Abstract
Environmental exposures (e.g. pesticides, air pollution, and environmental tobacco smoke) during prenatal and early postnatal development have been linked to a growing number of childhood diseases including allergic disorders and leukemia. Because the immune response plays a critical role in each of these diseases, it is important to study the effects of toxicants on the developing immune system. Children's unique susceptibility to environmental toxicants has become an important focus of the field of immunotoxicology and the use of immune biomarkers in molecular epidemiology of children's environmental health is a rapidly expanding field of research. In this review, we discuss how markers of immune status and immunotoxicity are being applied to pediatric studies, with a specific focus on the various methods used to analyze T-helper-1/2 (Th1/Th2) cytokine profiles. Furthermore, we review recent data on the effects of children's environmental exposures to volatile organic compounds, metals, and pesticides on Th1/Th2 cytokine profiles and the associations of Th1/Th2 profiles with adverse health outcomes such as pediatric respiratory diseases, allergies, cancer and diabetes. Although cytokine profiles are increasingly used in children's studies, there is still a need to acquire distribution data for different ages and ethnic groups of healthy children. These data will contribute to the validation and standardization of cytokine biomarkers for future studies. Application of immunological markers in epidemiological studies will improve the understanding of mechanisms that underlie associations between environmental exposures and immune-mediated disorders.
Keywords: biomarkers, immune system, environmental exposures, pesticides, asthma, health outcomes
1. Importance of using biomarkers to study children's environmental health
More than 33% of diseases in children less than 5 years of age are caused by environmental exposures (WHO, 2006). The main risk factors include pesticides, air and water pollution, lead, environmental tobacco smoke, infections, and inadequate diet (EPA, 2006). A growing number of childhood diseases such as allergic disorders (e.g. allergic rhinitis, atopic dermatitis, asthma), cancer (e.g. acute leukemia and myeloid leukemia), and others (e.g. Type 1 Diabetes) have been linked to environmental exposures during prenatal and early postnatal development. Because the immune response plays a critical role in each of these diseases, it is important to consider the effects of toxicants on children's developing immune system. In the recent years, children's susceptibility to environmental exposures has become an important focus of the field of immunotoxicology (Garry, 2004, Holsapple et al., 2004).
Despite the recognition that early childhood represents a critical period of immune development there is a dearth of data on the distributions of immune parameters in healthy children. Most of the data available for infants and children have been acquired from pediatric populations afflicted with either acute or chronic health conditions, or they have been extrapolated from adult studies. However, children's immune systems are less developed and, thus potentially more susceptible to environmental exposures (Kovarik and Siegrist, 1998). Moreover, immunological markers and reference values that have been used in adult occupational health studies (Colosio et al., 1999, Vogt 1991) are being applied in children's studies, a situation that also is not always appropriate since immune function evolves over time after exposures to environmental antigens. Finally, collecting blood from a pediatric population poses an important ethical issue and while most of the current immunological assays require approximately less than 1 ml of blood, given the invasive nature of blood collection, careful planning is required to maximize the use of these samples (Holland et al. 2003, Neri et al., 2006).
Luster et al. (2005) recently summarized current efforts to identify and implement tests of immune function (e.g. cytokine profiles) in children with various diseases of the immune system. However, several issues remain with this relatively new application of cytokine measurements in children's studies: a) lack of data on the distribution of different cytokine levels in normal, healthy children, b) lack of standardized methods; and c) the fact that cytokine levels measured on a single occasion represent only a “snapshot” that may not reflect the response that occurs at the target organ.
In this review, examples from the literature and recent data from two studies conducted at the University of California, Berkeley, the Northern California study of childhood leukemia (Ma et al. 2002; Buffler et al. 2005) and the CHAMACOS birth cohort of Latino mothers and children from agricultural community (Eskenazi et al. 2003) are presented to illustrate how cytokine markers have been used to link environmental exposures to cytokine profiles and how these immunological biomarkers can be applied in the study of adverse health outcomes in children.
2. Biomarkers help link environmental exposures to disease outcome
A biological marker (biomarker) is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological response to a therapeutic intervention” (NIH Biomarkers Definitions Working Group, 2001). Additionally, biological markers are important tools for molecular epidemiology and human biomonitoring studies (NRC, 2006). They have been used in exposure assessment (Metcalf and Orloff, 2004, Aprea et al.,2002, Anwar, 1997) and health risk prediction (Bonassi and Au, 2002). Biological markers of exposure to bioaerosols (e.g. allergens), air pollution, metals, pesticides etc. can provide specific evidence of exposures (Figure 1) and their relation to outcomes and, thus, aid in the study of how environmental exposures contribute to the development of adverse human health effects.
Figure 1.
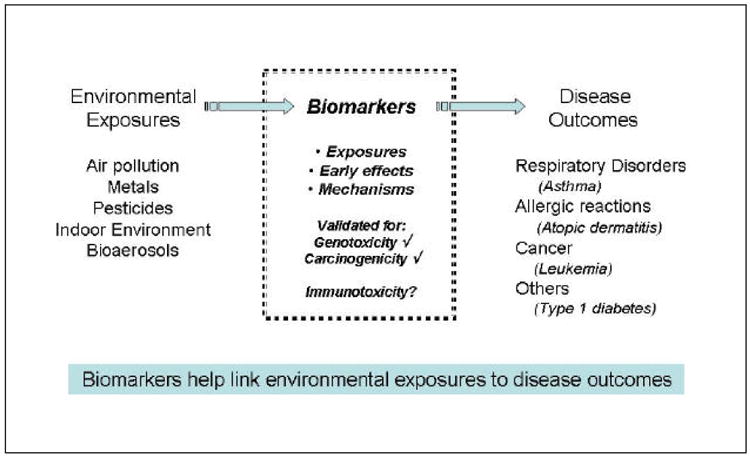
Role of biomarkers in children's environmental studies
As has been defined previously for all biomarkers (NIH Biomarkers Definitions Working Group, 2001), a useful immunological biomarker should have the following attributes: 1) clinical relevance (i.e. related to the disease or pathophysiological process of interest), 2) strong, mechanistic molecular or biochemical basis in the pathophysiology of a disease, 3) sensitivity and specificity with respect to treatment or exposure, 4) reliability, 5) practicality (level of assay invasiveness), and 6) simplicity in use and application (Metcalf and Orloff, 2004, Lesko and Atkinson, 2001). There continues to be a need for human cell-based methods that can be used to assess the immunotoxicity of xenobiotics in a simple, fast, economical and reliable way (Langezaal et al., 2002).
