MLRF, are a portable signal for N-terminal acetylation by MAK3. Amino acids 2, 3, and 4 are each important for acetylation by the MAK3 enzyme. In yeast cells, only three mitochondrial proteins are known to have the MAK3 acetylation signal, suggesting an explanation for the slow growth of mak3 mutants on nonfermentable carbon sources.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dihanich M., van Tuinen E., Lambris J. D., Marshallsay B. Accumulation of viruslike particles in a yeast mutant lacking a mitochondrial pore protein. Mol Cell Biol. 1989 Mar;9(3):1100–1108. doi: 10.1128/mcb.9.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Wickner R. B. Ribosomal frameshifting efficiency and gag/gag-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J Virol. 1992 Jun;66(6):3669–3676. doi: 10.1128/jvi.66.6.3669-3676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Elliott R. C., Liu P. S., Koduri R. K., Weickmann J. L., Lee J. H., Blair L. C., Ghosh-Dastidar P., Bradshaw R. A., Bryan K. M. Specificity of cotranslational amino-terminal processing of proteins in yeast. Biochemistry. 1987 Dec 15;26(25):8242–8246. doi: 10.1021/bi00399a033. [DOI] [PubMed] [Google Scholar]
- Kendall R. L., Yamada R., Bradshaw R. A. Cotranslational amino-terminal processing. Methods Enzymol. 1990;185:398–407. doi: 10.1016/0076-6879(90)85035-m. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. Identification of methionine Nalpha-acetyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1990 Mar 5;265(7):3603–3606. [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. Model peptides reveal specificity of N alpha-acetyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1990 Jul 15;265(20):11576–11580. [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. Molecular cloning and sequencing of a cDNA encoding N alpha-acetyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1989 Jul 25;264(21):12339–12343. [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. N alpha acetylation is required for normal growth and mating of Saccharomyces cerevisiae. J Bacteriol. 1989 Nov;171(11):5795–5802. doi: 10.1128/jb.171.11.5795-5802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. J., Lin L. W., Smith J. A. N alpha-acetyltransferase deficiency alters protein synthesis in Saccharomyces cerevisiae. FEBS Lett. 1989 Oct 9;256(1-2):139–142. doi: 10.1016/0014-5793(89)81734-x. [DOI] [PubMed] [Google Scholar]
- Moerschell R. P., Hosokawa Y., Tsunasawa S., Sherman F. The specificities of yeast methionine aminopeptidase and acetylation of amino-terminal methionine in vivo. Processing of altered iso-1-cytochromes c created by oligonucleotide transformation. J Biol Chem. 1990 Nov 15;265(32):19638–19643. [PubMed] [Google Scholar]
- Mullen J. R., Kayne P. S., Moerschell R. P., Tsunasawa S., Gribskov M., Colavito-Shepanski M., Grunstein M., Sherman F., Sternglanz R. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 1989 Jul;8(7):2067–2075. doi: 10.1002/j.1460-2075.1989.tb03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. M., Crivellone M. D., Tzagoloff A. Assembly of the mitochondrial membrane system. MRP1 and MRP2, two yeast nuclear genes coding for mitochondrial ribosomal proteins. J Biol Chem. 1987 Mar 5;262(7):3388–3397. [PubMed] [Google Scholar]
- Repetto B., Tzagoloff A. Structure and regulation of KGD1, the structural gene for yeast alpha-ketoglutarate dehydrogenase. Mol Cell Biol. 1989 Jun;9(6):2695–2705. doi: 10.1128/mcb.9.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Tsunasawa S. Methionine or not methionine at the beginning of a protein. Bioessays. 1985 Jul;3(1):27–31. doi: 10.1002/bies.950030108. [DOI] [PubMed] [Google Scholar]
- Takakura H., Tsunasawa S., Miyagi M., Warner J. R. NH2-terminal acetylation of ribosomal proteins of Saccharomyces cerevisiae. J Biol Chem. 1992 Mar 15;267(8):5442–5445. [PubMed] [Google Scholar]
- Tercero J. C., Riles L. E., Wickner R. B. Localized mutagenesis and evidence for post-transcriptional regulation of MAK3. A putative N-acetyltransferase required for double-stranded RNA virus propagation in Saccharomyces cerevisiae. J Biol Chem. 1992 Oct 5;267(28):20270–20276. [PubMed] [Google Scholar]
- Tercero J. C., Wickner R. B. MAK3 encodes an N-acetyltransferase whose modification of the L-A gag NH2 terminus is necessary for virus particle assembly. J Biol Chem. 1992 Oct 5;267(28):20277–20281. [PubMed] [Google Scholar]
- Tsunasawa S., Stewart J. W., Sherman F. Amino-terminal processing of mutant forms of yeast iso-1-cytochrome c. The specificities of methionine aminopeptidase and acetyltransferase. J Biol Chem. 1985 May 10;260(9):5382–5391. [PubMed] [Google Scholar]
- Whiteway M., Szostak J. W. The ARD1 gene of yeast functions in the switch between the mitotic cell cycle and alternative developmental pathways. Cell. 1985 Dec;43(2 Pt 1):483–492. doi: 10.1016/0092-8674(85)90178-3. [DOI] [PubMed] [Google Scholar]
- Wu M., Tzagoloff A. Mitochondrial and cytoplasmic fumarases in Saccharomyces cerevisiae are encoded by a single nuclear gene FUM1. J Biol Chem. 1987 Sep 5;262(25):12275–12282. [PubMed] [Google Scholar]



