Abstract
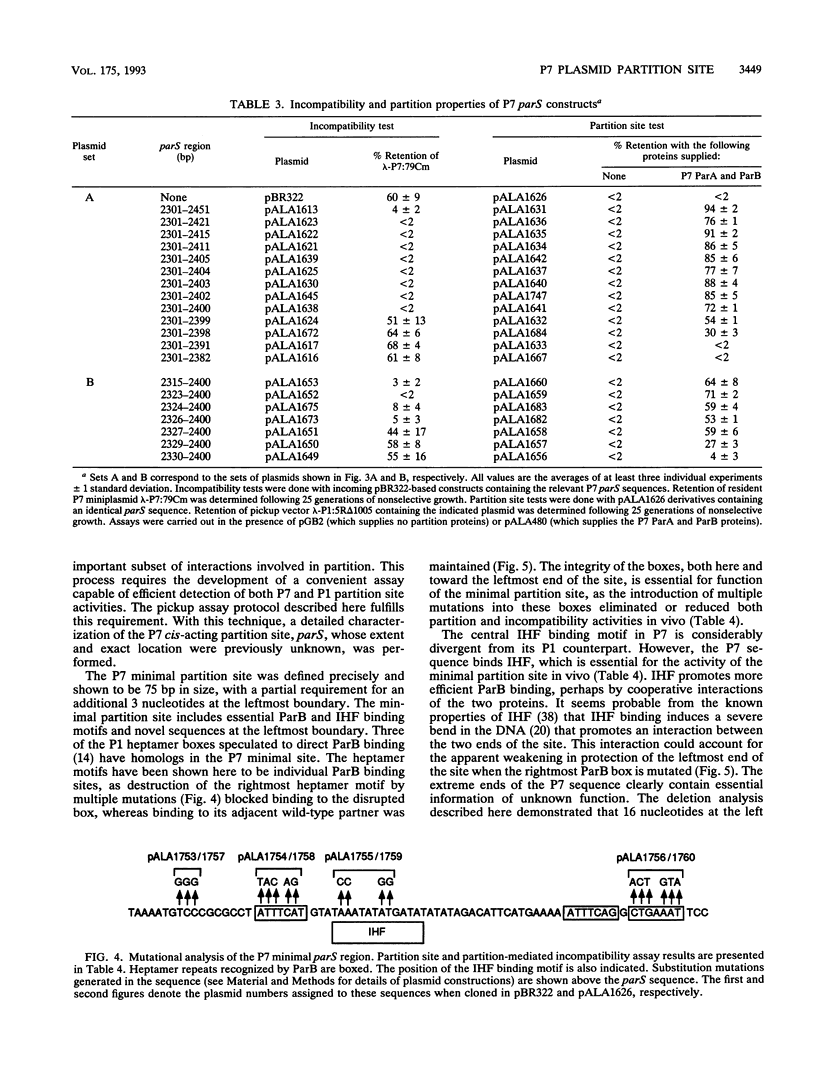
The par region of bacteriophage P7 is responsible for active partition of the P7 plasmid prophage into daughter cells. The cis-acting partition site was defined precisely as a 75-bp sequence that was necessary and sufficient to promote correct segregation of an unstable vector plasmid when the two P7 partition proteins, ParA and ParB, were supplied in trans. Roughly the same region was necessary to exert partition-mediated incompatibility. The minimal site contains an integration host factor (IHF) protein binding site bracketed by regions containing heptamer repeat sequences that individually bind ParB. An additional sequence forms the left boundary of the site. Site-directed mutations in the latter sequence, as well as the IHF motif and the rightmost ParB box, blocked site function. Although the P7 site shares 55% sequence identity with its counterpart in bacteriophage P1, functional interactions between the partition sites and the Par proteins of the two plasmids were entirely species specific in vivo. The P1 sequence has similar IHF and ParB binding motifs, but the left boundary sequence differs radically and may define a point of species-specific contact with the Par proteins. No evidence was found for the existence of a functional P7 analog of the P1 parS core, a small subregion of the P1 site that, in isolation, acts as an enfeebled partition site with modified incompatibility properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Friedman S. A., Austin S. J. Partition of unit-copy miniplasmids to daughter cells. III. The DNA sequence and functional organization of the P1 partition region. J Mol Biol. 1985 Sep 20;185(2):261–272. doi: 10.1016/0022-2836(85)90402-4. [DOI] [PubMed] [Google Scholar]
- Austin S. J., Eichorn B. G. Random diffusion can account for topA-dependent suppression of partition defects in low-copy-number plasmids. J Bacteriol. 1992 Aug;174(16):5190–5195. doi: 10.1128/jb.174.16.5190-5195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Abeles A. Partition of unit-copy miniplasmids to daughter cells. I. P1 and F miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J Mol Biol. 1983 Sep 15;169(2):353–372. doi: 10.1016/s0022-2836(83)80055-2. [DOI] [PubMed] [Google Scholar]
- Austin S., Abeles A. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J Mol Biol. 1983 Sep 15;169(2):373–387. doi: 10.1016/s0022-2836(83)80056-4. [DOI] [PubMed] [Google Scholar]
- Austin S., Hart F., Abeles A., Sternberg N. Genetic and physical map of a P1 miniplasmid. J Bacteriol. 1982 Oct;152(1):63–71. doi: 10.1128/jb.152.1.63-71.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Ziese M., Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981 Sep;25(3):729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G., Belin D., Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984 Nov;31(1-3):165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A., Austin S. J. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 1988 Jun;7(6):1881–1888. doi: 10.1002/j.1460-2075.1988.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. A., Martin K. A., Austin S. J. Biochemical activities of the parA partition protein of the P1 plasmid. Mol Microbiol. 1992 May;6(9):1141–1147. doi: 10.1111/j.1365-2958.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Martin K. A., Austin S. J. Specificity switching of the P1 plasmid centromere-like site. EMBO J. 1990 Apr;9(4):991–998. doi: 10.1002/j.1460-2075.1990.tb08201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennert G., Henning U. Tyrosine-incorporating amber suppressors in Escherichia coli K12. J Mol Biol. 1968 Apr 14;33(1):327–329. doi: 10.1016/0022-2836(68)90300-8. [DOI] [PubMed] [Google Scholar]