3. Markers of immune status and immunotoxicity
The endpoints most commonly used to study human immune function include cell counts, cell surface activation markers, immunoglobulin levels, responses to mitogen, and expression and secretion of cytokines (Table 1). The choice of immune test depends on the research question. Enumeration of immune cell subsets or a complete blood count (CBC) is used to obtain a general idea of the status of the immune response (i.e. elevated CBC indicates infection). To test for immunosuppression, lymphocytes are treated with mitogens and their proliferative ability is measured. Detection of specific cytokines reveals the state of immune response at that given time (e.g. elevated TNF-α levels reveal a state of inflammation). The applications of these markers in adult clinical and epidemiological studies have been reviewed previously (Vial et al., 1996, Voccia et al., 1999, Colosio et al., 1999, Vogt 1991). Therefore, only a brief summary of each endpoint will be provided with updated information on cytokine endpoints:
Table 1.
Parameters used to investigate changes in children's immune system
| Markers and Functional Tests | Parameters | Primary Method of Detection |
|---|---|---|
| Antibodies | IgM, IgD, IgG, IgA, IgE, | Plasma levels detected by ELISA |
| Cellular phenotype and activation markers | CD3, CD4, CD8, CD11c, CD19, CD25, CD56, CD14, basophils, neutrophils; Activation markers: CD69, CD45RO, CD45RA | Blood sample collected and cells that bind antigen specific flurochrome-labeled antibodies detected by flow cytometry |
| Proliferation Tests | Thymidine incorporation, mitogenic stimulation (PHA, Concavalin A) | Functional assays that require cultures of cells obtained from subject |
| Cytokines | IL-2, IL-4, IL-5, IL-10, IL-13, IFN-γ, TNF-α, GM -CSF | Serum or plasma levels, with or without activation, detected by ELISA; Flow cytometric detection of intracellular cytokine production |
| Chemokines | RANTES, IP-10, MIP-1a, MIP-1"β, MDC, TARC | Serum or plasma levels, with or without activation, detected by ELISA; PCR |
Cellular phenotype/cell counts
The immune response generally is divided into two categories: innate and adaptive immunity. Macrophages, dendritic, natural killer (NK) cells and the complement system are the main components of the innate branch, whereas B- and T-lymphocytes, which can mature into memory antigen-specific effector cells, are the main effectors of the adaptive branch. However, it now is known that considerable interaction occurs between the two branches (Steinman et al. 2006, Chan et al. 2006).Thus, as a broad and general indicator of the immune response, one of the initial methods is to determine whether the proportions of cells fall within the normal range; this can be done using fluorochrome-labeled antibodies that are specific for the cluster of differentiation (CD) surface antigen marker specific for each cell type. For example, in humans, B-cells are identified by the CD19 surface antigen marker, whereas T-cells are identified using CD3.
Antibodies
Antibodies, also called immunoglobulin (Ig), are glycoprotein molecules produced by B-lymphocytes. These include IgA, IgG1-4, IgD, IgM, and IgE. These antibodies perform various effector functions such as opsonization of antigens for phagocytosis by macrophages and neutrophils (IgG), activation of the classical complement pathway (IgM), mucosal immunity (IgA), and mast cell degranulation during immediate hypersensitivity reactions (IgE) (Abbas and Lichtman, 2005).
Proliferation Tests
This assay tests the ability of T-lymphocytes to proliferate in response to stimulation with their cognate antigen or a polyclonal stimulation such as phytohemaglutinin A (PHA) mitogen or anti-CD3 antibody. If T-cells fail to proliferate in response to these stimuli, or if the response is markedly reduced, this finding suggests that the immune response of the subject has been suppressed or compromised.
Cytokines
Cytokines are small molecular weight proteins secreted by immune cells that regulate the duration and intensity of the immune response (Table 2). For example, Type 1 cytokines (IFN -γ, IL-12) mediate the removal of malignant cells and cells infected with viruses, whereas Type 2 cytokines (IL-4, IL-5, IL-13) mediate the removal of soluble bacterial antigen. Secretion of Type 1 and Type 2 cytokines promote development of subsets of memory T-helper-1 (Th1) and T-helper-2 (Th2) cells, respectively (Trinchieri et al., 1996, O'Garra, 1998). Two functionally distinct T-helper lymphocyte subsets are differentiated by their signature cytokines: IFN-γ for Th1 lymphocytes and IL-4 for Th2 lymphocytes (Mosmann and Coffman, 1989). Elevated expression of IL-4, IL-5, and IL-13 from basophils, mast cells, and Th2-cells promotes the synthesis of IgE antibodies in B-cells (Yssel et al., 1998). ELISA is the most common method used to assay cytokines in biological samples, however several cytokine assays are now available; these include Luminex and LINCOPlex, a multi-plexed ELISA, RT-PCR, Taqman® real-time PCR, and immunohistochemistry (Whiteside, 2002, Pala et al., 2000, Jason and Larned, 1997).
Table 2.
Examples of cytokines involved in Th1 and Th2 development and function
| Name | Secreted by | Target cells | Primary activity |
|---|---|---|---|
| IL-1β | Macrophages
Epithelial cells |
T cells
Macrophages |
Activates macrophages and T cells; induces fever |
| IL-2 | Th1 | Th, Tc
NK |
Induces proliferation
Enhances activity |
| IL-4 | Th2
NK Mast cells |
B
Mast cells Macrophages |
Induces class switch to IgE and IgG1; up-regulates MHC II expression
Stimulates growth Up-regulates MHC II expression; increases phagocytic activity |
| IL-5 | Th2, Mast cells, basophils | B | Stimulates proliferation and activation of eosinophils; induces class switch to IgA |
| IL-6 | Th2
Monocytes Macrophages |
B
Hepatocytes |
Promotes terminal differentiation into plasma cells Induces synthesis of acute-phase proteins |
| IL-10 | Th2 | Macrophages
Regulatory T |
Anti-inflammatory; Inhibits synthesis of TNF-α, IL-1, IL-6, IL-2, IFN-γ, TNF-β (from Th1cells); down-regulates MHC II; Promotes development of Regulatory T cells (Tr1) |
| IL-12 | Macrophages
B-cells Dendritic cells |
Tc
Th1 NK |
Induces differentiation of naïve T cells to Th1 and Tc1 effector cells; Stimulates production of Th1 cytokines; inhibits Th2 cytokine production |
| IL-13 | Th2 | Macrophages
T cells |
Inhibits activation and release of inflammatory cytokine production; Inhibits development of Th1 cells; Promotes B cell grown and differentiation |
| IL-17 | Memory Th | T cells
Regulatory T |
Recent findings suggest that production of IL-17 by effector T cells (newly recognized subset called Th17 cells) plays a role in inflammation |
| IL-18 | Activated macrophages | T cells
NK |
Induces IFN-γ production by T cells and NK cells; Favors Th1 induction and later Th2 responses |
| IFN-γ | Th1
Tc NK |
Macrophages
Many cells |
Activates macrophages; Increases expression of MHCI and II; Inhibits viral replication; Suppresses Th2 development; Inhibits Th17 development |
| TNF-α | Macrophages
Mast cells |
Tumor cells
Inflammatory cells |
Induces expression of autocrine growth factors, nuclear proto-oncogenes, and induces signaling pathways that lead to proliferation, stimulates IL-6 |
| TGF-β | Monocytes; T cells | T cells | Anti-inflammatory; Induces IgA secretion; Inhibits cell growth; Promote development of Foxp3+ regulatory T-cells; Promotes development of Th17 effector cells |
The majority of epidemiological studies of pediatric immune response traditionally have used ELISA-based methods to detect cytokine levels in peripheral blood collected from subjects. More recently, analysis of cytokine production has been performed with intracellular staining followed by flow cytometry, a more informative and reliable approach since it ascertains cytokine production at the single cell level, and thus achieves higher specificity. Flow cytometric detection of intracellular cytokines is a functional assay that measures the ability of individual immune cells to express Type 1 and Type 2 cytokines after polyclonal stimulation with mitogens (Pala et al., 2000, Prussin, 1997, Jung et al., 1993). Efforts to develop non-invasive collection methods include analysis of cytokines in saliva (Winkler 2001) or induced sputum (Simpson 2005) and exhaled breath condensate (Robroeks, 2006). However, these methods still require further optimization and validation before they are ready to be used widely. Most importantly, the sensitivity of the assay requires improvement, perhaps at the collection stage, to stabilize cytokines in sputum or saliva, since several cytokines are detected at levels too low to differentiate between control and disease states.
