Abstract
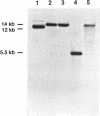
The products of the bvgAS locus activate expression of a majority of the known Bordetella virulence factors but also exert negative control over a class of genes called vrg genes (bvg-repressed genes). BvgAS negatively controls the production of flagella and the phenotype of motility in Bordetella bronchiseptica. In this study flaA, the flagellin gene, was cloned and characterized to facilitate studies of this negative control pathway. An internal flaA probe detected hybridizing sequences on genomic Southern blots of Bordetella pertussis, Bordetella parapertussis, and Bordetella avium, although B. pertussis and B. parapertussis are nonmotile. FlaA is similar to the FliC flagellins of Salmonella typhimurium and Escherichia coli, and flaA complemented an E. coli flagellin mutant. Insertional inactivation of the chromosomal flaA locus eliminated motility, which was restored by complementation with the wild-type locus. Analysis of flaA mRNA production by Northern (RNA) blotting and primer extension indicated that negative regulation by BvgAS occurs at the level of transcription. The transcriptional start site of flaA mapped near a consensus site for the alternative sigma factor, sigma F, encoded by fliA in E. coli and S. typhimurium. Consistent with a role for a fliA analog in B. bronchiseptica, transcriptional activation of a flaA-lacZ fusion in E. coli required fliA and a flaA-linked locus designated frl.frl also efficiently complemented mutations in the flagellar master regulatory locus, flhDC, of E. coli. Our analysis of the motility phenotype of B. bronchiseptica suggests that the Bordetella virulence control system mediates transcriptional control of flaA through a regulatory hierarchy that includes the frl locus and an alternative sigma factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerley B. J., Monack D. M., Falkow S., Miller J. F. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol. 1992 Feb;174(3):980–990. doi: 10.1128/jb.174.3.980-990.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricò B., Gross R., Smida J., Rappuoli R. Evolutionary relationships in the genus Bordetella. Mol Microbiol. 1987 Nov;1(3):301–308. doi: 10.1111/j.1365-2958.1987.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Aricò B., Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987 Jun;169(6):2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N., Chamberlin M. J. Secondary sigma factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):830–834. doi: 10.1073/pnas.86.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti D. N. Regulation of Escherichia coli sigma F RNA polymerase by flhD and flhC flagellar regulatory genes. J Bacteriol. 1990 Jul;172(7):4106–4108. doi: 10.1128/jb.172.7.4106-4108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D. H., Frantz B. B., Matsumura P. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5' consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988 Apr;170(4):1575–1581. doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. T., Knapp S., Mekalanos J. J. Evidence that modulation requires sequences downstream of the promoters of two vir-repressed genes of Bordetella pertussis. J Bacteriol. 1990 Dec;172(12):6997–7004. doi: 10.1128/jb.172.12.6997-7004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. T., Mahan M. J., Mekalanos J. J. Repressor binding to a regulatory site in the DNA coding sequence is sufficient to confer transcriptional regulation of the vir-repressed genes (vrg genes) in Bordetella pertussis. J Bacteriol. 1993 Jan;175(2):519–527. doi: 10.1128/jb.175.2.519-527.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. T., Shahin R., Mekalanos J. J. A vir-repressed gene of Bordetella pertussis is required for virulence. Infect Immun. 1992 Feb;60(2):571–577. doi: 10.1128/iai.60.2.571-577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis D. A., Greisen H. A., Appel M. J. Bacteriological variation among Bordetella bronchiseptica isolates from dogs and other species. J Clin Microbiol. 1977 Apr;5(4):471–480. doi: 10.1128/jcm.5.4.471-480.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. F., Helmann J. D. Restoration of motility to an Escherichia coli fliA flagellar mutant by a Bacillus subtilis sigma factor. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5123–5127. doi: 10.1073/pnas.89.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P., Sugasawara R. J., Schantz A. Identification of a common enterobacterial flagellin epitope with a monoclonal antibody. J Gen Microbiol. 1990 Feb;136(2):337–342. doi: 10.1099/00221287-136-2-337. [DOI] [PubMed] [Google Scholar]
- Gillen K. L., Hughes K. T. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J Bacteriol. 1991 Apr;173(7):2301–2310. doi: 10.1128/jb.173.7.2301-2310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Gober J. W., Champer R., Reuter S., Shapiro L. Expression of positional information during cell differentiation of Caulobacter. Cell. 1991 Jan 25;64(2):381–391. doi: 10.1016/0092-8674(91)90646-g. [DOI] [PubMed] [Google Scholar]
