Abstract
Several studies support an association between EEG voltage and alcohol dependence. However, the distribution of EEG variants also appears to differ depending on an individual’s ethnic heritage, suggesting significant genetic stratification of this EEG phenotype. The present study’s aims were to investigate the incidence of EEG alpha variants and spectral power in the alpha frequency range in Mexican American young adults based on gender, personal and family history of alcohol dependence. Clinical ratings (high, medium and low alpha voltage variants) and spectral characteristics of the EEG in the alpha frequency range (7.5–12 Hz) were investigated in young adult (age 18–25 yrs) Mexican American men (n=98) and women (n=138) who were recruited from the community. Nineteen percent (n=45) of the participants had a low voltage alpha EEG variant, 18% had a high voltage variant, and 63% had a medium voltage variant. There were no significant differences in the distribution of the EEG variants based on family history of alcohol dependence. There was a significant relationship between gender and the three alpha variants (chi square: = 9.7, df=2; p<0.008), and there were no male participants with alcohol dependence with high alpha variants (chi square: 5.8; df=2; p<0.056). Alcohol dependence, but not a family history of alcohol dependence, was associated with lower spectral power in the alpha frequency range in the right (F=4.4; df=1,96; p<0.04) and left (F=5.3; df=1,96; p<0.02) occipital areas in the men but not in the women. In conclusion, in this select population of Mexican American young adults, male gender and alcohol dependence are associated with an absence of high voltage alpha variants and lower alpha power in the EEG. These data suggest that EEG low voltage, a highly heritable trait, may represent an important endophenotype in male Mexican Americans that may aid in linking brain function with genetic factors underlying alcohol dependence in this ethnic group.
Keywords: Mexican Americans, alcohol dependence, EEG variants, EEG spectral power
Introduction
Several features of the EEG have been shown to be genetically influenced and EEG phenotypes based on frequency and amplitude characteristics have been suggested (Rangaswamy et al., 2002, 2004; Vogel, 1962). Evidence from twin studies confirm that a significant proportion of the variance in the EEG is genetically determined (Christian et al., 1996; Juel-Nielson & Harvald, 1958; Lykken et al., 1982; Stassen et al., 1987; van Beijsterveldt & Boomsma, 1994; Vogel, 1962). EEG patterns appear to remain highly stable over most of an adult’s lifespan (Vogel, 1970), with variation within an individual studied on two different occasions being not greater than that observed between monozygotic twins (Lykken et al., 1982).
One relatively common EEG pattern or variant, low voltage alpha (LVA), appears to follow a simple Mendelian mode of inheritance and to have high penetrance (Anokhin et al., 1992; Steinlein et al., 1992; Vogel, 1958, 1970; Vogel et al., 1979a, b). Genetic linkage studies that have localized the LVA EEG trait to chromosome 20q in some families (Anokhin et al., 1992) have intensified the interest in relating this electrophysiological phenotype to behavioral disorders. While the functional significance of the presence of LVA appears unclear at present, several studies support a cross-sectional association between having a low voltage EEG and alcohol dependence (Arentsen & Sindrup, 1963; Coger et al., 1978; Jones & Holmes, 1976; Varga and Nagy, 1960). This characteristic is four times more common in some types of alcoholics, particularly those with anxiety, as compared to non-alcoholics (Enoch et al., 1995, 1999). Additionally, it has been associated with anxiety and the catechol-O-methyltransferase polymorphism in women (Enoch et al., 2003).
Like most genetically regulated traits, the prevalence of low voltage alpha records also appears to be stratified over population groups. Anokhin et al. (1992) reviewed 11 studies published over a 50 year time period that together represented over 23,000 cases of individuals studied in Europe, the Far East, the United States and Central Asia. The prevalence of LVA was found to vary from 3–13% depending on the study. However, the authors cautioned that the samples differed in definition of the trait, as well as the subjects’: age, sex and ethnic background, which was not always disclosed (Anokhin et al., 1992).
The prevalence of alcohol abuse and alcohol dependence among different ethnic groups also varies. Certain tribes of Native Americans have very high rates of alcoholism and other alcohol-related disease when compared to Euro Americans, African Americans, and Asian Americans (Burns, 1995; U.S. Indian Health Service, 1997; Young, 1991). In a small study of Native American adult males, half of the men were classified as either LVA (22%) or as borderline LVA (23%), whereas 22% were classified as monomorphic alpha (high voltage continuous alpha (MA)). LVA was found to be significantly associated with high degrees of Native American heritage (Ehlers et al., 1999), a characteristic that has also been associated with alcohol dependence in a large sample of adults from that Indian population (Ehlers et al., 2004c).
