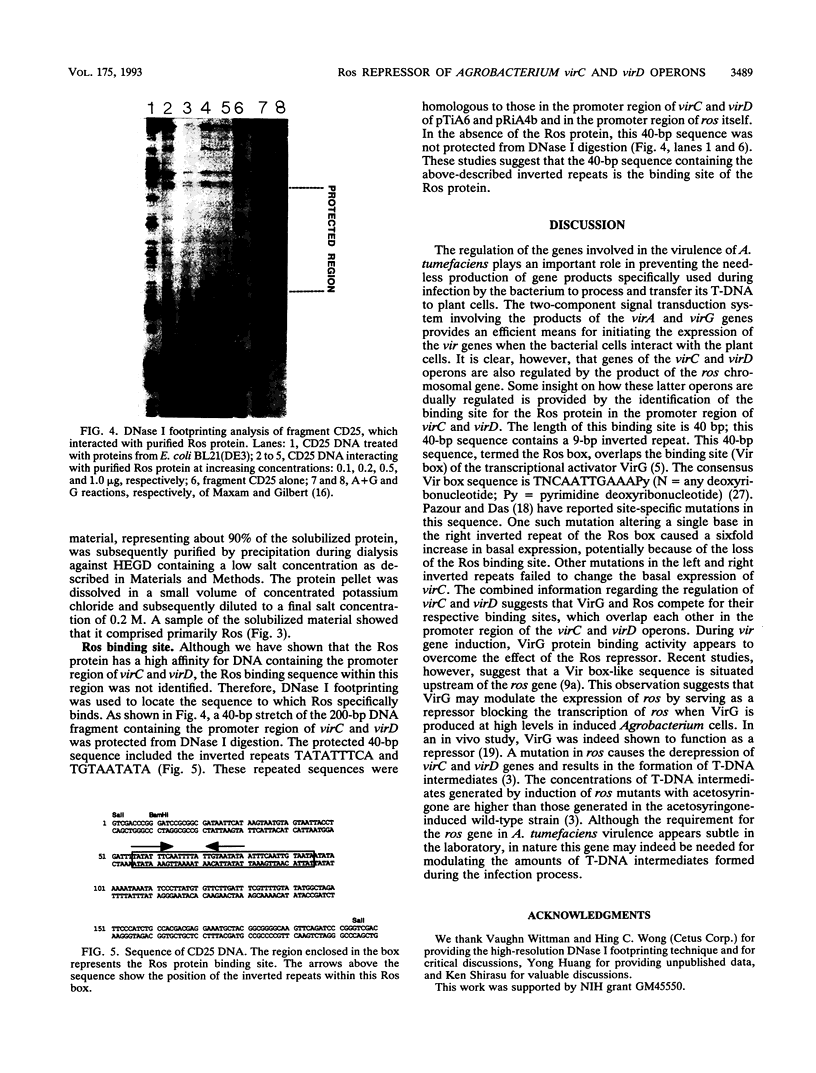
Abstract
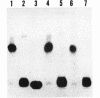
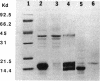
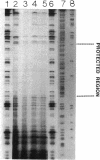
The virulence genes of the Agrobacterium tumefaciens Ti plasmid are regulated both positively and negatively. The products of the genes of the virC and virD operons play an important role in host specificity and T-DNA processing. These operons are transcribed in opposite directions and therefore bear diametrically oriented promoters. These promoters are positively regulated by the VirG protein, which is believed to be activated through phosphorylation by a histidine kinase encoded by the virA gene. The virC and virD operons are also regulated by a 15.5-kDa repressor protein encoded by the ros chromosomal gene. A mutation in ros causes the constitutive expression of virC and virD in the complete absence of the VirG protein. It appears, therefore, that the Ros repressor interacts with the regulatory region of these operons. The Ros repressor is shown here to bind to an upstream sequence (Ros box) comprising 40 bp bearing a 9-bp inverted repeat, TATATTTCA/TGTAATATA, in the promoter region of these operons. The affinity for this sequence is specific and tenacious, since the addition of at least a 20,000-fold excess of competitor DNA failed to remove the Ros protein coding sequence from the Ros box. DNase I footprint analysis showed that the Ros box overlaps the binding site of VirG (Vir box). This result suggests that virC and virD transcription is modulated by Ros and VirG proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cangelosi G. A., Ankenbauer R. G., Nester E. W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Rogowsky P. M., Kado C. I., Winans S. C., Yanofsky M. F., Nester E. W. Dual control of Agrobacterium tumefaciens Ti plasmid virulence genes. J Bacteriol. 1987 Nov;169(11):5113–5118. doi: 10.1128/jb.169.11.5113-5118.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Tait R. C., Kado C. I. Regulation of Ti plasmid virulence genes by a chromosomal locus of Agrobacterium tumefaciens. J Bacteriol. 1985 Nov;164(2):774–781. doi: 10.1128/jb.164.2.774-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley M. B., D'Souza M. R., Kado C. I. The virC and virD operons of the Agrobacterium Ti plasmid are regulated by the ros chromosomal gene: analysis of the cloned ros gene. J Bacteriol. 1991 Apr;173(8):2608–2616. doi: 10.1128/jb.173.8.2608-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley M. B., Kado C. I. Mapping of the ros virulence regulatory gene of A. tumefaciens. Mol Gen Genet. 1991 Nov;230(1-2):24–27. doi: 10.1007/BF00290645. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley N., Hohn B., Ramos C., Kado C., Rogowsky P. DNA transfer from Agrobacterium to Zea mays or Brassica by agroinfection is dependent on bacterial virulence functions. Mol Gen Genet. 1989 Jun;217(2-3):309–316. doi: 10.1007/BF02464898. [DOI] [PubMed] [Google Scholar]
- Hoey T., Dynlacht B. D., Peterson M. G., Pugh B. F., Tjian R. Isolation and characterization of the Drosophila gene encoding the TATA box binding protein, TFIID. Cell. 1990 Jun 29;61(7):1179–1186. doi: 10.1016/0092-8674(90)90682-5. [DOI] [PubMed] [Google Scholar]
