Abstract
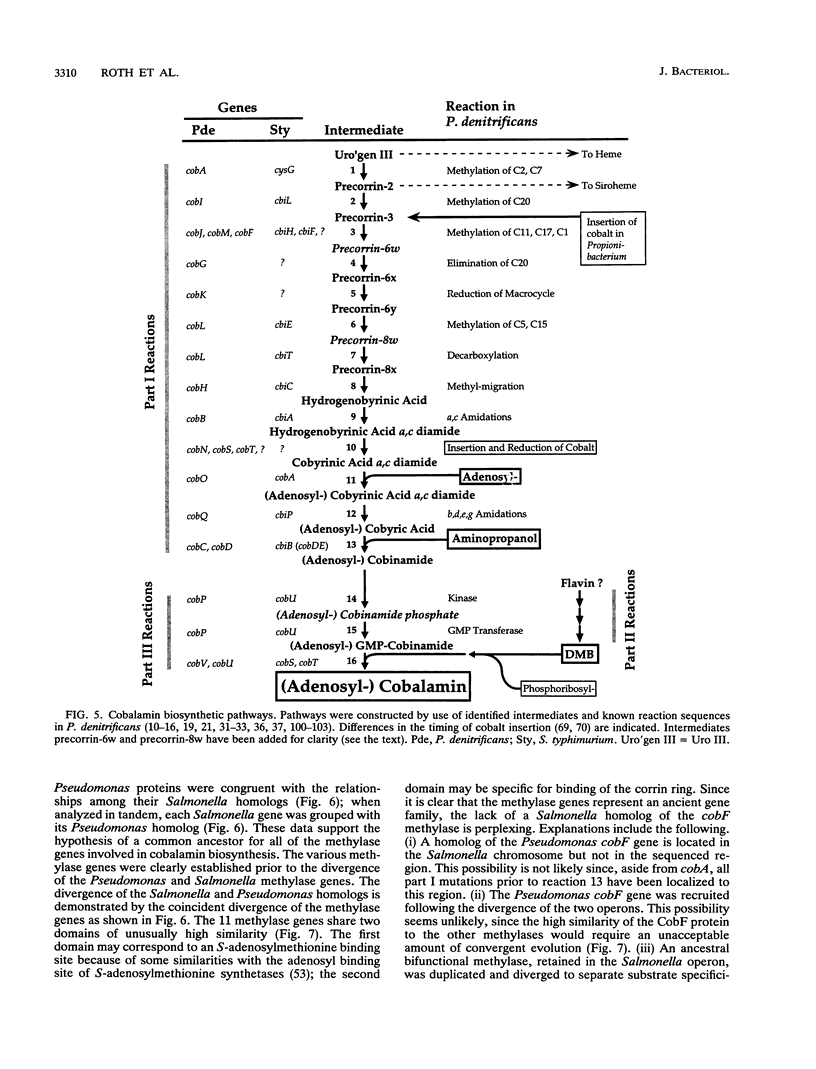
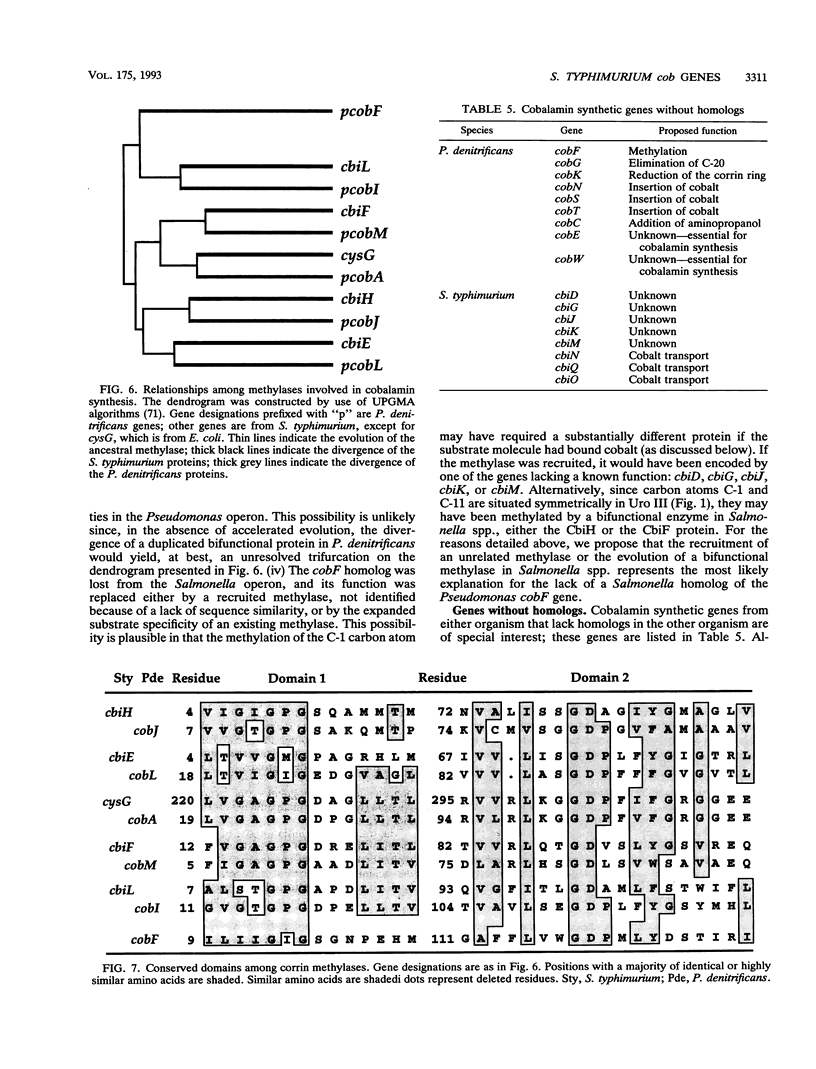
Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic conditions. Of the 30 cobalamin synthetic genes, 25 are clustered in one operon, cob, and are arranged in three groups, each group encoding enzymes for a biochemically distinct portion of the biosynthetic pathway. We have determined the DNA sequence for the promoter region and the proximal 17.1 kb of the cob operon. This sequence includes 20 translationally coupled genes that encode the enzymes involved in parts I and III of the cobalamin biosynthetic pathway. A comparison of these genes with the cobalamin synthetic genes from Pseudomonas denitrificans allows assignment of likely functions to 12 of the 20 sequenced Salmonella genes. Three additional Salmonella genes encode proteins likely to be involved in the transport of cobalt, a component of vitamin B12. However, not all Salmonella and Pseudomonas cobalamin synthetic genes have apparent homologs in the other species. These differences suggest that the cobalamin biosynthetic pathways differ between the two organisms. The evolution of these genes and their chromosomal positions is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Jensen R. A. The phylogenetic origin of the bifunctional tyrosine-pathway protein in the enteric lineage of bacteria. Mol Biol Evol. 1988 May;5(3):282–297. doi: 10.1093/oxfordjournals.molbev.a040496. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andersson D. I. Involvement of the Arc system in redox regulation of the Cob operon in Salmonella typhimurium. Mol Microbiol. 1992 Jun;6(11):1491–1494. doi: 10.1111/j.1365-2958.1992.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Roth J. R. Mutations affecting regulation of cobinamide biosynthesis in Salmonella typhimurium. J Bacteriol. 1989 Dec;171(12):6726–6733. doi: 10.1128/jb.171.12.6726-6733.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D. I., Roth J. R. Redox regulation of the genes for cobinamide biosynthesis in Salmonella typhimurium. J Bacteriol. 1989 Dec;171(12):6734–6739. doi: 10.1128/jb.171.12.6734-6739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner S. A., Ellington A. D., Tauer A. Modern metabolism as a palimpsest of the RNA world. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7054–7058. doi: 10.1073/pnas.86.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Couder M., Debussche L., Thibaut D., Cameron B., Crouzet J. Biosynthesis of vitamin B12: stepwise amidation of carboxyl groups b, d, e, and g of cobyrinic acid a,c-diamide is catalyzed by one enzyme in Pseudomonas denitrificans. J Bacteriol. 1991 Oct;173(19):6046–6051. doi: 10.1128/jb.173.19.6046-6051.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Debussche L., Famechon A., Thibaut D., Cameron B., Crouzet J. A bifunctional protein from Pseudomonas denitrificans carries cobinamide kinase and cobinamide phosphate guanylyltransferase activities. J Bacteriol. 1991 Oct;173(19):6052–6057. doi: 10.1128/jb.173.19.6052-6057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Debussche L., Thibaut D., Crouzet J., Cameron B. Purification and characterization of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1989 Aug;171(8):4222–4231. doi: 10.1128/jb.171.8.4222-4231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Famechon A., Thibaut D., Debussche L., Cameron B., Crouzet J. Biosynthesis of vitamin B12 in Pseudomonas denitrificans: the biosynthetic sequence from precorrin-6y to precorrin-8x is catalyzed by the cobL gene product. J Bacteriol. 