Few such data are available in Hispanics. Hispanic American males, like Native Americans, as a group, are more likely to drink frequently and to consume larger quantities of alcohol than Whites or Blacks (Caetano, 1984; Caetano & Kaskutas, 1995; Dawson, 1998; Nielsen, 2000; Stinson et al., 1998). Additionally, the total lifetime prevalence rate of alcoholism (alcohol abuse and dependence) has been found to be higher among Hispanic American men than among White men in the Epidemiologic Catchment Area (ECA) study (Helzer et al., 1991). However, the relationship between alpha variants, alpha power and risk for alcoholism in Hispanic Americans, either males or females has been little investigated. In an investigation of a small sample of Hispanic American college students, all of whom had a family history of alcohol dependence, but no personal history of alcohol dependence, Hispanic Americans were found to have a lower percentage of individuals in the low-voltage alpha group and more individuals in the medium-voltage alpha group when compared with findings for white non-Hispanic Americans. Additionally, women were found to have higher overall power in the alpha frequency ranges (Ehlers et al., 2004a).
Hispanic American subgroups bring with them a diversity of racial heritage, as well as cultures that vary in psychosocial, religious, and economic bases. The importance of specifying subgroups of Hispanics to avoid inaccurate generalizations has been stressed (Caetano et al., 1998). Mexican Americans represent the largest subgroup of Hispanic Americans, nearly two thirds of the total U.S. Hispanic population, followed by Puerto Ricans, Cubans, Caribbeans, Central and South Americans. The present study was designed to extend our previous investigations of EEG alpha variants and alpha power to a community sample of Mexican American young adults. The aims of the present study were to evaluate EEG alpha variants and alpha power as a function of personal and family history of alcohol dependence. Additionally, exploratory analyses tested whether the findings were different in men versus women.
Materials and Methods
Participants were recruited using a commercial mailing list that provided the addresses of individuals with Hispanic surnames in 11 zip codes in San Diego County that were identified as having a population that was over 20% Hispanic heritage and were within 25 miles of the research site. The mailed invitation stated that potential participants must be of Mexican American heritage, be between the ages of 18 and 30 years, be residing in the United States legally, and be able to read and write in English. Potential participants were requested to phone research staff for more information. During the phone interview, potential participants were screened for the presence of the inclusion criteria as listed on the invitation, and were excluded it they were: pregnant or nursing, currently had a major medical or neurological disorder, or a head injury that might bias the EEG testing. Participants were asked to refrain from alcohol or any other substance use for 24 hours prior to testing.
On the test day, after a complete description of the study to the participants, written informed consent was obtained using a protocol approved by The Institutional Review Board of The Scripps Research Institute. Information on demography, personal, medical and psychiatric history, and family history of alcohol and other substance dependence was obtained using a family history interview and the face-to-face Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA), a reliable poly diagnostic psychiatric interview (Bucholz et al., 1994; Hesselbrock et al., 1999). Participants were eliminated from the current data analyses if they were taking psychoactive medication that may affect the EEG or had a positive breath-analyzer test on the day of the evaluation. Lifetime history of alcohol dependence in this population was defined by DSM-III-R criteria. Family history of alcoholism was assessed using the Family History Assessment Module (FHAM). A participant was classified as family history positive if they had a first degree relative with alcohol dependence. Family history negative participants had no first degree relatives with alcohol dependence.
EEG collection and spectral analyses
Six channels of bipolar EEG (F3-C3, C3-P3, P3-01 and F4-C4, C4-P4, P4-02, international 10–20 system) were obtained using an electrode cap (impedance < 5K ohms), as described. Bipolar recordings were obtained for comparison to previous studies in a wide range of ethnic groups (see Ehlers & Schuckit, 1990, 1991; Ehlers et al., 1999; Wall et al., 1993). A forehead ground electrode and an electrode placed left lateral infraorbitally and referenced to the left earlobe were used to monitor both horizontal and vertical eye movement. Resting EEG was recorded in a temperature and noise controlled room while a participant was comfortably sitting on a chair. Participants were instructed to relax and keep their eyes closed, but to remain awake throughout the EEG recording. Ten to 15 minutes of EEG was collected on paper (Nihon Kohden, high-low pass filters 1–70 Hz) and also digitized for subsequent analyses. EEG records were monitored during all recordings for signs of drowsiness or artifact. Ten minutes of artifact-free, drowsiness-free EEG, as defined by Daly & Pedley (1990), was computer analyzed for each channel. Time of recording with respect to the menstrual cycle was not controlled, as previous studies have demonstrated that the EEG variables under study are not sensitive to time during the cycle (see Ehlers et al., 1996).
Records were digitized at 128 Hz. The Fourier transform of consecutive four second epochs was calculated and the power spectrum produced using a PC computer with software developed by Ehlers & Havstad (1982). Power density is calculated in microvolts squared per octave, a transformation that expands amplitudes at high frequencies and reduces them at low frequencies producing a spectrum with less 1/f characteristics (Ehlers et al., 1989). For statistical analysis, data from the two posterior channels were used where alpha is of the highest amplitude (P3-O1, P4-O2). The transformed data were compressed into frequency bands. Mean spectral power density (microvolts squared/octave) in the alpha frequency band (7.5–12 Hz) was calculated by summing the raw power spectral values within the band, multiplying by a scale factor derived from the calibration signal to produce the total power in the band in microvolts squared, and dividing by the width of the band in octaves. This width is the logarithm of the ratio of the maximum and minimum frequencies in the band, divided by the log of two. The details of the spectral analysis procedures have been previously described (Ehlers & Havstad, 1982).
