Abstract
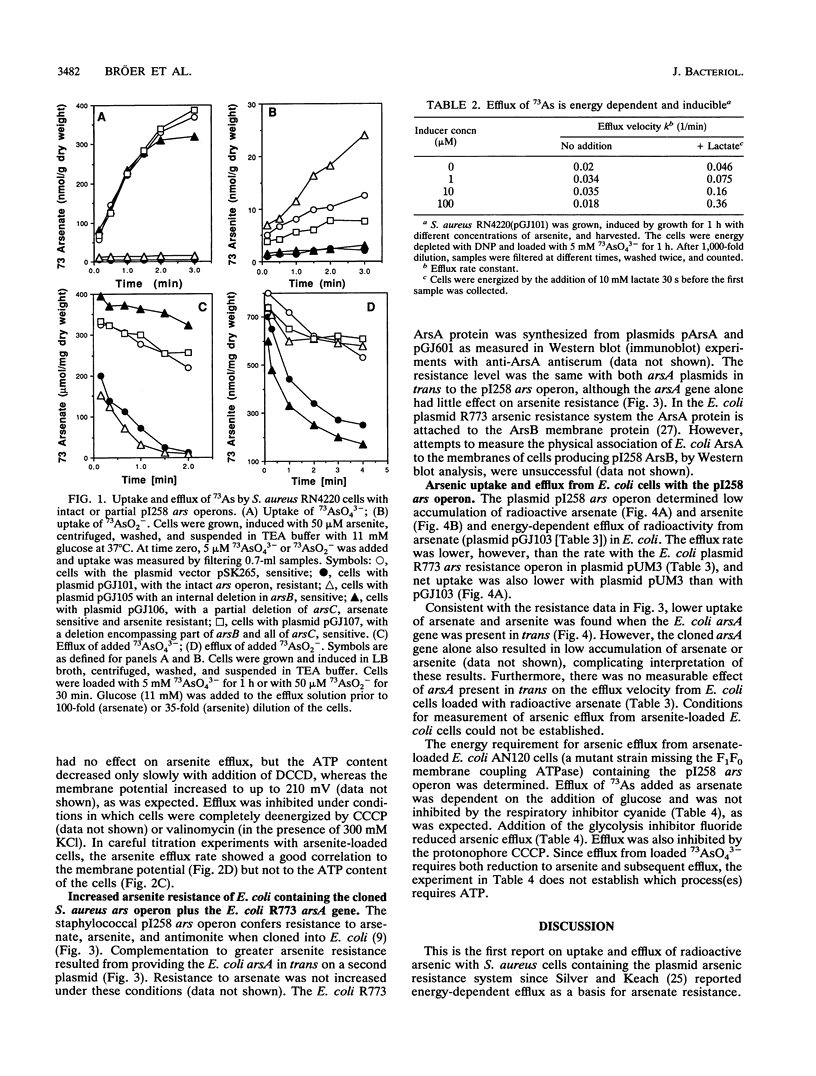
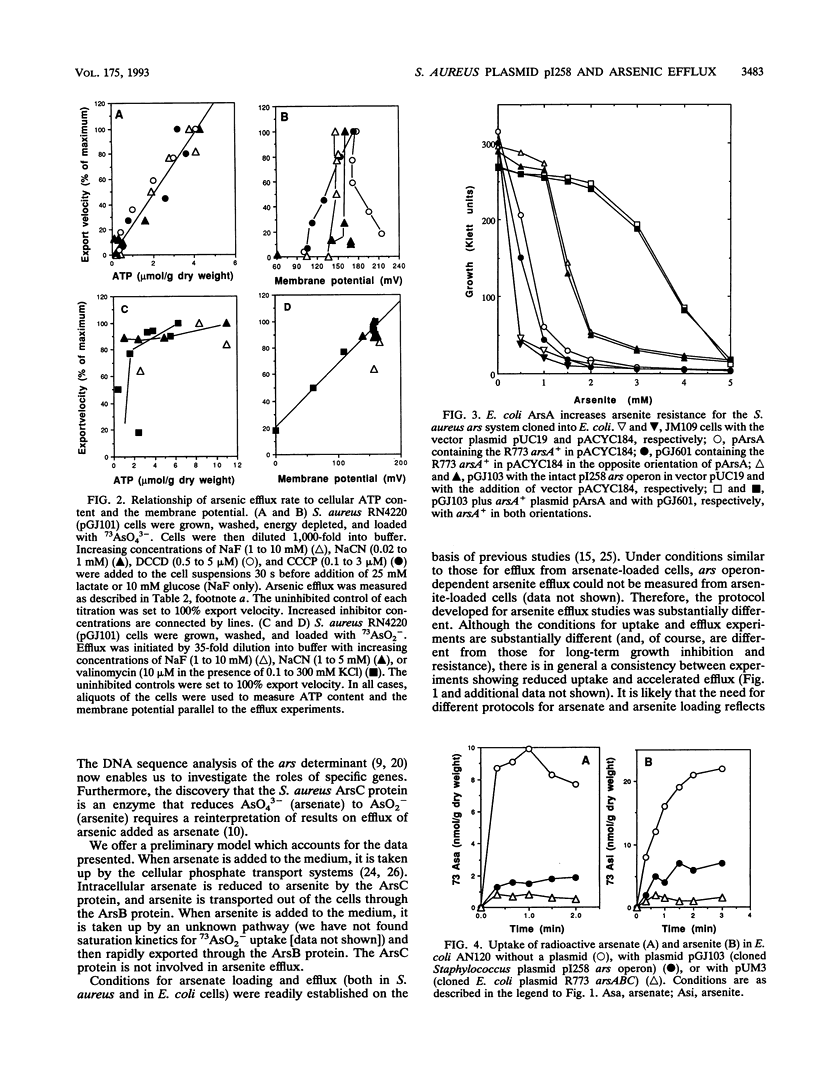
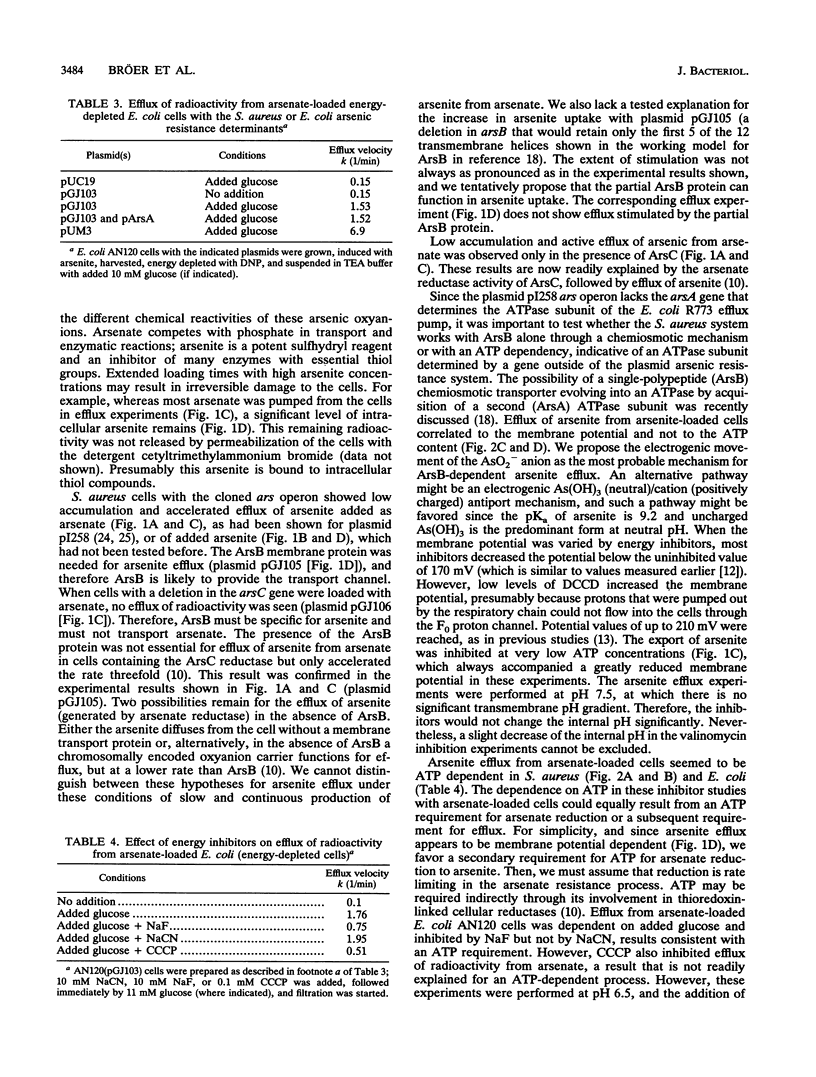
The arsenic resistance operon of Staphylococcus aureus plasmid pI258 determined lowered net cellular uptake of 73As by an active efflux mechanism. Arsenite was exported from the cells; intracellular arsenate was first reduced to arsenite and then transported out of the cells. Resistant cells showed lower accumulation of 73As originating from both arsenate and arsenite. Active efflux from cells loaded with arsenite required the presence of the plasmid-determined arsB gene. Efflux of arsenic originating as arsenate required the presence of the arsC gene and occurred more rapidly with the addition of arsB. Inhibitor studies with S. aureus loaded with arsenite showed that arsenite efflux was energy dependent and appeared to be driven by the membrane potential. With cells loaded with 73AsO4(3-), a requirement for ATP for energy was observed, leading to the conclusion that ATP was required for arsenate reduction. When the staphylococcal arsenic resistance determinant was cloned into Escherichia coli, lowered accumulation of arsenate and arsenite and 73As efflux from cells loaded with arsenate were also found. Cloning of the E. coli plasmid R773 arsA gene (the determinant of the arsenite-dependent ATPase) in trans to the S. aureus gene arsB resulted in increased resistance to arsenite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bröer S., Krämer R. Lysine excretion by Corynebacterium glutamicum. 2. Energetics and mechanism of the transport system. Eur J Biochem. 1991 Nov 15;202(1):137–143. doi: 10.1111/j.1432-1033.1991.tb16354.x. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Misra T. K., Silver S., Rosen B. P. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J Biol Chem. 1986 Nov 15;261(32):15030–15038. [PubMed] [Google Scholar]