1992 Feb;174(3):1050–1052. doi: 10.1128/jb.174.3.1050-1052.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Maton L., Debussche L., Thibaut D. Purification and characterization of Cob(II)yrinic acid a,c-diamide reductase from Pseudomonas denitrificans. J Bacteriol. 1992 Nov;174(22):7452–7454. doi: 10.1128/jb.174.22.7452-7454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Thibaut D., Couder M., Muller J. C. Identification and quantitation of corrinoid precursors of cobalamin from Pseudomonas denitrificans by high-performance liquid chromatography. Anal Biochem. 1990 Aug 15;189(1):24–29. doi: 10.1016/0003-2697(90)90038-b. [DOI] [PubMed] [Google Scholar]
- Blanche F., Thibaut D., Famechon A., Debussche L., Cameron B., Crouzet J. Precorrin-6x reductase from Pseudomonas denitrificans: purification and characterization of the enzyme and identification of the structural gene. J Bacteriol. 1992 Feb;174(3):1036–1042. doi: 10.1128/jb.174.3.1036-1042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik T. A., Ailion M., Roth J. R. A single regulatory gene integrates control of vitamin B12 synthesis and propanediol degradation. J Bacteriol. 1992 Apr;174(7):2253–2266. doi: 10.1128/jb.174.7.2253-2266.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey R. N., Banner C. D., Wolf J. B. Cloning of multiple genes involved with cobalamin (Vitamin B12) biosynthesis in Bacillus megaterium. J Bacteriol. 1986 Aug;167(2):623–630. doi: 10.1128/jb.167.2.623-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B., Blanche F., Rouyez M. C., Bisch D., Famechon A., Couder M., Cauchois L., Thibaut D., Debussche L., Crouzet J. Genetic analysis, nucleotide sequence, and products of two Pseudomonas denitrificans cob genes encoding nicotinate-nucleotide: dimethylbenzimidazole phosphoribosyltransferase and cobalamin (5'-phosphate) synthase. J Bacteriol. 1991 Oct;173(19):6066–6073. doi: 10.1128/jb.173.19.6066-6073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B., Briggs K., Pridmore S., Brefort G., Crouzet J. Cloning and analysis of genes involved in coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1989 Jan;171(1):547–557. doi: 10.1128/jb.171.1.547-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B., Guilhot C., Blanche F., Cauchois L., Rouyez M. C., Rigault S., Levy-Schil S., Crouzet J. Genetic and sequence analyses of a Pseudomonas denitrificans DNA fragment containing two cob genes. J Bacteriol. 1991 Oct;173(19):6058–6065. doi: 10.1128/jb.173.19.6058-6065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Chang J. T. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature. 1975 Mar 13;254(5496):150–151. doi: 10.1038/254150a0. [DOI] [PubMed] [Google Scholar]