Data Analyses
Data analyses focused on the specific aim that was generated, based on previous EEG research investigating LVA (see Enoch et al., 1999), as well as studies in populations at varying risk for the development of alcoholism (Ehlers & Schuckit, 1990, 1991; Ehlers et al., 1999, 2004a, Ehlers et al., b; Wall et al., 1993). The aims were to investigate the relationship between EEG alpha variants, EEG alpha power, and alcohol dependence and family history of alcohol dependence. Exploratory analyses were also conducted to evaluate whether men and women differed in their relationship between alpha variants, alpha power and alcohol dependence/family history of alcohol dependence.
While there is no consensus on an exact definition of LVA (Niedermeyer & Lopes da Silva, 1999), it is generally identified as a record with the absence of rhythmical alpha activity combined with an overall low EEG amplitude usually not exceeding 20 microvolts. It has been suggested that an emphasis should be placed, in making the identification of LVA, on the absence of rhythmical alpha activity, particularly in the occipital leads, rather than reduced overall EEG amplitude (Anokhin et al., 1992). It is also readily acknowledged that some EEG records clearly represent “borderline” cases. Attempts have also been made to discriminate between LVA and borderline LVA (BLVA) using computer and statistical techniques (see Dunki et al., 1996; Vogel et al., 1981).
In the present study, visual scoring of the EEG for clinical abnormalities, impressionistic classification, and spectral analyses were used to initially identify EEG alpha variants. Impressionistic classification was conducted by a Registered EEG Technician (E.P) who blindly rated the entire EEG record for amplitude and waveform characteristics into three categories, according to the criteria described by Vogel and colleagues (1979a, b): 1) monomorphic alpha (MA), 2) medium voltage variant, and 3) low voltage alpha (LVA). Spectral analysis methods were used to further quantify EEG alpha power in the left occipital lead, to verify the impressionistic classification system, and then all records were classified into the 3 categories based on spectral analyses (high voltage alpha > 75 microvolts squared per octave, medium voltage 11–74 microvolts squared per octave, LVA ≤ 10 microvolts squared per octave). Concordance between visual scoring and EEG power has been determined to be high (Cohen’s Kappa 0.83) (Ehlers et al., 1999, 2004a). In this case, the cut-off values for classifications were based on previous studies in Asian Americans, Euro Americans, Native Americans and Hispanic Americans (Ehlers & Phillips, 2003; Ehlers & Schuckit, 1990, 1991; Ehlers et al., 1999, 2004a; Wall et al., 1993) for comparison to previous studies. The relationship between alcohol dependence and family history of alcohol dependence and the three alpha variants was explored using Chi square analyses or Fishers exact test depending on cell size. Exploratory analyses were conducted for these variables in men and women separately.
The second aim of this study was focused on whether significant EEG power differences in the alpha frequency ranges could be found based on alcohol dependence and family history of alcohol dependence. The EEG dependent variables were mean power in the alpha frequency range (7.5–9 Hz) in the two posterior leads. An ANOVA was applied to data evaluating the association between EEG power and alcohol dependence, and family history of alcoholism. Statistical significance was set at the probability level: p >0.05. Power analyses indicated there was sufficient power (0.80) at alpha=0.05 to detect differences in our primary analyses. Exploratory analyses were conducted to see if an association between alpha power, family history and alcohol dependence could be observed in men and women analyzed separately. Comparisons between demographic, drinking variables, diagnoses and EEG were conducted using ANOVA or Pearson correlation for 1 or 2 continuous variables respectably and Chi square analyses for 2 dichotomous variables.
Results
The young adults who participated in the study included 59% women and the sample had a mean age of 23 years (SD= + 3.9 years). Demographic data including age, gender, number of years of education, marriage, and current drinking history (quantity and frequency/month) are presented in Table 1. There were no significant differences in age, number of years of education, income, or current frequency or quantity of drinking between those individuals who were family history positive and those who had no first degree relatives with alcohol dependence.
Table 1.