In summary, there are many aspects to immune dysfunction and, subsequently, possibilities of markers and assays. The choice of immune marker depends on the research question as each of these assays (counts of lymphocyte subsets, mitogen assays, and Th1/Th2 cytokine profiles) provides different information. The counts and ratios of lymphocyte subsets serve as a general indicator of immune status. Mitogen tests and cytokine profiles reveal how these cells function. Although the assays complement one another, the intracellular cytokine assay can provide a more mechanistic examination of the effect of exposure because it identifies memory Th1 or Th2 cells that have been generated as a result of exposure to a specific antigen. In studies of diseases that involve a dysregulated immune response, the addition of a cytokine profile endpoint to the relatively few number of immune function assays currently available can help to evaluate whether the immune response is compromised or deviated from a normal response. Specific examples will be presented to illustrate how these immune markers have been used in the study of childhood diseases including allergic disorders, cancer, and others such as Type 1 diabetes (Table 3).
Table 3.
Applications of Th1/Th2 cytokine in studies of children's immune-mediated disorders
| Disorder | Subjects | Cytokines Assayed | Method Used | Biological Sample | Findings | Reference |
|---|---|---|---|---|---|---|
| ASTHMA | Birth cohort (n=172) | IFN-γ, TNF-a, IL-4, IL-5, IL-9, IL-10, IL-13 | ELISA | Peripheral Blood | Atopy is associated with increased Th2; Bronchial hyper-responsiveness is associated with increased Th1 | Heaton et al., 2005 |
|
| ||||||
| Children with asthma (n=33) vs. healthy control | IFN-γ, TNF-a, IL-2, IL-4, IL-5, IL-10 | FC* | Exhaled breath condensate | Cytokine levels detectable, but low and collection and processing method needs improvement | Robroeks et al., 2006 | |
|
| ||||||
| ALLERGIC RHINITIS | Children with allergic rhinitis (n=29) undergoing immunotherapy | IL-2, IL-4, IL-13, IFN-γ, IL-10, IL-5 | ICS/FC*
ELISA |
Peripheral Blood | Sublingual immunotherapy in children with rhinitis did not alter cytokine profiles. | Rolinck-Werninghaus et al. 2005 |
|
| ||||||
| ATOPIC DERMATITIS | Children with atopic dermatitis (n=9) vs. health control | IFN-γ, IL-4 | ICS/FC | Peripheral Blood | Compared to controls, atopic dermatitis had higher IL-4 Th2 cells | Kawamoto et al., 2006 |
|
| ||||||
| Children with atopic dermatitis vs. controls | IFN-γ, IL-4, IL-2, TNF-α | ICS/FC | Peripheral Blood | Children with AD had significantly lower IL-4 and TNF-α positive cells; no evidence of imbalance towards Th2 in children with AD | Machura et al., 2006 | |
|
| ||||||
| LEUKEMIA | Children diagnosed with B-ALL (n=50) vs. healthy control | IFN-γ
IL-4 |
ICS/FC | Peripheral Blood | ALL associated with increased Th2 | Luzynski et al, 2005 |
|
| ||||||
| Children diagnosed with B-ALL (n=45) vs. healthy control | IFN-γ
IL-4; IL-2 |
ICS/FC | Peripheral Blood | ALL associated with increased Th2 and decreased Th1 | Zhang et al., 2000 | |
|
| ||||||
| TYPE 1 DIABETES | Children diagnosed with Type 1 diabetes (n=10) | IFN-γ
IL-4 |
mRNA
ELISA |
Peripheral Blood | Th1 IFN-γ levels low at diagnosis but increased with disease progression | Karlsson Faresjo et al., 2004 |
|
| ||||||
| Newly diagnosed diabetes (n=22) vs. health controls | IFN-γ
IL-4 |
mRNA
ELISA |
Peripheral blood | Increased IFN-γ and IL-4 in mRNA in subjects with new diabetes but no difference in protein | Halminen et al., 2001 | |
ICS=Intracellular cytokine staining; FC = Flow cytometry
4. Th1/Th2 cytokines in the study of children's diseases
In a comprehensive study by Heaton et al., (2005), multiple immune markers (Table 3), were used to differentiate between various airway disease phenotypes in children. The authors reported that atopic children were more likely to have increased Th2 cytokines such as IL-4, IL-5, IL-13 whereas children with bronchial hyper-reactivity (one component of the asthma phenotype that also is observed in children without asthma) were more likely to have elevated IFN-γ, a Th1 cytokine (Heaton 2005). However, these associations of Th1/Th2 are not consistent for all allergic disorders suggesting that different mechanisms are responsible for various types of allergic disorders. For example, in atopic dermatitis (AD), one study reports that AD is associated with increased IL-4 Th2 cells (Kawamoto et al., 2006) whereas Machura et al. (2006) report that children with AD have significantly lower IL-4 Th2 cells and TNF-α Th1 cells and therefore no distinct bias towards Th1 or Th2 profiles.
In a study of potential immune mechanisms in the pathogenesis of pediatric leukemia, Zhang et al. (2000), applied the flow cytometric method to detect intracellular Th1/Th2 cytokine production and reported that children diagnosed with acute lymphocytic leukemia (ALL) had decreased numbers of Th1 lymphocytes that produce IL-2 and IFN-γ and increased numbers of Th2 cells that produce IL-4 (Table 3). Since the detection and removal of leukemic cells requires a pro-inflammatory immune response produced by T-helper, T-cytotoxic, natural killer, and macrophage cells, the reduced numbers of Th1 cells that produce pro-inflammatory IFN-γ potentially could contribute to the lack of inflammatory response to leukemic cells.