- Dabbs E. R., Sole G. J. Plasmid-borne resistance to arsenate, arsenite, cadmium, and chloramphenicol in a Rhodococcus species. Mol Gen Genet. 1988 Jan;211(1):148–154. doi: 10.1007/BF00338406. [DOI] [PubMed] [Google Scholar]
- Dou D., Owolabi J. B., Dey S., Rosen B. P. Construction of a chimeric ArsA-ArsB protein for overexpression of the oxyanion-translocating ATPase. J Biol Chem. 1992 Dec 25;267(36):25768–25775. [PubMed] [Google Scholar]
- Hedges R. W., Baumberg S. Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol. 1973 Jul;115(1):459–460. doi: 10.1128/jb.115.1.459-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. M., Rosen B. P. Characterization of the catalytic subunit of an anion pump. J Biol Chem. 1989 Oct 15;264(29):17349–17354. [PubMed] [Google Scholar]
- Ji G., Silver S. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9474–9478. doi: 10.1073/pnas.89.20.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G., Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992 Jun;174(11):3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Krämer R., Lambert C. Uptake of glutamate in Corynebacterium glutamicum. 2. Evidence for a primary active transport system. Eur J Biochem. 1990 Dec 27;194(3):937–944. doi: 10.1111/j.1432-1033.1990.tb19489.x. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Rosen B. P. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6119–6122. doi: 10.1073/pnas.79.20.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Borbolla M. G. A plasmid-encoded arsenite pump produces arsenite resistance in Escherichia coli. Biochem Biophys Res Commun. 1984 Nov 14;124(3):760–765. doi: 10.1016/0006-291x(84)91023-4. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Dey S., Dou D., Ji G., Kaur P., Ksenzenko MYu, Silver S., Wu J. Evolution of an ion-translocating ATPase. Ann N Y Acad Sci. 1992 Nov 30;671:257–272. doi: 10.1111/j.1749-6632.1992.tb43801.x. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., Weigel U., Karkaria C., Gangola P. Molecular characterization of an anion pump. The arsA gene product is an arsenite(antimonate)-stimulated ATPase. J Biol Chem. 1988 Mar 5;263(7):3067–3070. [PubMed] [Google Scholar]