- Childs J. D., Smith D. A. New methionine structural gene in Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):377–382. doi: 10.1128/jb.100.1.377-382.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Kieffer-Higgins S. Multiplex DNA sequencing. Science. 1988 Apr 8;240(4849):185–188. doi: 10.1126/science.3353714. [DOI] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Thibaut D., Debussche L. Genetic and sequence analysis of an 8.7-kilobase Pseudomonas denitrificans fragment carrying eight genes involved in transformation of precorrin-2 to cobyrinic acid. J Bacteriol. 1990 Oct;172(10):5980–5990. doi: 10.1128/jb.172.10.5980-5990.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Cauchois L., Blanche F., Debussche L., Thibaut D., Rouyez M. C., Rigault S., Mayaux J. F., Cameron B. Nucleotide sequence of a Pseudomonas denitrificans 5.4-kilobase DNA fragment containing five cob genes and identification of structural genes encoding S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase and cobyrinic acid a,c-diamide synthase. J Bacteriol. 1990 Oct;172(10):5968–5979. doi: 10.1128/jb.172.10.5968-5979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Levy-Schil S., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Debussche L., Thibaut D. Nucleotide sequence and genetic analysis of a 13.1-kilobase-pair Pseudomonas denitrificans DNA fragment containing five cob genes and identification of structural genes encoding Cob(I)alamin adenosyltransferase, cobyric acid synthase, and bifunctional cobinamide kinase-cobinamide phosphate guanylyltransferase. J Bacteriol. 1991 Oct;173(19):6074–6087. doi: 10.1128/jb.173.19.6074-6087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDA J., PEDZIWILK Z., ZODROW K. Badania nad wystepowaniem witaminu B12 u roŝlin motyikowych. Acta Microbiol Pol. 1957;6(3):233–238. [PubMed] [Google Scholar]
- Das A., Yanofsky C. Restoration of a translational stop-start overlap reinstates translational coupling in a mutant trpB'-trpA gene pair of the Escherichia coli tryptophan operon. Nucleic Acids Res. 1989 Nov 25;17(22):9333–9340. doi: 10.1093/nar/17.22.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Couder M., Thibaut D., Cameron B., Crouzet J., Blanche F. Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1992 Nov;174(22):7445–7451. doi: 10.1128/jb.174.22.7445-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Couder M., Thibaut D., Cameron B., Crouzet J., Blanche F. Purification and partial characterization of Cob(I)alamin adenosyltransferase from Pseudomonas denitrificans. J Bacteriol. 1991 Oct;173(19):6300–6302. doi: 10.1128/jb.173.19.6300-6302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Thibaut D., Cameron B., Crouzet J., Blanche F. Purification and characterization of cobyrinic acid a,c-diamide synthase from Pseudomonas denitrificans. J Bacteriol. 1990 Nov;172(11):6239–6244. doi: 10.1128/jb.172.11.6239-6244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Johnson M. G., Roth J. R. The CobII and CobIII regions of the cobalamin (vitamin B12) biosynthetic operon of Salmonella typhimurium. J Bacteriol. 1992 Jan;174(1):24–29. doi: 10.1128/jb.174.1.24-29.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Roth J. R. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987 May;169(5):2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B., McCloskey J., Kersten W., Kersten H. New function of vitamin B12: cobamide-dependent reduction of epoxyqueuosine to queuosine in tRNAs of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2078–2082. doi: 10.1128/jb.170.5.2078-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Scott A. I. On B12 biosynthesis and evolution. J Theor Biol. 1977 Nov 21;69(2):381–384. doi: 10.1016/0022-5193(77)90145-x. [DOI] [PubMed] [Google Scholar]