We used the same method of flow cytometry to evaluate Th1/Th2 cytokines in ALL cases versus acute myeloid leukemia (AML) (Figure 2). Children diagnosed with AML in this pilot study were more likely to have decreased Th1 but slightly increased Th2 levels (Figure 3). These observed differences in cytokine values were not statistically significant possibly because of the broad inter-individual variability and a relatively small sample size (n=32). A power calculation performed using this data indicated that approximately 100 samples would have to be evaluated to differentiate cytokine levels found in the AML and ALL groups reliably. Together, these studies demonstrate that Th1/Th2 biomarkers can be used to examine mechanisms of disease progression and differentiate between subtypes of a disorder.
Figure 2. Flow cytometric detection of intracellular Th1/Th2 cytokines in childhood leukemia.
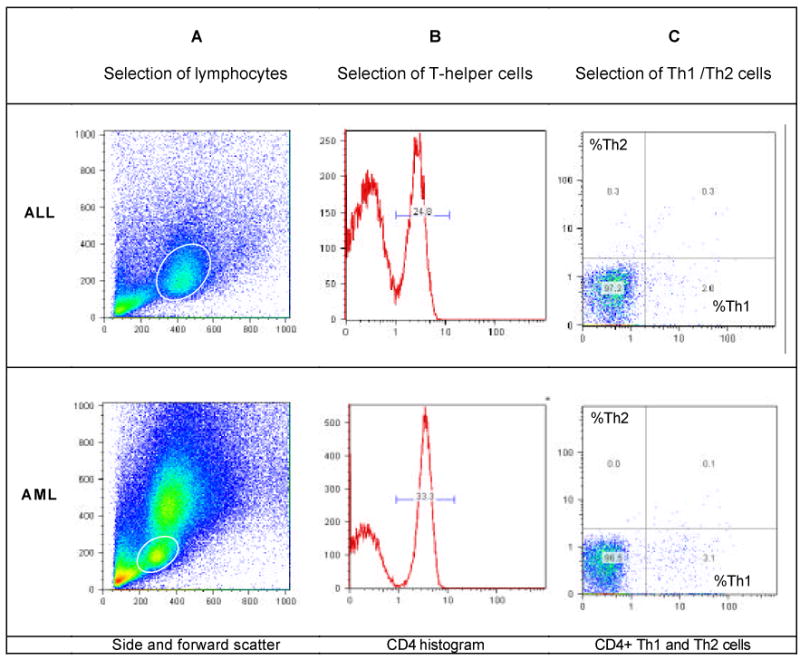
Th1/Th2 cytokines were detected by flow cytometry (Duramad et al., 2004) in samples from patients with acute lymphoid leukemia (ALL) and acute myeloid leukemia (AML). Whole blood stimulated with PMA/ionomycin was stained with cytokine-specific antibodies (IFN -γ-FITC for T-helper-1 cells and IL-4-PE for T-helper-2 cells). In the scatter plot of all cells (A), a circular gate was placed around the live lymphocyte population, based on size and granularity. The granulocyte population is more pronounced in the AML sample. Of total lymphocytes, only the CD4+ T-helper cells were selected (right peak, B). These T-helper cells were examined for Th1/Th2 cytokine production (C), with cells that stained positive for IFN-γ only (lower right quadrant) classified as Th1 cells and those that stained positive for IL-4 only (upper left quadrant) classified as Th2 cells.
Figure 3. Th1/Th2 cytokines in patients with childhood acute lymphoid leukemia (ALL) and acute myeloid leukemia (AML).
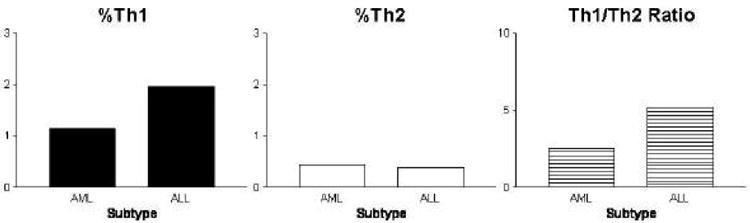
Intracellular Th1/Th2 cytokines were detected by flow cytometry (Duramad et al, 2004a) in a subset of pediatric peripheral blood samples (n=32, age 0-14 years) collected by the Northern California Childhood Leukemia Study (Buffler et al., 2005). Acute lymphoid leukemia (ALL, n=26) subjects had consistently higher %Th1 but slightly lower %Th2 compared to subjects diagnosed with acute myeloid leukemia (AML, n=6), however these differences were not statistically significant (Duramad et al. 2004b). Females (n=20) and males (n=12) had similar %Th1 (1.6 vs. 2.0; p=0.8), and %Th2 (0.3 vs. 0.6; p=0.2).
5. Sources of Th1/Th2 variability
Many factors can contribute to the variability of immune biomarkers including host factors (sex, age, ethnicity, nutrition) and exposure factors (chemicals, bioaerosols, season etc). These need to be addressed before the biomarker is used in large-scale epidemiological studies. When lymphocyte subsets were analyzed in 807 children who range in age from newborns to 18 years old, age was found to be an important factor in distributions of cell types. For example, percent CD4 was highest at 0-3 months of age (52%) but slowly declined over time whereas percent CD8 (18% at 0-3 months of age) increased over time (Shearer et al., 2003). With significant efforts undertaken by the researchers to control for inter- and intra-laboratory variability in their methods, the variance within age groups was substantial, even after the outliers (10th percentile) were discarded. Thus, variability can not be completely explained by methodological differences because genetic, environmental and socio-economic factors may play significant roles (Marti et al., 2002, NRC 2006).
Prior to analysis of the intracellular Th1/Th2 cytokines profiles in a birth cohort of Latino children in an agricultural community studied in the Children's Environmental Health Center at UC Berkeley, CHAMACOS (Eskenazi et al., 2003), we conducted a validation study to evaluate and optimize the precision of our method. We determined the normal healthy reference values for children and adult samples collected over a one-year period, calculated the assay coefficient of variance and found low intra-assay variability (Duramad et al., 2004). We found that age is an important variable to consider when measuring Th1/Th2 cytokine profiles in children. When we compared these data with the results from sub-cohort of these children at 60 month (Figure 4) a significant increase of Th1 and a slight decrease of Th2 was observed with age. This finding corroborates observations of an increase in Th1/Th2 ratio from birth till adulthood with a slight decline in the late years of life (Duramad et al., 2004, Kawamoto et al., 2006, McNerlan et al., 2002). More studies will be needed to establish a normal range of cytokines and other immune markers in children of different age, ethnic groups and life conditions, and epidemiologic analyses of biomarkers in relation to health should always include appropriate adjustments for such covariates. Significant inter-individual variability in healthy subjects may be related to a number of factors including environmental and life style exposures.