- Rosenstein R., Peschel A., Wieland B., Götz F. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol. 1992 Jun;174(11):3676–3683. doi: 10.1128/jb.174.11.3676-3683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco M. J., Hope C. L., Owolabi J. B., Tisa L. S., Rosen B. P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990 Feb 11;18(3):619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco M. J., Tisa L. S., Rosen B. P. Identification of the membrane component of the anion pump encoded by the arsenical resistance operon of R-factor R773. Mol Microbiol. 1989 Jan;3(1):15–21. doi: 10.1111/j.1365-2958.1989.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Silver S., Budd K., Leahy K. M., Shaw W. V., Hammond D., Novick R. P., Willsky G. R., Malamy M. H., Rosenberg H. Inducible plasmid-determined resistance to arsenate, arsenite, and antimony (III) in escherichia coli and Staphylococcus aureus. J Bacteriol. 1981 Jun;146(3):983–996. doi: 10.1128/jb.146.3.983-996.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Keach D. Energy-dependent arsenate efflux: the mechanism of plasmid-mediated resistance. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6114–6118. doi: 10.1073/pnas.79.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992 Mar;56(1):195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa L. S., Rosen B. P. Molecular characterization of an anion pump. The ArsB protein is the membrane anchor for the ArsA protein. J Biol Chem. 1990 Jan 5;265(1):190–194. [PubMed] [Google Scholar]
- Wu J., Rosen B. P. The ArsR protein is a trans-acting regulatory protein. Mol Microbiol. 1991 Jun;5(6):1331–1336. doi: 10.1111/j.1365-2958.1991.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Wu J., Tisa L. S., Rosen B. P. Membrane topology of the ArsB protein, the membrane subunit of an anion-translocating ATPase. J Biol Chem. 1992 Jun 25;267(18):12570–12576. [PubMed] [Google Scholar]