Demographic and drinking variables in Mexican American men and women (n=237)
| No alcohol dependence | Alcohol Dependence | |||||
|---|---|---|---|---|---|---|
| Variable | Males (n=68) | Females (n=108) | All (n=176) | Males (n=30) | Females (n=31) | All (n=61) |
| Age (mean ± S.D.) | 23.0 ± 3.8 | 23.1 ± 3.8 | 23.1 ± 3.8 | 23.8 ± 4.1 | 24.3 ± 3.9 | 24.0 ± 4.0 |
| Years of education | 13.4 ± 1.6 | 13.4 ± 1.7 | 13.4 ± 1.7 | 13.7 ± 1.6 | 14.0 ± 1.8 | 13.9 ± 1.7 |
| Income (median) | $40 – <$50K | $40 – <$50K | $40 – <$50K | $50K – <$75K | $30K – <40K | $50K – <$75K |
| Not Married (n) | 58 | 81 | 139 | 27 | 30 | 57 |
| Not Employed | 19 | 32 | 51 | 7 | 8 | 15 |
| Drinking Quantity (drinks per occasion) | 3.3 ± 2.9 | 2.2 ± 1.7 | 2.7 ± 2.3 | 5.6 ± 3.6 | 2.9 ± 2.5 | 4.2 ± 3.4 |
| Drinking Frequency (drinks per month) | 2.5 ± 3.4 | 2.7 ± 3.4 | 2.6 ± 3.4 | 4.4 ± 5.8 | 4.3 ± 5.0 | 4.3 ± 5.4 |
Participants with alcohol dependence did not differ on age, income or number of years of education, however, they were less likely to be married (Chi square=6.9; df=2; p<0.008), reported drinking more often (F=7.4, df=1,195; p<0.007), and more drinks per occasion (F=15.0; df=1,195; p<0.0001) than those participants without an alcohol dependence diagnosis. There were no significant differences in age, number of years of education, income, employment or marriage status between men and women. With respect to the drinking variables, women did not report drinking significantly less frequently, however, men reported drinking two more drinks per occasion than women (F=21.9; df=1,195; p<0.0001). The relationships between the two drinking variables (drinking quantity, drinking frequency) and the main outcome variables, EEG alpha variants and EEG alpha power in the two leads, were explored and found to be non-significant.
To address the first major research question, EEG records were evaluated for the distribution of EEG variants. None of the records were found to contain EEG abnormalities. Nineteen percent of the participants were classified as LVA and 18% were classified as High voltage alpha. The remainder (63%) had medium voltage records. Chi square revealed that the three EEG groups did not differ with respect to a participant’s age, number of years of education or their quantity or frequency of drinking. There were also no significant differences in the distribution of the EEG variants based on the presence of a family history of alcoholism. However, as seen in figure 1, women were more likely than men to have a high alpha EEG variant (Chi Square=9.7, df=2; p<0.008). Additionally, more males with alcohol dependence had a low voltage variant and no alcohol dependent males had a high voltage EEG variant (chi square: 5.8, df=2, p<0.056).
Figure 1.
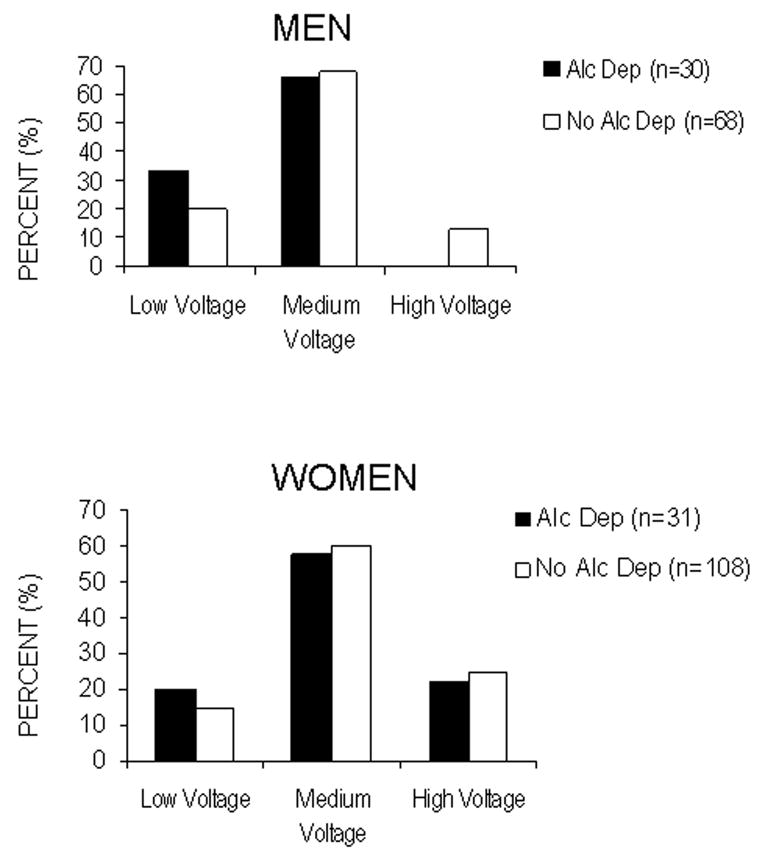
Distribution of three electroencephalogram (EEG) variants (high-voltage alpha, medium-voltage alpha, and low-voltage alpha; as determined by spectral analyses) by diagnosis (alcohol dependent (Alc Dep), no alcohol dependence (No Alc Dep), in men in the upper graph and women in the lower. Data are presented as the percentage of individuals of each gender in each of the three EEG variant groups. There was a significant relationship between gender and the three alpha variants (chi square: = 9.7, df=2; p<0.008), and there were no male participants with alcohol dependence with high alpha variants (chi square: 5.8; df=2; p<0.056)
Evaluation of spectral power in the alpha frequency range (7.5–12 Hz) revealed that alcohol dependence, but not a family history of alcohol dependence, was associated with lower spectral power in the alpha frequency range. As seen in figure 2, EEG power in the right (F= 4.4; df=1, 235; p<0.04) and left occipital areas (F= 4.5; f=1,235; p<0.035) was significantly lower in participants with alcohol dependence. Exploratory analyses also revealed that alcohol dependence was significantly associated with lower alpha levels in the right (F=4.4; df=1,96; p<0.04) and left (F=5.3; df=1,96; p<0.02) occipital areas in the men, but not in the women. Additionally, alpha power was not found to be significantly associated with a family history of alcohol dependence.