Figure 4. Changes in Th1 and Th2 with age.
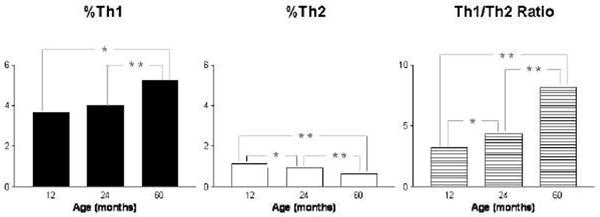
Intracellular Th1/Th2 cytokines were detected by flow cytometry (Duramad et al, 2004) in a subset of peripheral blood samples (N=39 for 12 months old, N=239 for 24 months old, and N=153 for 60 months old) from Latino children enrolled in CHAMACOS study (Eskenazi et al. 2003). A statistically significant increase in Th1 and Th1/Th2 ratio but decrease in Th2 was observed between ages 12 and 60 months. (* p<0.05 and ** p<0.001).
6. Environmental exposures and Th1/Th2
Vine et al., (2000) examined the relationship between exposure to environmental toxicants (e.g. organochlorine pesticides, volatile organic compounds, and metals) found in a Superfund site in Aberdeen, North Carolina, on DNA damage and immune function of inhabitants living near the site. Of the immune markers analyzed in the study population of over 200 subjects [these included cell counts, levels of immunoglobulins (Igs), skin prick test, and mitogen stimulation assays] only mitogen proliferation was signficantly lower in subjects who lived near the site. There was no significant difference in cell counts (6 analyzed) or Igs (4 analyzed). The authors acknowledged that these markers were relatively broad indicators of immune function and proposed more specific immune assays (cytokine analysis). However, they did not carry it out because of the cost constraints. Since then, numerous studies have used immune markers that include cytokine profiles, to evaluate the effect of environmental toxicants such as volatile organic compounds (VOCs), pesticides, polychlorinated biphenyls (PCBs), and metals on children's immunity (Table 4).
Table 4.
Cytokine and other immunological biomarkers used to evaluate potential immunotoxic effects of environmental exposures
| Environmental Toxicant | Subjects | Method of Exposure Assessment | Immune Parameters Measured | Findings | Reference |
|---|---|---|---|---|---|
| VOLATILE ORGANIC COMPOUNDS (VOCs) | Neonates (n=85) | Passive sampling of maternal indoor environment for VOCs | Intracellular IFN-γ and IL-4 cytokine analysis of T cells isolated from cord blood | Napthalene associated with increased IL-4 cytokine production; Trichloroethylene associated with decreased IFN-γ | Lehmann et al., 2002 |
| e.g. | |||||
| Benzene Napthalene Trichloroethylene | |||||
|
|
|||||
| Environmental Tobacco Smoke (ETS) | Atopic children Ages: 6-16 (n=24) vs. reference group | Questionnaire parental tobacco smoke | Nasopharangeal aspirate analyzed for IL-13 levels | ETS augments expression and secretion of IL-13, potentially increasing Th1 response | Feleszko et al., 2006 |
|
| |||||
| METALS | Children Ages: 6-10 years of age (n=90) | Arsenic levels detected in urine | Cell counts; Mitogen-induced proliferation; Cytokine production GM-CSF, IL-2, IL-4, IL-10, IFN-γ | Only GM -CSF levels were positively associated with elevated arsenic levels in children | Soto-Pena et al., 2006 |
| Arsenic | |||||
|
|
|||||
| Mercury | Newborns (n=47) vs. reference group | Methyl mercury measured in cord blood | Cord blood immune cell subsets and IL-10, TNF-α production | No difference in cell subsets; No association with cytokine secretion. | Bilrha et al., 2003 |
|
| |||||
| PESTICIDES | Children Age: 8 years (n=27) | Questionnaire; Detection of dioxin in mother's breast milk | Diagnosis of allergy; cell counts | Postnatal exposure associated with increased CD4 counts; negative association between exposure to dioxin and allergy. | ten Tusscher et al., 2003 |
| Polychlorinated Biphenyls (PCBs) | |||||
|
|
|||||
| Organophosphates (OPs) | Children Age: 1-2 years (n=239) | Questionnaire; Detection of OP metabolites in urine | Diagnosis of asthma, wheezing, Intracellular IFN-γ and IL-4 cytokine analysis | Increased IL-4 Th2 cells associated with asthma and wheeze; IFN-γ Th1 associated breastfeeding and parental occupation in agriculture | Duramad et al.,2006 |
|
|
|||||
| Organochlorines PCBs | Neonates (n=47) vs. reference group | Organochlorine (12 PCB cogeners) measured in cord blood | Cord blood immune cell subsets and IL-10, TNF-α production | Decreased TNFα in exposed group associated with DDE, PCB, and hexachlorobenzene exposures | Bilrha et al., 2003 |
| Hexachlorobenzene | |||||
|
|
|||||
| Propanil | Children (n=52) vs. reference group | Exposed vs. non-exposed determined by distance from fields sprayed with propanil | Cell counts, proliferation, IL-2 cytokine production | No significant difference between exposed and unexposed | McClure et al., 2001 |
In a study of the effects of VOCs on newborn immune development (n=85), VOC exposure was ascertained with passive sampling of the maternal indoor environment and immune function was measured by detection of intracellular IFN-γ and IL-4 cytokine production (Lehmann et al., 2002). The authors report that exposure to naphthalene was associated with increased IL-4 cytokine production and decreased IFN -γ production was associated with trichloroethylene In other studies, no association was found between children's exposures to mercury (Bilhra et al., 2003) and IL-10 and TNF-a levels, however increased levels of GM-CSF was associated with children's exposure to arsenic (Soto-Pena et al., 2006). In a large longitudinal birth cohort study (N=540) from Czech Republic (Sram, 2001), lymphocyte immunophenotyping was conducted in two groups of mothers and their newborns that lived either in clean air area (Prachatice) or in the area polluted from power plants and coal home heating (Teplice) (Hertz-Picciotto et al., 2006). A significantly lower percent of T lymphocytes and the %CD4 of the total T-cells were observed in both maternal and cord blood in polluted area with a more pronounced decline during winter months. Additionally, after three years of follow up, children born in Teplice experienced a significantly higher rate of otitis media, respiratory and gastrointestinal infections, and pneumonia that indicates air pollution affected their susceptibility. Karmaus et al. (2005) reported immune function biomarkers (cell counts and antibody production) in children exposed to lead and organochlorine compounds in a cross-sectional study. Their findings indicate that lead is associated with increased IgE production, corroborating a prior report by Lutz et al. (1999). However, cytokines profiles were not evaluated. Since IgE production is a direct result of IL-4 (and IL-13) production, it would be important to examine cytokine levels in children exposed to lead in future studies. In CHAMACOS, a longitudinal birth cohort in Salinas Valley, California (Eskenazi et al, 2003), the relations between early childhood exposures to an agricultural environment and Th1 and Th2 cytokines were investigated in 24 month old children (n=239) (Duramad et al, 2006). Exclusive breastfeeding at one month and pet ownership were associated with increased Th1 whereas increased Th2 was associated with maternal agricultural work and the presence of gas stove in the home. Bilhra et al., (2003) found that newborns exposed to organochlorines in utero had significantly decreased production of TNF-a, an important proinflammatory cytokine, suggesting that these children may be more susceptible to infections. These data indicate further that early exposures to environmental toxicants can directly impact the development and function of children's immunity.
