Abstract
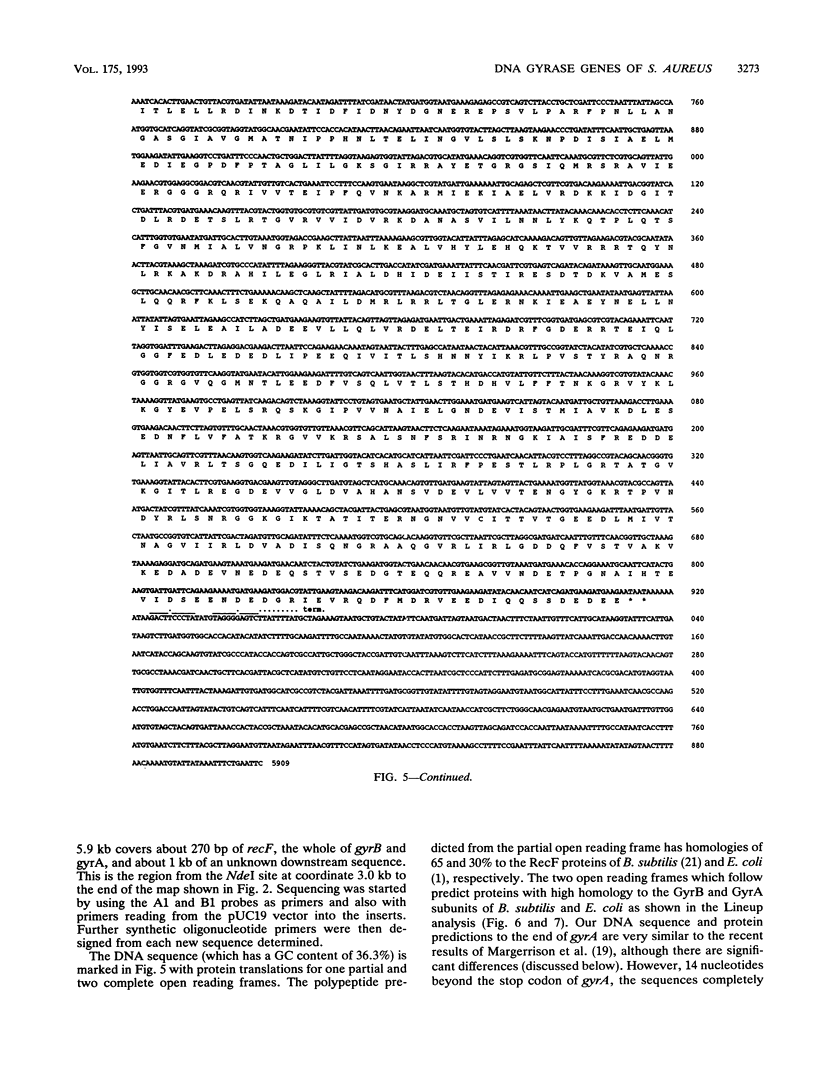
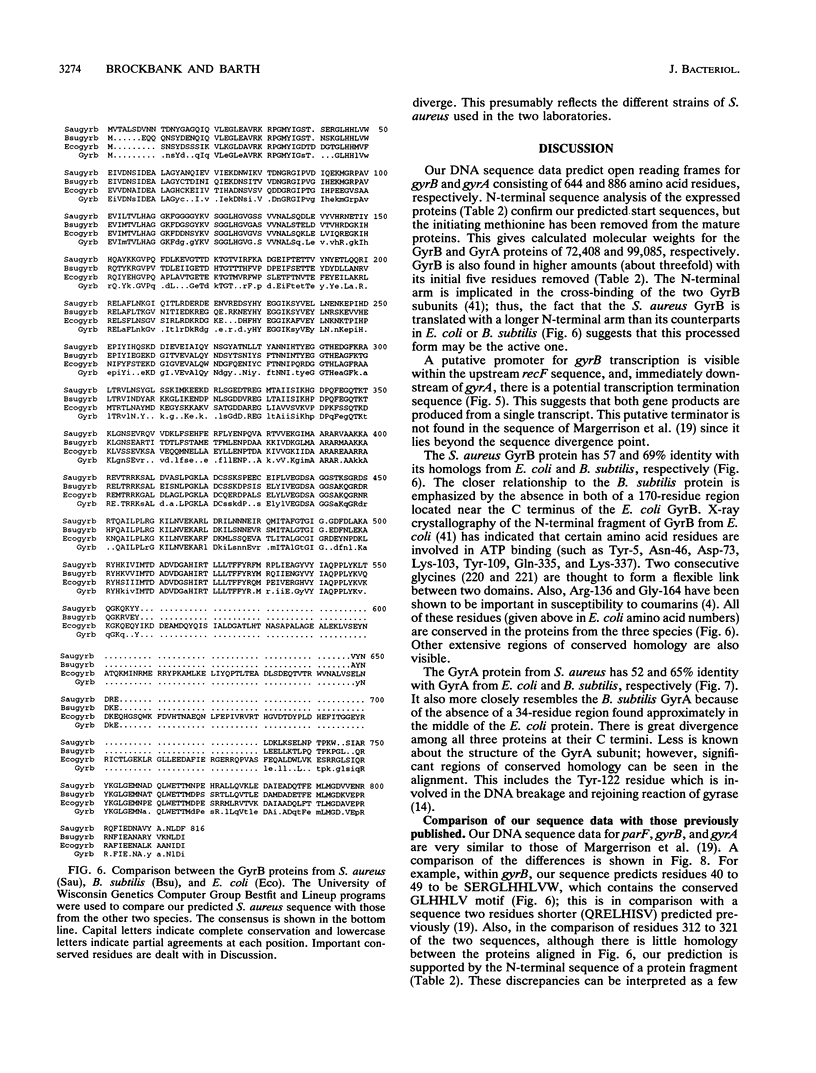
We have isolated and cloned the gyrA and gyrB genes from Staphylococcus aureus. These adjacent genes encode the subunits of DNA gyrase. The nucleotide sequence of a 5.9-kb region which includes part of an upstream recF gene, the whole of gyrB and gyrA, and about 1 kb of unknown downstream sequence has been determined. The gyrB and gyrA gene sequences predict proteins of 886 and 644 amino acid residues, respectively, which have significant homologies with the gyrase subunits of Escherichia coli and Bacillus subtilis. Residues thought to be important to the structure and function of the subunits are conserved. These genes have been expressed separately by using a T7 promoter vector. N-terminal sequencing of the cloned gene products suggests that the mature GyrB subunit exists mainly with its initial five residues removed. Protein sequencing also supports the interpretation of our DNA sequencing data, which are inconsistent in several placed with the recently published sequence of the same genes (E. E. C. Margerrison, R. Hopewell, and L. M. Fisher, J. Bacteriol. 174:1596-1603, 1992).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Mizuuchi M., Robinson E. A., Appella E., O'Dea M. H., Gellert M., Mizuuchi K. DNA sequence of the E. coli gyrB gene: application of a new sequencing strategy. Nucleic Acids Res. 1987 Jan 26;15(2):771–784. doi: 10.1093/nar/15.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Fujii T., Fujimaki K., Inoue M., Mitsuhashi S. Purification of Citrobacter freundii DNA gyrase and inhibition by quinolones. Antimicrob Agents Chemother. 1988 Jan;32(1):104–109. doi: 10.1128/aac.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman S. D., Hu P. C., Bott K. F. Mycoplasma pneumoniae DNA gyrase genes. Mol Microbiol. 1990 Jul;4(7):1129–1134. doi: 10.1111/j.1365-2958.1990.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Contreras A., Maxwell A. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol Microbiol. 1992 Jun;6(12):1617–1624. doi: 10.1111/j.1365-2958.1992.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G. P., Das H. K. Cloning and sequence analysis of gyrA gene of Klebsiella pneumoniae. Nucleic Acids Res. 1990 Jan 11;18(1):151–156. doi: 10.1093/nar/18.