Discussion
Data from large international studies suggest that the prevalence of LVA EEG in the general adult population may range from 3–13% (Anokhin, 1987; Niedermeyer, 1987). This trait was seen in 19% of the present select population of Mexican Americans. This is a similar percentage to what was found in a previous study of Hispanic college students who were all family history positive for alcoholism (21%) (Ehlers et al., 2004a). The present study also confirms the findings of a number of other investigations that have found reduced EEG alpha activity or low voltage records in alcoholics (Enoch et. al., 1995, 1999, 2002, 2003; Funderburk, 1949; Jones & Holmes, 1976; Little & McAvoy, 1952; Propping et al., 1981). There were also gender differences observed in EEG alpha variants and alpha power in the present study. Women were more likely to have a high voltage EEG variant and had higher alpha power when compared to men. This finding also confirms findings from our previous studies in Hispanics (Ehlers et al., 2004a,b), and those of others in mixed ethnic groups who have also noted that women in general have higher amplitudes in the resting EEG when compared to men (Veldhuizen et al., 1993; Wada et al., 1994). Additionally, we found that men, but not women alcoholics, were more likely to have low voltage alpha.
Studies in children of alcoholics, in several different ethnic groups, suggest that low voltage alpha may also be phenotypic markers of the risk for alcoholism. In one study, consisting of primarily Euro American participants, individuals with a family history of alcoholism were found to have reduced alpha power when compared to those participants with no family history of alcohol dependence (Finn & Justus, 1999). In another study investigating Native American Indian men, at high risk for the development of alcoholism, but with no personal history of alcohol dependence, an excess of LVA EEGs was observed (22% of the records). Additionally, in that study, LVA was significantly associated with higher degrees of Native American heritage (Ehlers et al., 1999), a characteristic that has been associated with alcohol dependence in a large sample of adults from that Indian population (Ehlers et al., 2004c). However, in the present study, and in a previous study of Hispanic college students (Ehlers et al., 2004a,b), family history of alcoholism was not found to correlate with either alpha variants or EEG alpha power. Cohen et al. (1991) and Fein & Allen (2005) also failed to find an association between family history of alcohol dependence and EEG alpha measures. Additionally, several studies in a range of ethnic groups have also provided data to suggest that high voltage alpha may be associated with a family history of alcohol dependence (Ehlers & Phillips, 2003; Ehlers & Schuckit, 1988, 1991; Ehlers et al., 1996, 1999).
The reason that in some studies an excess of lower voltage EEGs are observed associated with alcohol dependence/family history of alcoholism and in other studies alcohol dependence or family history of alcoholism is associated with higher alpha voltage is as yet unknown. Fein & Allen (2005) have suggested that higher voltage alpha may be a trait marker for alcoholism that is present early in an alcoholic’s lifetime but that decreases in EEG power may be found later in an alcoholic’s life as a result of long-term severe alcohol abuse. However, in the present study of young adults, no relationship was found between EEG voltage and drinking history. An alternative explanation takes into account the fact that alcohol dependence has a heterogeneous etiology and that both high voltage alpha and low voltage alpha may be endophenotypes for separate traits that both increase the risk for alcoholism. In support of this supposition, Enoch et al. (1995, 1999, 2003) have suggested that LVA may be associated with alcoholism and anxiety in women. Whereas, in two separate studies, high voltage alpha was found to be associated with a subjectively reported lower level of response to an alcohol challenge (Ehlers & Schuckit, 1991; Ehlers et al., 2004b), a trait shown to be highly associated with risk for alcoholism in a longitudinal study of men (Schuckit & Smith, 1996). In the present study, low voltage alpha was most strongly associated with a male-limited type of alcoholism. These data suggest that clinical heterogeneity, ethnic heritage and gender should be taken into consideration when interpreting phenotypic markers of alcoholism risk.
It has been suggested that human electrophysiological measures (e.g. EEG, Event-related potentials (ERPs), event-related oscillations (EROs)) may provides some of the most informative phenotypes or endophenotypes (intermediate phenotypes) for the genetic analysis of psychiatric disorders (Begleiter & Porjesz, 2006). The EEG is a highly heritable, quantitative, biological measure that is less complex and presumably closer to gene function than diagnostic and psychological measures of alcohol dependence. As such, several important studies have begun to identify genes associated with certain EEG phenotypes. Low voltage alpha, in females, has been reported to be associated with a genetic variant that leads to low activity of the enzyme that metabolizes dopamine and norepinephrine, catechol-o-methyltransferase (COMT) (Enoch et al., 2003). Low voltage alpha has also been reported to be linked to the GABAergic system, as an association has been found between the exon 7 variant of the GABAB receptor gene and alpha voltage (Winterer et al., 2003). Porjesz et. al. (2002) have also found significant associations between and the GABAergic system and the human EEG. They found significant genetic linkage between the beta frequency of the EEG and a cluster of GABAA receptor genes on chromosome 4P. Additionally, these same GABAA receptor genes were also found to be associated with a DSM-IV diagnosis of alcohol dependence (Edenberg et al., 2004). These genetic findings provide evidence that EEG measures are promising endophenotypes in the search for genes involved in alcohol dependence. Taken together with the data from the present study, it appears that EEG alpha voltage may be an important endophenotype for the future exploration of the genetic basis of alcohol dependence in Mexican American men.