7. Conclusions and Future Directions
In the last several years the field of immunotoxicology has advanced in the following areas: increased recognition of the susceptibility of children's immune systems to environmental exposures (Garry, 2004, Holsapple et al., 2004), extensive validation of the whole blood Th1/Th2 lymphocyte assay for epidemiological use (Hanekom et al., 2004, Godoy-Ramirez et al., 2004, Hoover et al., 2003, Langezaal et al., 2002, Bergeron et al., 2002) Duramad et al., 2004); proposal of immunotoxicity testing tiers (Holsapple et al., 2005), and the use of cytokine markers/assays to identify chemical allergens (Dearman et al., 2003a, Kimber et al., 2003).
Limitations exist for all biomarkers currently in use (NRC, 2006) and this is also the case for cytokines and other immunological biomarkers. Given the mechanistic relevance of cytokines in the altered immune response of children's disorders, this warrants additional validation studies for existing assays, as well as development of the novel methods for application in the rapidly developing area of molecular epidemiology of children's environmental health. More work is needed to acquire the distribution data for different age and ethnic groups, especially for novel assays. Further, the interpretation of the role of immune biomarkers can be strengthened in well characterized cohort studies that effectively explore a whole biomarker continuum (e.g. markers of exposure, effect and susceptibility). In summary, incorporation of immune biomarkers is useful for all children's studies focused on the effects of environmental exposures and diseases with significant environmental component, and could facilitate understanding of the mechanisms that underlie the associations between environmental exposures and immune-mediated disorders.
Acknowledgments
This research was supported by the following grants: 2P01ES009605, R01ES09137, PS42ES04705 and 5RO1ES12503 from NIH and R82670901 from EPA. Contribution to data analysis by Drs. M.Kwan and K.Harley, and helpful discussions with Drs. P.Buffler, B.Eskenazi, S.Selvin and M.Lipsett are sincerely appreciated. This paper's contents are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS or EPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas AK, Lichtman AH. Cellular and molecular immunology. Philadelphia, PA: Saunders Elsevier; 2005. [Google Scholar]
- Anwar WA. Biomarkers of human exposure to pesticides. Environ Health Perspect. 1997;105:801–806. doi: 10.1289/ehp.97105s4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprea C, Colosio C, Mammone T, Minoia C, Maroni M. Biological monitoring of pesticide exposure: a review of analytical methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;769:191–219. doi: 10.1016/s1570-0232(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M, Nicholson JK, Phaneuf S, Ding T, Soucy N, Badley AD, Hawley Foss NC, Mandy F. Selection of lymphocyte gating protocol has an impact on the level of reliability of T-cell subsets in aging specimens. Cytometry. 2002;50:53–61. doi: 10.1002/cyto.10092. [DOI] [PubMed] [Google Scholar]
- Bilrha H, Roy R, Moreau B, Belles-Isles M, Dewailly E, Ayotte P. In vitro activation of cord blood mononuclear cells and cytokine production in a remote coastal population exposed to organochlorines and methyl mercury. Environ Health Perspect. 2003;111:1952–1957. doi: 10.1289/ehp.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–346. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Au WW. Biomarkers in molecular epidemiology studies for health risk prediction. Mutat Res. 2002;511:73–86. doi: 10.1016/s1383-5742(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Buffler PA, Kwan ML, Reynolds P, Urayama KY. Environmental and genetic risk factors for childhood leukemia: appraising the evidence. Cancer Invest. 2005;23:60–75. [PubMed] [Google Scholar]
- Burchiel SW, Lauer FT, Gurule D, Mounho BJ, Salas VM. Uses and future applications of flow cytometry in immunotoxicity testing. Methods. 1999;19:28–35. doi: 10.1006/meth.1999.0824. [DOI] [PubMed] [Google Scholar]
- Burchiel SW. The effects of environmental and other chemicals on the human immune system: the emergence of immunotoxicology. Clin Immunol. 1999;90:285–286. doi: 10.1006/clim.1998.4692. [DOI] [PubMed] [Google Scholar]
- Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- Colosio C, Corsini E, Barcellini W, Maroni M. Immune parameters in biological monitoring of pesticide exposure: current knowledge and perspectives. Toxicol Lett. 1999;108:285–295. doi: 10.1016/s0378-4274(99)00100-9. [DOI] [PubMed] [Google Scholar]
- Dearman RJ, Smith S, Basketter DA, Kimber I. Classification of chemical allergens according to cytokine secretion profiles of murine lymph node cells biomarker. J Appl Toxicol. 1997;17:53–62. doi: 10.1002/(sici)1099-1263(199701)17:1<53::aid-jat393>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Dearman RJ, Betts CJ, Humphreys N, Flanagan BF, Gilmour NJ, Basketter DA, Kimber I. Chemical allergy: considerations for the practical application of cytokine profiling. Toxicol Sci. 2003;71:137–145. doi: 10.1093/toxsci/71.2.137. [DOI] [PubMed] [Google Scholar]
- Descotes J, Nicolas B, Vial T. Assessment of immunotoxic effects in humans. Clin Chem. 1995;41:1870–1873. [PubMed] [Google Scholar]
- Duramad P, McMahon CW, Hubbard A, Eskenazi B, Holland NT. Flow cytometric detection of intracellular TH1/TH2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2004a;13:1452–1458. [PubMed] [Google Scholar]
- Duramad P, Kwan M, Swearingen S, Buffler PA, Holland NT. Flow cytometric detection of Th1/Th2 Lymphocytes in Whole Blood Obtained from Children with Leukemia. Proceedings of the 95th Annual AACR meeting; 2004.2004b. p. 1084. #4694. [Google Scholar]
- Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, Tager IB. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–1922. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency, the United States (EPA) Children's Environmental Health. 2006. Report. [Google Scholar]
- Eskenazi B, Bradman A, Gladstone E, Jaramillo S, Birch K, Holland N. CHAMACOS, A Longitudinal Birth Cohort Study: Lessons from the Fields. J of Children's Health. 2003;1:3–27. [Google Scholar]
- Feleszko W, Zawadzka -Krajewska A, Matysiak K, Lewandowska D, Peradzynska J, Dinh QT, Hamelmann E, Groneberg DA, Kulus M. Parental tobacco smoking is associated with augmented IL-13 secretion in children with allergic asthma. J Allergy Clin Immunol. 2006;117:97–102. doi: 10.1016/j.jaci.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Garry VF. Pesticides and children. Toxicol Appl Pharmacol. 2004;198:152–163. doi: 10.1016/j.taap.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Godoy-Ramirez K, Franck K, Mahdavifar S, Andersson L, Gaines H. Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry. J Immunol Methods. 2004;292:1–15. doi: 10.1016/j.jim.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Halminen M, Juhela S, Vaarala O, Simell O, Ilonen J. Induction of interferon-gamma and IL-4 production by mitogen and specific antigens in peripheral blood lymphocytes of Type 1 diabetes patients. Autoimmunity. 2001;34:1–8. doi: 10.3109/08916930108994120. [DOI] [PubMed] [Google Scholar]
- Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, Murray RA, Hawkridge A, Haslett PA, Ress S, Hussey GD, Kaplan G. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods. 2004;291:185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, Serralha M, Holt BJ, Hollams E, Yerkovich S, Holt K, Sly PD, Goldblatt J, Le Souef P, Holt PG. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–149. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- Holland NT, Smith MT, Eskenazi B, Bastaki M. Biological sample collection and processing for molecular epidemiological studies. Mutat Res. 2003;543:217–234. doi: 10.1016/s1383-5742(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Paustenbach DJ, Charnley G, West LJ, Luster MI, Dietert RR, Burns-Naas LA. Symposium summary: children's health risk--what's so special about the developing immune system? Toxicol Appl Pharmacol. 2004;199:61–70. doi: 10.1016/j.taap.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Burns-Naas LA, Hastings KL, Ladics GS, Lavin AL, Makris SL, Yang Y, Luster MI. A proposed testing framework for developmental immunotoxicology (DIT) Toxicol Sci. 2005;83:18–24. doi: 10.1093/toxsci/kfh299. [DOI] [PubMed] [Google Scholar]
- Holt PG. Programming for responsiveness to environmental antigens that trigger allergic respiratory disease in adulthood is initiated during the perinatal period. Environ Health Perspect. 1998;106 3:795–800. doi: 10.1289/ehp.98106795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DR, Donnay A, Mitchell CS, Ziem G, Rose NR, Sabath DE, Yurkow EJ, Nakamura R, Vogt RF, Waxdal M, Margolick JB. Reproducibility of Immunological Tests Used To Assess Multiple Chemical Sensitivity Syndrome. Clin Diagn Lab Immunol. 2003;10:1029–1036. doi: 10.1128/CDLI.10.6.1029-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason J, Larned J. Single -cell cytokine profiles in normal humans: comparison of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997;207:13–22. doi: 10.1016/s0022-1759(97)00079-3. [DOI] [PubMed] [Google Scholar]
- Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- Karlsson Faresjo MG, Ernerudh J, Ludvigsson J. Cytokine profile in children during the first 3 months after the diagnosis of type 1 diabetes. Scand J Immunol. 2004;59:517–526. doi: 10.1111/j.0300-9475.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Brooks KR, Nebe T, Witten J, Obi-Osius N, Kruse H. Immune function biomarkers in children exposed to lead and organochlorine compounds: a cross-sectional study. Environ Health. 2005;4:5. doi: 10.1186/1476-069X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto N, Kaneko H, Takemura M, Seishima M, Sakurai S, Fukao T, Kasahara K, Iwasa S, Kondo N. Age -related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol. 2006;17:125–133. doi: 10.1111/j.1399-3038.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Kimber I, Dearman RJ. What makes a chemical an allergen? Ann Allergy Asthma Immunol. 2003;90:28–31. doi: 10.1016/s1081-1206(10)61645-6. [DOI] [PubMed] [Google Scholar]
- Koscher V, Milhe F, El Biaze M, Vervloet D, Magnan A. Variation of T-cell activation in allergic subjects during natural pollen exposure. Allergy. 2006;61(1):35–42. doi: 10.1111/j.1398-9995.2005.00922.x. [DOI] [PubMed] [Google Scholar]
- Kovarik J, Siegrist CA. Immunity in early life. Immunol Today. 1998;19:150–152. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langezaal I, Hoffmann S, Hartung T, Coecke S. Evaluation and Prevalidation of an Immunotoxicity Test Based on Human Whole -blood Cytokine Release. Altern Lab Anim. 2002;30:581–595. doi: 10.1177/026119290203000605. [DOI] [PubMed] [Google Scholar]
- Lehmann I, Thoelke A, Rehwagen M, Rolle -Kampczyk U, Schlink U, Schulz R, Borte M, Diez U, Herbarth O. The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environ Toxicol. 2002;17:203–210. doi: 10.1002/tox.10055. [DOI] [PubMed] [Google Scholar]
- Lesko LJ, Atkinson AJ., Jr Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol Toxicol. 2001;41:347–366. doi: 10.1146/annurev.pharmtox.41.1.347. [DOI] [PubMed] [Google Scholar]
- Luczynski W, Stasiak-Barmuta A, Krawczuk-Rybak M, Malinowska I. Assessment of selected co-stimulatory, adhesion and activatory molecules and cytokines of Th(1)/Th(2) balance in acute lymphoblastic leukemia in children. Arch Immunol Ther Exp (Warsz) 2005;53:357–363. [PubMed] [Google Scholar]
- Luster MI, Johnson VJ, Yucesoy B, Simeonova PP. Biomarkers to assess potential developmental immunotoxicity in children. Toxicol Appl Pharmacol. 2005;206:229–236. doi: 10.1016/j.taap.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Lutz PM, Wilson TJ, Ireland J, Jones AL, Gorman JS, Gale NL, Johnson JC, Hewett JE. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology. 1999;134:63–78. doi: 10.1016/s0300-483x(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, Reynolds P. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect. 2002;110:955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti GE, Zenger VE, Vogt R, Gaigalas A. Quantitative flow cytometry: history, practice, theory, consensus, inter-laboratory variation and present status. Cytotherapy. 2002;4:97–98. doi: 10.1080/146532402317251626. [DOI] [PubMed] [Google Scholar]
- Machura E, Mazur B, Kwiecien J, Karczewska K. Intracellular production of IL-2, IL-4, IFN-gamma, and TNF-alpha by peripheral blood CD3(+) and CD4 (+) T cells in children with atopic dermatitis. Eur J Pediatr. 2006 doi: 10.1007/s00431-006-0319-5. [DOI] [PubMed] [Google Scholar]
- McClure GY, Helm RM, Stine K, Burks AW, Jones SM, Gandy J. Evaluation of immune parameters in propanil-exposed farm families. Arch Environ Contam Toxicol. 2001;41:104–111. doi: 10.1007/s002440010226. [DOI] [PubMed] [Google Scholar]
- McNerlan SE, Alexander HD, Rea IM. Age -related reference intervals for lymphocyte subsets in whole blood of healthy individuals. Scand J Clin Lab Invest. 1999;59:89–92. doi: 10.1080/00365519950185805. [DOI] [PubMed] [Google Scholar]
- McNerlan SE, Rea IM, Alexander HD. A whole blood method for measurement of intracellular TNF-alpha, IFN- gamma and IL-2 expression in stimulated CD3+ lymphocytes: differences between young and elderly subjects. Exp Gerontol. 2002;37:227–234. doi: 10.1016/s0531-5565(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Metcalf SW, Orloff KG. Biomarkers of exposure in community settings. J Toxicol Environ Health A. 2004;67:715–726. doi: 10.1080/15287390490428198. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Myrianthefs P, Karatzas S, Venetsanou K, Grouzi E, Evagelopoulou P, Boutzouka E, Fildissis G, Spiliotopoulou I, Baltopoulos G. Seasonal variation in whole blood cytokine production after LPS stimulation in normal individuals. Cytokine. 2003;24(6):286–92. doi: 10.1016/j.cyto.2003.08.005. [DOI] [PubMed] [Google Scholar]
- National Institute of Health (NIH) Biomarkers Definitions Working Group, 2001.