- Huang Y., Morel P., Powell B., Kado C. I. VirA, a coregulator of Ti-specified virulence genes, is phosphorylated in vitro. J Bacteriol. 1990 Feb;172(2):1142–1144. doi: 10.1128/jb.172.2.1142-1144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Roitsch T., Ankenbauer R. G., Gordon M. P., Nester E. W. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. doi: 10.1128/jb.172.2.525-530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H. J., White F. F., Iyer V. N., Gordon M. P., Nester E. W. Mutational analysis of the virulence region of an Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1983 Feb;153(2):878–883. doi: 10.1128/jb.153.2.878-883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leroux B., Yanofsky M. F., Winans S. C., Ward J. E., Ziegler S. F., Nester E. W. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. doi: 10.1002/j.1460-2075.1987.tb04830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers L. S., Regensburg-Tuïnk A. J., Schilperoort R. A., Hooykaas P. J. Specificity of signal molecules in the activation of Agrobacterium virulence gene expression. Mol Microbiol. 1989 Jul;3(7):969–977. doi: 10.1111/j.1365-2958.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Pazour G. J., Das A. Characterization of the VirG binding site of Agrobacterium tumefaciens. Nucleic Acids Res. 1990 Dec 11;18(23):6909–6913. doi: 10.1093/nar/18.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell B. S., Kado C. I. Specific binding of VirG to the vir box requires a C-terminal domain and exhibits a minimum concentration threshold. Mol Microbiol. 1990 Dec;4(12):2159–2166. doi: 10.1111/j.1365-2958.1990.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Rogowsky P. M., Close T. J., Chimera J. A., Shaw J. J., Kado C. I. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol. 1987 Nov;169(11):5101–5112. doi: 10.1128/jb.169.11.5101-5112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowsky P. M., Powell B. S., Shirasu K., Lin T. S., Morel P., Zyprian E. M., Steck T. R., Kado C. I. Molecular characterization of the vir regulon of Agrobacterium tumefaciens: complete nucleotide sequence and gene organization of the 28.63-kbp regulon cloned as a single unit. Plasmid. 1990 Mar;23(2):85–106. doi: 10.1016/0147-619x(90)90028-b. [DOI] [PubMed] [Google Scholar]
- Shimoda N., Toyoda-Yamamoto A., Nagamine J., Usami S., Katayama M., Sakagami Y., Machida Y. Control of expression of Agrobacterium vir genes by synergistic actions of phenolic signal molecules and monosaccharides. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6684–6688. doi: 10.1073/pnas.87.17.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Morel P., Kado C. I. Vir box sequences in Agrobacterium tumefaciens pTiC58 and A6. Nucleic Acids Res. 1988 Sep 12;16(17):8736–8736. doi: 10.1093/nar/16.17.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Kado C. I. Regulation of the virC and virD promoters of pTiC58 by the ros chromosomal mutation of Agrobacterium tumefaciens. Mol Microbiol. 1988 May;2(3):385–392. doi: 10.1111/j.1365-2958.1988.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Toro N., Datta A., Carmi O. A., Young C., Prusti R. K., Nester E. W. The Agrobacterium tumefaciens virC1 gene product binds to overdrive, a T-DNA transfer enhancer. J Bacteriol. 1989 Dec;171(12):6845–6849. doi: 10.1128/jb.171.12.6845-6849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]