It is important to consider some of the present study’s limitations. First, the findings may not generalize to all Mexican Americans, or all Hispanic young adult Americans. Over half of the participants in the present study were women and thus the findings may not generalize to previous studies that have focused on samples of entirely male participants. It has been suggested that the EEG of cocaine-abusing women may be more normal than that of cocaine-abusing men (King et al., 2000), suggesting that women may have less EEG effects or “markers” of drug abuse than men. Second, the study was limited to young adults between the ages of 18 and 30 years. This allowed an investigation of the association between parental alcohol dependence and EEG patterns; however, since not all of the participants in the study had passed through the age of risk, the association of EEG traits with personal drinking history and alcohol use disorders may be limited. Further studies employing a longitudinal design will be required to test the relationship of EEG amplitude and eventual alcohol related morbidity and mortality. Despite these limitations, this report represents an important first step in an ongoing investigation to determine risk and protective factors associated with the development of substance use disorders in Mexican Americans.
Table 2.
EEG alpha power in Mexican American young adults with and without alcohol dependence (mean ± sem, microvolts squared per octave).
| ALCOHOL DEPENDENT: | MEN | WOMEN | ALL |
|---|---|---|---|
| P3-01 (7.5–12 Hz) | 23.7 ± 5.1* | 43.1 ± 10.2 | 33.6 ± 6.2* |
| P4-02 (7.5–12 Hz) | 22.8 ± 5.6* | 43.4 ± 12.6 | 33.2 ± 7.4* |
| NON-ALCOHOL DEPENDENT: | |||
| P3-01 (7.5–12 Hz) | 36.6 ± 3.4* | 55.9 ± 5.5 | 48.5 ± 3.6* |
| P4-02 (7.5–12 Hz) | 38.2 ± 3.7* | 59.9 ± 6.7 | 51.5 ± 4.4* |
* p <0.05
Acknowledgments
Supported in part by the National Institute of Alcoholism and Alcohol Abuse Grants: 04620 and 10201 and the Stein Endowment fund. The computer programs were written by D. James Havstad. The authors would like to thank Phil Lau and David Gilder, for assistance in data analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anokhin AP. On the genetic nature of individual peculiarities of the whole brain EEG organization. Sov J Psychol. 1987;1:649–657. [Google Scholar]
- Anokhin A, Steinlein O, Fischer C, Mao Y, Vogt P, Schalt E, Vogel F. A genetic study of the human low-voltage electroencephalogram. Hum Genet. 1992;90:99–112. doi: 10.1007/BF00210751. [DOI] [PubMed] [Google Scholar]
- Arentsen K, Sindrup E. Electroencephalographic investigation of alcoholics. Acta Psychiatr Scand. 1963;39:371–383. doi: 10.1111/j.1600-0447.1963.tb07471.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Burns TR. How does IHS relate administratively to the high alcoholism mortality rate? Am Indian Alsk Native Ment Health Res. 1995;6:31–45. doi: 10.5820/aian.0603.1995.31. [DOI] [PubMed] [Google Scholar]
- Caetano R. A note on arrest statistics for alcohol-related offenses. Drinking Pract Surv. 1984;19:12–17. [Google Scholar]
- Caetano R, Kaskutas LA. Changes in drinking patterns among whites, blacks and Hispanics, 1984–1992. J Stud Alcohol. 1995;56:558–565. doi: 10.15288/jsa.1995.56.558. [DOI] [PubMed] [Google Scholar]
- Caetano R, Clark CL, Tam T. Alcohol consumption among racial/ethnic minorities: theory and research. Alcohol Health Res World. 1998;22:233–241. [PMC free article] [PubMed] [Google Scholar]
- Christian JC, Morzorati S, Norton JA, Jr, Williams CJ, O’Connor S, Li TK. Genetic analysis of the resting electroencephalographic power spectrum in human twins. Psychophysiology. 1996;33:584–591. doi: 10.1111/j.1469-8986.1996.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Coger RW, Dymond AM, Serafetinides EA, Lowenstam I, Pearson D. EEG signs of brain impairment in alcoholism. Biol Psychiatry. 1978;13:729–739. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H. EEG characteristics in males at risk for alcoholism. Alcohol Clin Exp Res. 1991;15:858–861. doi: 10.1111/j.1530-0277.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Daly DD, Pedley TA. Current practice of clinical electroencephalography. 2. New York: Raven Press; 1990. [Google Scholar]
- Dawson DA. Beyond black, white and Hispanic: race, ethnic origin and drinking patterns in the United States. J Subst Abuse. 1998;10:321–339. doi: 10.1016/s0899-3289(99)00009-7. [DOI] [PubMed] [Google Scholar]
- Dunki RM, Schmid GB, Scheidegger P, Stassen HH, Bomben G, Propping P. Reliable computer-assisted classification of the EEG: EEG variants in index cases and their first degree relatives. Am J Med Genet. 1996;67:1–8. doi: 10.1002/(SICI)1096-8628(19960216)67:1<1::AID-AJMG1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variation in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW. Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacology Bull. 1982;18:43–47. [Google Scholar]