- National Research Council (NRC) Human Biomonitoring for Environmental Chemicals. The National Academy Press; Washington, DC: 2006. pp. 1–213. report. [Google Scholar]
- Neri M, Bonassi S, Knudsen LE, Sram RJ, Holland N, Ugolini D, Merlo DF. Children's exposure to environmental pollutants and biomarkers of genetic damage. I. Overview and critical issues. Mutat Res. 2006;612:1–13. doi: 10.1016/j.mrrev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- Pala P, Hussell T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–124. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- Perez F, Oyarzun A, Carrasco E, Angel B, Albala C, Santos JL. Plasma levels of interleukin-1beta, interleukin-2 and interleukin-4 in recently diagnosed type 1 diabetic children and their association with beta-pancreatic autoantibodies. Rev Med Chil. 2004;132:413–420. doi: 10.4067/s0034-98872004000400002. [DOI] [PubMed] [Google Scholar]
- Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single - cell level. J Clin Immunol. 1997;17:195–204. doi: 10.1023/a:1027350226435. [DOI] [PubMed] [Google Scholar]
- Robroeks CM, Jobsis Q, Damoiseaux JG, Heijmans PH, Rosias PP, Hendriks HJ, Dompeling E. Cytokines in exhaled breath condensate of children with asthma and cystic fibrosis. Ann Allergy Asthma Immunol. 2006;96:349–355. doi: 10.1016/S1081-1206(10)61247-1. [DOI] [PubMed] [Google Scholar]
- Rolinck-Werninghaus C, Kopp M, Liebke C, Lange J, Wahn U, Niggemann B. Lack of detectable alterations in immune responses during sublingual immunotherapy in children with seasonal allergic rhinoconjunctivitis to grass pollen. Int Arch Allergy Immunol. 2005;136:134–141. doi: 10.1159/000083320. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Wood LG, Gibson PG. Inflammatory mediators in exhaled breath, induced sputum and saliva. Clin Exp Allergy. 2005;35:1180–1185. doi: 10.1111/j.1365-2222.2005.02327.x. [DOI] [PubMed] [Google Scholar]
- Soto-Pena GA, Luna AL, Acosta-Saavedra L, Conde P, Lopez-Carrillo L, Cebrian ME, Bastida M, Calderon-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. Faseb J. 2006;20:779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Sram R, editor. Teplice Program: Impact of Air pollution on Human health. Academia; Prague: 2001. pp. 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- ten Tusscher GW, Steerenberg PA, van Loveren H, Vos JG, von dem Borne AE, Westra M, van der Slikke JW, Olie K, Pluim HJ, Koppe JG. Persistent hematologic and immunologic disturbances in 8-year-old Dutch children associated with perinatal dioxin exposure. Environ Health Perspect. 2003;111:1519–1523. doi: 10.1289/ehp.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G, Peritt D, Gerosa F. Acute induction and priming for cytokine production in lymphocytes. Cytokine Growth Factor Rev. 1996;7:123–132. doi: 10.1016/1359-6101(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Van Loveren H, Steerenberg PA, Vos JG. Early detection of immunotoxicity: from animal studies to human biomonitoring. Toxicol Lett. 1995;77:73–80. doi: 10.1016/s0378-4274(97)84688-7. [DOI] [PubMed] [Google Scholar]
- Vial T, Nicolas B, Descotes J. Clinical immunotoxicity of pesticides. J Toxicol Environ Health. 1996;48:215–229. doi: 10.1080/009841096161294. [DOI] [PubMed] [Google Scholar]
- Vine MF, Stein L, Weigle K, Schroeder J, Degnan D, Tse CK, Hanchette C, Backer L. Effects on the immune system associated with living near a pesticide dump site. Environ Health Perspect. 2000;108:1113–1124. doi: 10.1289/ehp.001081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voccia I, Blakley B, Brousseau P, Fournier M. Immunotoxicity of pesticides: a review. Toxicol Ind Health. 1999;15:119–132. doi: 10.1177/074823379901500110. [DOI] [PubMed] [Google Scholar]
- Vogt RF., Jr Use of laboratory tests for immune biomarkers in environmental health studies concerned with exposure to indoor air pollutants. Environ Health Perspect. 1991;95:85–91. doi: 10.1289/ehp.919585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos JG, van Loveren H. Markers for immunotoxic effects in rodents and man. Toxicol Lett. 1995;82-83:385–394. doi: 10.1016/0378-4274(95)03489-7. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Cytokine assays. Biotechniques. 2002;(Suppl):4–8. 10, 12–15. [PubMed] [Google Scholar]
- World Health Organization (WHO) Report, 2006.
- Winkler O, Hadnagy W, Idel H. Cytokines detectable in saliva of children as appropriate markers of local immunity of the oral cavity--an approach for the use in air pollution studies. Int J Hyg Environ Health. 2001;204:181–184. doi: 10.1078/1438-4639-00092. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Komada Y, Chipeta J, Li QS, Inaba H, Azuma E, Yamamoto H, Sakurai M. Intracellular cytokine profile of T cells from children with acute lymphoblastic leukemia. Cancer Immunol Immunother. 2000;49:165–172. doi: 10.1007/s002620050616. [DOI] [PMC free article] [PubMed] [Google Scholar]