- Grabau C., Roth J. R. A Salmonella typhimurium cobalamin-deficient mutant blocked in 1-amino-2-propanol synthesis. J Bacteriol. 1992 Apr;174(7):2138–2144. doi: 10.1128/jb.174.7.2138-2144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillerez J., Gazeau M., Dreyfus M. In the Escherichia coli lacZ gene the spacing between the translating ribosomes is insensitive to the efficiency of translation initiation. Nucleic Acids Res. 1991 Dec 25;19(24):6743–6750. doi: 10.1093/nar/19.24.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson C., Lindström P. H., Hagervall T. G., Esberg K. B., Björk G. R. The trmA promoter has regulatory features and sequence elements in common with the rRNA P1 promoter family of Escherichia coli. J Bacteriol. 1991 Mar;173(5):1757–1764. doi: 10.1128/jb.173.5.1757-1764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E., Higgins E., Chen J. W., Umbarger H. E. Translational coupling between the ilvD and ilvA genes of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4798–4807. doi: 10.1128/jb.170.10.4798-4807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth K., Rex G., Surin B., Zinck R., McCarthy J. E. Translational coupling varying in efficiency between different pairs of genes in the central region of the atp operon of Escherichia coli. Mol Microbiol. 1991 Apr;5(4):813–824. doi: 10.1111/j.1365-2958.1991.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Horikawa S., Sasuga J., Shimizu K., Ozasa H., Tsukada K. Molecular cloning and nucleotide sequence of cDNA encoding the rat kidney S-adenosylmethionine synthetase. J Biol Chem. 1990 Aug 15;265(23):13683–13686. [PubMed] [Google Scholar]
- Jensen R. A. Biochemical pathways in prokaryotes can be traced backward through evolutionary time. Mol Biol Evol. 1985 Mar;2(2):92–108. doi: 10.1093/oxfordjournals.molbev.a040338. [DOI] [PubMed] [Google Scholar]
- Jeter R. M. Cobalamin-dependent 1,2-propanediol utilization by Salmonella typhimurium. J Gen Microbiol. 1990 May;136(5):887–896. doi: 10.1099/00221287-136-5-887. [DOI] [PubMed] [Google Scholar]
- Jeter R. M., Olivera B. M., Roth J. R. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter R. M., Roth J. R. Cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J Bacteriol. 1987 Jul;169(7):3189–3198. doi: 10.1128/jb.169.7.3189-3198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. G., Escalante-Semerena J. C. Identification of 5,6-dimethylbenzimidazole as the Co alpha ligand of the cobamide synthesized by Salmonella typhimurium. Nutritional characterization of mutants defective in biosynthesis of the imidazole ring. J Biol Chem. 1992 Jul 5;267(19):13302–13305. [PubMed] [Google Scholar]
- Karlin S., Altschul S. F. Methods for assessing the statistical significance of molecular sequence features by using general scoring schemes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2264–2268. doi: 10.1073/pnas.87.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. G., Ochman H., Hartl D. L. Molecular and evolutionary relationships among enteric bacteria. J Gen Microbiol. 1991 Aug;137(8):1911–1921. doi: 10.1099/00221287-137-8-1911. [DOI] [PubMed] [Google Scholar]