- Goldman S., Hanski E., Fish F. Spontaneous phase variation in Bordetella pertussis is a multistep non-random process. EMBO J. 1984 Jun;3(6):1353–1356. doi: 10.1002/j.1460-2075.1984.tb01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow R. A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980 Dec;44(4):722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyard S., Ullmann A. Analysis of Bordetella pertussis cya operon regulation by use of cya-lac fusions. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):251–256. doi: 10.1016/0378-1097(91)90561-n. [DOI] [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Genetics of pertussis toxin. Mol Microbiol. 1989 Jan;3(1):119–124. doi: 10.1111/j.1365-2958.1989.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Huh Y. J., Weiss A. A. A 23-kilodalton protein, distinct from BvgA, expressed by virulent Bordetella pertussis binds to the promoter region of vir-regulated toxin genes. Infect Immun. 1991 Jul;59(7):2389–2395. doi: 10.1128/iai.59.7.2389-2395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M. The flagellar filament protein. Can J Microbiol. 1988 Apr;34(4):452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobisch M., Novotny P. Identification of a 68-kilodalton outer membrane protein as the major protective antigen of Bordetella bronchiseptica by using specific-pathogen-free piglets. Infect Immun. 1990 Feb;58(2):352–357. doi: 10.1128/iai.58.2.352-357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y. Fusions of flagellar operons to lactose genes on a mu lac bacteriophage. J Bacteriol. 1982 Apr;150(1):16–26. doi: 10.1128/jb.150.1.16-26.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Suzuki H., Ishidsu J. I., Iino T. The role of cAMP in flagellation of Salmonella typhimurium. Mol Gen Genet. 1976 Dec 31;142(4):289–298. doi: 10.1007/BF00271253. [DOI] [PubMed] [Google Scholar]
- Kutsukake K., Ohya Y., Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E., Roberts M., Kenimer J. G., Charles I. G., Fairweather N., Novotny P., Brennan M. J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. L., Ezaki T., Miura H., Matsui K., Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988 Aug;56(8):1967–1973. doi: 10.1128/iai.56.8.1967-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livey I., Wardlaw A. C. Production and properties of Bordetella pertussis heat-labile toxin. J Med Microbiol. 1984 Feb;17(1):91–103. doi: 10.1099/00222615-17-1-91. [DOI] [PubMed] [Google Scholar]
- Melton A. R., Weiss A. A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989 Nov;171(11):6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Johnson S. A., Black W. J., Beattie D. T., Mekalanos J. J., Falkow S. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J Bacteriol. 1992 Feb;174(3):970–979. doi: 10.1128/jb.174.3.970-979.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Roy C. R., Falkow S. Analysis of Bordetella pertussis virulence gene regulation by use of transcriptional fusions in Escherichia coli. J Bacteriol. 1989 Nov;171(11):6345–6348. doi: 10.1128/jb.171.11.6345-6348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D. M., Arico B., Rappuoli R., Falkow S. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol. 1989 Dec;3(12):1719–1728. doi: 10.1111/j.1365-2958.1989.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Hewlett E. L., Peppler M. S., Selander R. K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986 Apr;166(1):230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A., Ohta N. Regulation of the cell division cycle and differentiation in bacteria. Annu Rev Microbiol. 1990;44:689–719. doi: 10.1146/annurev.mi.44.100190.003353. [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Kutsukake K., Suzuki H., Iino T. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet. 1990 Apr;221(2):139–147. doi: 10.1007/BF00261713. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: analysis of motility mutants in three animal models. Infect Immun. 1991 Aug;59(8):2727–2736. doi: 10.1128/iai.59.8.2727-2736.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Falkow S. Identification of Bordetella pertussis regulatory sequences required for transcriptional activation of the fhaB gene and autoregulation of the bvgAS operon. J Bacteriol. 1991 Apr;173(7):2385–2392. doi: 10.1128/jb.173.7.2385-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Miller J. F., Falkow S. Autogenous regulation of the Bordetella pertussis bvgABC operon. Proc Natl Acad Sci U S A. 1990 May;87(10):3763–3767. doi: 10.1073/pnas.87.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Miller J. F., Falkow S. The bvgA gene of Bordetella pertussis encodes a transcriptional activator required for coordinate regulation of several virulence genes. J Bacteriol. 1989 Nov;171(11):6338–6344. doi: 10.1128/jb.171.11.6338-6344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato V., Prugnola A., Aricò B., Rappuoli R. The bvg-dependent promoters show similar behaviour in different Bordetella species and share sequence homologies. Mol Microbiol. 1991 Oct;5(10):2493–2498. doi: 10.1111/j.1365-2958.1991.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Scarlato V., Prugnola A., Aricó B., Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974 Dec;120(3):1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Aaronson W., Monack D., Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989 Mar 16;338(6212):266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Garletts T. L. Derivation of a physical map of the chromosome of Bordetella pertussis Tohama I. J Bacteriol. 1992 Dec;174(23):7770–7777. doi: 10.1128/jb.174.23.7770-7777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibitz S., Yang M. S. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991 Jul;173(14):4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar T. M., Bleumink-Pluym N. M., van der Zeijst B. A. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991 Aug;10(8):2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Willems R., Paul A., van der Heide H. G., ter Avest A. R., Mooi F. R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990 Sep;9(9):2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]