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin T. J., 4th, Kolodner R. D. Purification and preliminary characterization of the Escherichia coli K-12 recF protein. J Bacteriol. 1990 Nov;172(11):6291–6299. doi: 10.1128/jb.172.11.6291-6299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. L., Dyall-Smith M. L. Mutations in DNA gyrase result in novobiocin resistance in halophilic archaebacteria. J Bacteriol. 1991 Jan;173(2):642–648. doi: 10.1128/jb.173.2.642-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
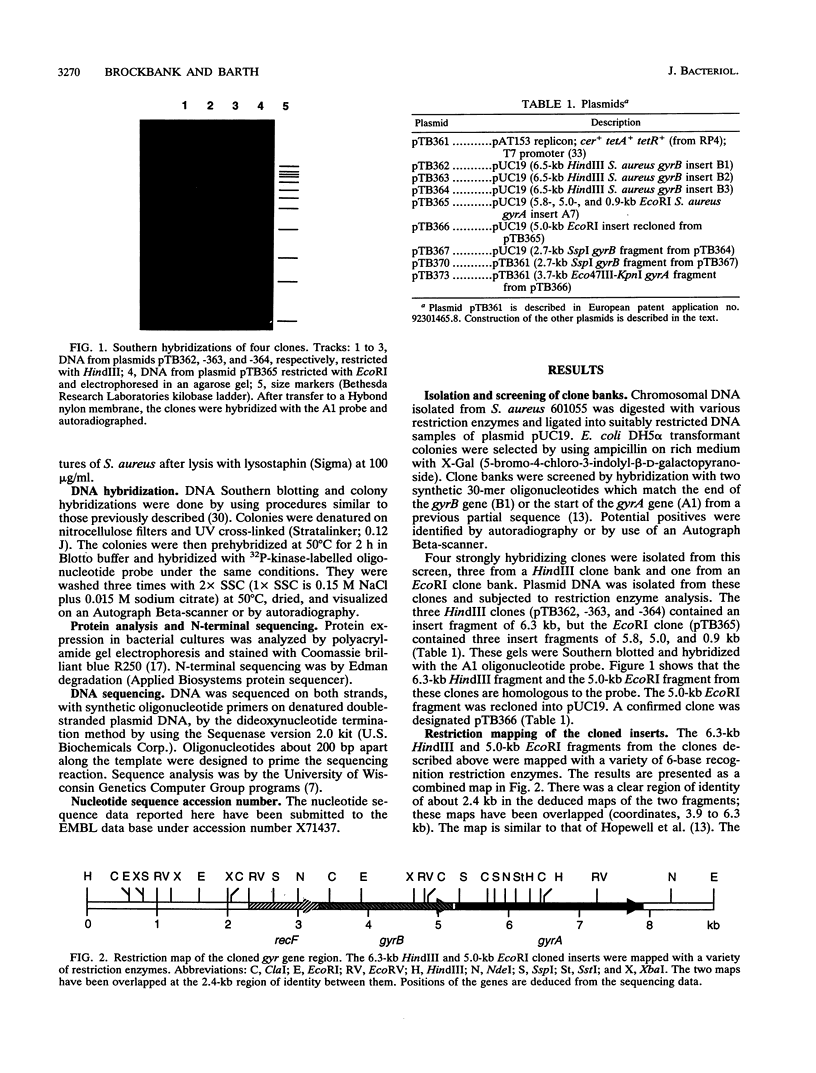
- Hopewell R., Oram M., Briesewitz R., Fisher L. M. DNA cloning and organization of the Staphylococcus aureus gyrA and gyrB genes: close homology among gyrase proteins and implications for 4-quinolone action and resistance. J Bacteriol. 1990 Jun;172(6):3481–3484. doi: 10.1128/jb.172.6.3481-3484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Klevan L., Wang J. C. Deoxyribonucleic acid gyrase-deoxyribonucleic acid complex containing 140 base pairs of deoxyribonucleic acid and an alpha 2 beta 2 protein core. Biochemistry. 1980 Nov 11;19(23):5229–5234. doi: 10.1021/bi00564a012. [DOI] [PubMed] [Google Scholar]
- Krueger S., Zaccai G., Wlodawer A., Langowski J., O'Dea M., Maxwell A., Gellert M. Neutron and light-scattering studies of DNA gyrase and its complex with DNA. J Mol Biol. 1990 Jan 5;211(1):211–220. doi: 10.1016/0022-2836(90)90021-D. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Micrococcus luteus DNA gyrase: active components and a model for its supercoiling of DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2098–2102. doi: 10.1073/pnas.75.5.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerrison E. E., Hopewell R., Fisher L. M. Nucleotide sequence of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J Bacteriol. 1992 Mar;174(5):1596–1603. doi: 10.1128/jb.174.5.1596-1603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Yoshida S., Wakebe H., Inoue M., Yamaguchi T., Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Dec;35(12):2562–2567. doi: 10.1128/aac.35.12.