- Little S., Hyde S., Campbell C. J., Lilley R. J., Robinson M. K. Translational coupling in the threonine operon of Escherichia coli K-12. J Bacteriol. 1989 Jun;171(6):3518–3522. doi: 10.1128/jb.171.6.3518-3522.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Altered cobalamin metabolism in Escherichia coli btuR mutants affects btuB gene regulation. J Bacteriol. 1989 Jan;171(1):154–161. doi: 10.1128/jb.171.1.154-161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundrigan M. D., Köster W., Kadner R. J. Transcribed sequences of the Escherichia coli btuB gene control its expression and regulation by vitamin B12. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1479–1483. doi: 10.1073/pnas.88.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. The enzymic conversion of threonine to aminoacetone. Biochim Biophys Acta. 1960 Jun 17;41:164–165. doi: 10.1016/0006-3002(60)90388-7. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Miozzari G. F., van Cleemput M., Bennett G. N., Yanofsky C. Nucleotide sequences of the trpG regions of Escherichia coli, Shigella dysenteriae, Salmonella typhimurium and Serratia marcescens. J Mol Biol. 1980 Oct 5;142(4):503–517. doi: 10.1016/0022-2836(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Nishimura Y., Hirota Y., Nishimura S. Isolation and characterization of an Escherichia coli mutant lacking tRNA-guanine transglycosylase. Function and biosynthesis of queuosine in tRNA. J Biol Chem. 1982 Jun 10;257(11):6544–6550. [PubMed] [Google Scholar]
- Obradors N., Badía J., Baldomà L., Aguilar J. Anaerobic metabolism of the L-rhamnose fermentation product 1,2-propanediol in Salmonella typhimurium. J Bacteriol. 1988 May;170(5):2159–2162. doi: 10.1128/jb.170.5.2159-2162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peijnenburg A. A., Venema G., Bron S. Translational coupling in a penP-lacZ gene fusion in Bacillus subtilis and Escherichia coli: use of AUA as a restart codon. Mol Gen Genet. 1990 Apr;221(2):267–272. doi: 10.1007/BF00261730. [DOI] [PubMed] [Google Scholar]
- Poston J. M. Leucine 2,3-aminomutase: a cobalamin-dependent enzyme present in bean seedlings. Science. 1977 Jan 21;195(4275):301–302. doi: 10.1126/science.195.4275.301. [DOI] [PubMed] [Google Scholar]
- Richter-Dahlfors A. A., Andersson D. I. Analysis of an anaerobically induced promoter for the cobalamin biosynthetic genes in Salmonella typhimurium. Mol Microbiol. 1991 Jun;5(6):1337–1345. doi: 10.1111/j.1365-2958.1991.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Richter-Dahlfors A. A., Andersson D. I. Cobalamin (vitamin B12) repression of the Cob operon in Salmonella typhimurium requires sequences within the leader and the first translated open reading frame. Mol Microbiol. 1992 Mar;6(6):743–749. doi: 10.1111/j.1365-2958.1992.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Rickes E. L., Brink N. G., Koniuszy F. R., Wood T. R., Folkers K. Crystalline Vitamin B12. Science. 1948 Apr 16;107(2781):396–397. doi: 10.1126/science.107.2781.396. [DOI] [PubMed] [Google Scholar]
- Roessner C. A., Warren M. J., Santander P. J., Atshaves B. P., Ozaki S., Stolowich N. J., Iida K., Scott A. I. Expression of 9 Salmonella typhimurium enzymes for cobinamide synthesis. Identification of the 11-methyl and 20-methyl transferases of corrin biosynthesis. FEBS Lett. 1992 Apr 13;301(1):73–78. doi: 10.1016/0014-5793(92)80213-z. [DOI] [PubMed] [Google Scholar]