- Friedman S. A., Austin S. J. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid. 1988 Mar;19(2):103–112. doi: 10.1016/0147-619x(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Funnell B. E. Participation of Escherichia coli integration host factor in the P1 plasmid partition system. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6657–6661. doi: 10.1073/pnas.85.18.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B. E. The P1 plasmid partition complex at parS. The influence of Escherichia coli integration host factor and of substrate topology. J Biol Chem. 1991 Aug 5;266(22):14328–14337. [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Chromosome and plasmid partition in Escherichia coli. Annu Rev Biochem. 1992;61:283–306. doi: 10.1146/annurev.bi.61.070192.001435. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A. PCR-generated probes for the study of DNA-protein interactions. Biotechniques. 1992 Jun;12(6):830–832. [PubMed] [Google Scholar]
- Ludtke D. N., Austin S. J. The plasmid-maintenance functions of the P7 prophage. Plasmid. 1987 Jul;18(1):93–98. doi: 10.1016/0147-619x(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Ludtke D. N., Eichorn B. G., Austin S. J. Plasmid-partition functions of the P7 prophage. J Mol Biol. 1989 Oct 5;209(3):393–406. doi: 10.1016/0022-2836(89)90005-3. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Davis M. A., Austin S. Fine-structure analysis of the P1 plasmid partition site. J Bacteriol. 1991 Jun;173(12):3630–3634. doi: 10.1128/jb.173.12.3630-3634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. A., Friedman S. A., Austin S. J. Partition site of the P1 plasmid. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8544–8547. doi: 10.1073/pnas.84.23.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motallebi-Veshareh M., Rouch D. A., Thomas C. M. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol. 1990 Sep;4(9):1455–1463. doi: 10.1111/j.1365-2958.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- Nordström K., Austin S. J. Mechanisms that contribute to the stable segregation of plasmids. Annu Rev Genet. 1989;23:37–69. doi: 10.1146/annurev.ge.23.120189.000345. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Hoppensteadt F. C. On plasmid incompatibility. Plasmid. 1978 Sep;1(4):421–434. doi: 10.1016/0147-619x(78)90001-x. [DOI] [PubMed] [Google Scholar]
- Pal S. K., Mason R. J., Chattoraj D. K. P1 plasmid replication. Role of initiator titration in copy number control. J Mol Biol. 1986 Nov 20;192(2):275–285. doi: 10.1016/0022-2836(86)90364-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesman J., Rizzino A. A rapid and reliable method for the purification of high-quality plasmid DNA for double-stranded sequencing. Biotechniques. 1991 Mar;10(3):319, 326-8. [PubMed] [Google Scholar]
- Williams D. R., Thomas C. M. Active partitioning of bacterial plasmids. J Gen Microbiol. 1992 Jan;138(1):1–16. doi: 10.1099/00221287-138-1-1. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Nash H. A. The interaction of E. coli IHF protein with its specific binding sites. Cell. 1989 Jun 2;57(5):869–880. doi: 10.1016/0092-8674(89)90801-5. [DOI] [PubMed] [Google Scholar]