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., von Meyenburg K., Hansen F. G., Yoshikawa H. Conservation of genes and their organization in the chromosomal replication origin region of Bacillus subtilis and Escherichia coli. EMBO J. 1985 Dec 1;4(12):3345–3350. doi: 10.1002/j.1460-2075.1985.tb04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Yoshikawa H. Genes and their organization in the replication origin region of the bacterial chromosome. Mol Microbiol. 1992 Mar;6(5):629–634. doi: 10.1111/j.1365-2958.1992.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Piercy E. A., Barbaro D., Luby J. P., Mackowiak P. A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 1989 Jan;33(1):128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Clark A. J. The dependence of postreplication repair on uvrB in a recF mutant of Escherichia coli K-12. Mol Gen Genet. 1977 Oct 24;155(3):279–286. doi: 10.1007/BF00272806. [DOI] [PubMed] [Google Scholar]
- Sanzey B. Modulation of gene expression by drugs affecting deoxyribonucleic acid gyrase. J Bacteriol. 1979 Apr;138(1):40–47. doi: 10.1128/jb.138.1.40-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Danaher R. J., Cook T. M. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1991 Apr;35(4):622–626. doi: 10.1128/aac.35.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg S. L., Wang J. C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987 Oct 20;197(4):729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Sato K., Kimura Y., Hayakawa I., Osada Y., Nishino T. Inhibition by quinolones of DNA gyrase from Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Jul;35(7):1489–1491. doi: 10.1128/aac.35.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiara A. S., Cundliffe E. Cloning and characterization of a DNA gyrase B gene from Streptomyces sphaeroides that confers resistance to novobiocin. EMBO J. 1988 Jul;7(7):2255–2259. doi: 10.1002/j.1460-2075.1988.tb03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. J., Hansen S. L., Tatem B. A., Auger F., Standiford H. C. Activity of novobiocin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1985 Apr;15(4):435–440. doi: 10.1093/jac/15.4.435. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wigley D. B., Davies G. J., Dodson E. J., Maxwell A., Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991 Jun 20;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]