- Rondon M. R., Escalante-Semerena J. C. The poc locus is required for 1,2-propanediol-dependent transcription of the cobalamin biosynthetic (cob) and propanediol utilization (pdu) genes of Salmonella typhimurium. J Bacteriol. 1992 Apr;174(7):2267–2272. doi: 10.1128/jb.174.7.2267-2272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof D. M., Roth J. R. Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium. J Bacteriol. 1989 Jun;171(6):3316–3323. doi: 10.1128/jb.171.6.3316-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. A., Childs J. D. Methionine genes and enzymes of Salmonella typhimurium. Heredity (Edinb) 1966 May;21(2):265–286. doi: 10.1038/hdy.1966.22. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Automatic generation of primary sequence patterns from sets of related protein sequences. Proc Natl Acad Sci U S A. 1990 Jan;87(1):118–122. doi: 10.1073/pnas.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Effect of manganese ions on the incorporation of dideoxynucleotides by bacteriophage T7 DNA polymerase and Escherichia coli DNA polymerase I. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4076–4080. doi: 10.1073/pnas.86.11.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Neuhard J. Translational coupling in the pyrF operon of Salmonella typhimurium. Mol Gen Genet. 1990 Jul;222(2-3):345–352. doi: 10.1007/BF00633839. [DOI] [PubMed] [Google Scholar]
- Thibaut D., Couder M., Crouzet J., Debussche L., Cameron B., Blanche F. Assay and purification of S-adenosyl-L-methionine:precorrin-2 methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1990 Nov;172(11):6245–6251. doi: 10.1128/jb.172.11.6245-6251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut D., Couder M., Famechon A., Debussche L., Cameron B., Crouzet J., Blanche F. The final step in the biosynthesis of hydrogenobyrinic acid is catalyzed by the cobH gene product with precorrin-8x as the substrate. J Bacteriol. 1992 Feb;174(3):1043–1049. doi: 10.1128/jb.174.3.1043-1049.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut D., Debussche L., Blanche F. Biosynthesis of vitamin B12: isolation of precorrin-6x, a metal-free precursor of the corrin macrocycle retaining five S-adenosylmethionine-derived peripheral methyl groups. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8795–8799. doi: 10.1073/pnas.87.22.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Honda S., Fukui S. Fermentation of 1,2-propanediol with 1,2-ethanediol by some genera of Enterobacteriaceae, involving coenzyme B12-dependent diol dehydratase. J Bacteriol. 1979 Jul;139(1):39–47. doi: 10.1128/jb.139.1.39-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. J., Stolowich N. J., Santander P. J., Roessner C. A., Sowa B. A., Scott A. I. Enzymatic synthesis of dihydrosirohydrochlorin (precorrin-2) and of a novel pyrrocorphin by uroporphyrinogen III methylase. FEBS Lett. 1990 Feb 12;261(1):76–80. doi: 10.1016/0014-5793(90)80640-5. [DOI] [PubMed] [Google Scholar]
- Wolf J. B., Brey R. N. Isolation and genetic characterizations of Bacillus megaterium cobalamin biosynthesis-deficient mutants. J Bacteriol. 1986 Apr;166(1):51–58. doi: 10.1128/jb.166.1.51-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Guchte M., Kok J., Venema G. Distance-dependent translational coupling and interference in Lactococcus lactis. Mol Gen Genet. 1991 May;227(1):65–71. doi: 10.1007/BF00260708. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]