- Ehlers CL, Schuckit MA. EEG response to ethanol in sons of alcoholics. Psychopharmacol Bull. 1988;24:434–437. [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr Clin Neurophysiol. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. EEG fast frequency activity in the sons of alcoholics. Biol Psychiatry. 1990;27:631–641. doi: 10.1016/0006-3223(90)90531-6. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. Evaluation of EEG alpha activity in sons of alcoholics. Neuropsychopharmacology. 1991;4:199–205. [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Parry BL. Electrophysiological findings during the menstrual cycle in women with and without late luteal phase dysphoric disorder: relationship to risk for alcoholism? Biol Psychiatry. 1996;39:720–732. doi: 10.1016/0006-3223(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Cloutier D, Phillips E. Electroencephalographic responses to alcohol challenge in Native American Mission Indians. Biol Psychiatry. 1999;45:776–787. doi: 10.1016/s0006-3223(98)00113-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. EEG low voltage variants and alpha power in African American young adults: Relation to family history of alcoholism. Alcohol Clin Exp Res. 2003;27:765–772. doi: 10.1097/01.ALC.0000065439.09492.67. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Schuckit MA. EEG alpha variants and alpha power in Hispanic American and white non-Hispanic American young adults with a family history of alcohol dependence. Alcohol. 2004a;33:99–106. doi: 10.1016/j.alcohol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Wall TL, Wilhelmsen K, Schuckit MA. EEG alpha and level of response to alcohol in Hispanic- and non-Hispanic-American young adults with a family history of alcoholism. J Stud Alcohol. 2004b;65:301–308. doi: 10.15288/jsa.2004.65.301. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Betancourt M, Gilder DA. The clinical course of alcoholism in 243 Mission Indians. Am J Psychiatry. 2004c;161:1204–1210. doi: 10.1176/appi.ajp.161.7.1204. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Rohrbaugh JW, Davis EZ, Harris CR, Ellingson RJ, Andreason P, Moore V, Varner JL, Brown GL, Eckardt MJ. Relationship of genetically transmitted alpha EEG traits to anxiety disorders and alcoholism. Am J Med Genet. 1995;60:400–408. doi: 10.1002/ajmg.1320600510. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol Clin Exp Res. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Rohrbaugh JW, Goldman D. The relationship between two intermediate phenotypes for alcoholism: low voltage alpha EEG and low P300 ERP amplitude. J Stud Alcohol. 2002;63:509–517. doi: 10.15288/jsa.2002.63.509. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Fein G, Allen J. EEG spectral changes in treatment-naive, actively drinking alcoholics. Alcohol Clin Exp Res. 2005;29:538–546. doi: 10.1097/01.alc.0000159107.08471.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A. Reduced EEG alpha power in the male and female offspring of alcoholics. Alcohol Clin Exp Res. 1999;23:256–262. [PubMed] [Google Scholar]
- Funderburk WH. Electroencephalographic studies in chronic alcoholics. Electroencephalogr Clin Neurophysiol. 1949;1:369. [Google Scholar]
- Helzer JE, Burnam A, McEvoy LT. Alcohol abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric disorders in America: the epidemiologic catchment area study. New York: The Free Press; 1991. pp. 81–115. [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Jones FW, Holmes DS. Alcoholism, alpha production, and biofeedback. J Consult Clin Psychol. 1976;44:224–228. doi: 10.1037//0022-006x.44.2.224. [DOI] [PubMed] [Google Scholar]
- Juel-Nielsen N, Harvald B. The encephalogram in uniovular twins brought up apart. Acta Genet Statistica Medica (Basel) 1958;8:57–64. doi: 10.1159/000151054. [DOI] [PubMed] [Google Scholar]
- King DE, Herning RI, Gorelick DA, Cadet JL. Gender differences in the EEG of abstinent cocaine abusers. Neuropsychobiology. 2000;42:93–98. doi: 10.1159/000026678. [DOI] [PubMed] [Google Scholar]
- Little SC, McAvoy M. Electroencephalographic studies in alcoholism. J Stud Alcohol. 1952;13:9–15. [PubMed] [Google Scholar]
- Lykken DT, Tellegen A, Iacono WG. EEG spectra in twins: Evidence for a neglected mechanism of genetic determination. Physiol Psychol. 1982;10:60–65. [Google Scholar]
- Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Da Silva FL, editors. Electroencephalography, basic principles, clinical applications, and related fields. Baltimore: Urban & Schwarzenberg; 1987. pp. 97–117. [Google Scholar]
- Niedermeyer E, Lopes da Silva FH. Electroencephalography: basic principles, clinical applications, and related fields. 4. Baltimore, MD: Williams & Wilkins; 1999. [Google Scholar]
- Nielsen AL. Examining drinking patterns and problems among Hispanic groups: results from a national survey. J Stud Alcohol. 2000;61:301–310. doi: 10.15288/jsa.2000.61.301. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice J, O’Connor SJ, Rohrbaugh J, Kupferman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAa receptor gene locus. PNAS. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propping P, Kurger J, Mark N. Genetic disposition to alcoholism: an EEG study in alcoholics and their relatives. Hum Genet. 1981;59:51–59. doi: 10.1007/BF00278854. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, Rohrbaugh J, O’Connor SJ, Kuperman S, Reich T, Begleiter H. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52:831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Reich T, Begleiter H. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Bomben G, Propping P. Genetic aspects of the EEG: an investigation into the within-pair similarity of monozygotic and dizygotic twins with a new method of analysis. Electroencephalogr Clin Neurophysiol. 1987;66:489–501. doi: 10.1016/0013-4694(87)90095-2. [DOI] [PubMed] [Google Scholar]
- Steinlein O, Anokhin A, Yping M, Schalt E, Vogel F. Localization of a gene for the human low-voltage EEG on 20q and genetic heterogeneity. Genomics. 1992;12:69–73. doi: 10.1016/0888-7543(92)90408-k. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Yi H, Grant BF, Chou P, Dawson DA, Pickering R. Drinking in the United States: Main Findings from the 1992 National Longitudinal Alcohol Epidemiologic Survey (NLAES) 1998 [Google Scholar]
- United States, Indian Health Service. Trends in Indian health. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Indian Health Service; 1997. [Google Scholar]
- van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- Varga B, Nagy T. Analysis of alpha rhythms in the electroencephalgram of alcoholics. Electroencephalogr Clin Neurophysiol. 1960;12:933. [Google Scholar]
- Veldhuizen RJ, Jonkman EJ, Poortvliet DC. Sex differences in age regression parameters of healthy adults--normative data and practical implications. Electroencephalogr Clin Neurophysiol. 1993;86:377–384. doi: 10.1016/0013-4694(93)90133-g. [DOI] [PubMed] [Google Scholar]
- Vogel F. Uber die Erblichkeit des normalen Elektroenzephalogrammes; vergleichende Untersuchungne an ein- und zweieiigen Zwillingen. Stuttgart: Thieme; 1958. [Google Scholar]
- Vogel F. Erganzende Untersuchungen zur Genetik des menschlichen Niederspannungs-EEG. Deutsch Z Nervenheilk. 1962;185:105–111. [Google Scholar]
- Vogel F. The genetic basis of the normal human electroencephalogram (EEG) Humangenetik. 1970;10:91–114. doi: 10.1007/BF00295509. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J, Propping P, Lehnert KF. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examinations in healthy males with various inherited EEG variants. I. Rationale of the study. Material. Methods. Heritability of test parameters. Hum Genet. 1979a;47:1–45. doi: 10.1007/BF00295569. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examinations in healthy males with various inherited EEG variants. II. Results. Hum Genet. 1979b;47:47–80. doi: 10.1007/BF00295570. [DOI] [PubMed] [Google Scholar]
- Vogel F, Kruger J, Schalt E. [Characterization of genetic EEG-variations with the amplitude-interval-analysis. I. Variations of alpha-activity; flat-EEG; borderline flat EEG, occipital slow beta-waves; monotonous alpha-rhythm (author’s transl)] EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1981;12:33–44. [PubMed] [Google Scholar]
- Wada Y, Takizawa Y, Jiang ZY, Yamaguchi N. Gender differences in quantitative EEG at rest and during photic stimulation in normal young adults. Clin Electroencephalogr. 1994;25:81–85. doi: 10.1177/155005949402500209. [DOI] [PubMed] [Google Scholar]
- Wall TL, Gallen CC, Ehlers CL. Effects of alcohol on the EEG in Asian men with genetic variations of ALDH2. Biol Psychiatry. 1993;34:91–99. doi: 10.1016/0006-3223(93)90261-b. [DOI] [PubMed] [Google Scholar]
- Winterer G, Mahlberg R, Smolka MN, Samochowiec J, Ziller M, Rommelspacher HP, Hermann WM, Schmidt LG, Sander T. Association analysis of exonic variants of the GABA(B)-receptor gene and alpha electroencephalogram voltage in normal subjects and alcohol-dependent patients. Behav Genet. 2003;33:7–15. doi: 10.1023/a:1021043315012. [DOI] [PubMed] [Google Scholar]
- Young TJ. Native American drinking: a neglected subject of study and research. J Drug Educ. 1991;21:65–72. doi: 10.2190/04VJ-R84N-JRUD-B0LF. [DOI] [PubMed] [Google Scholar]


