Abstract
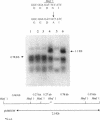
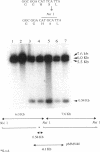
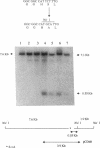
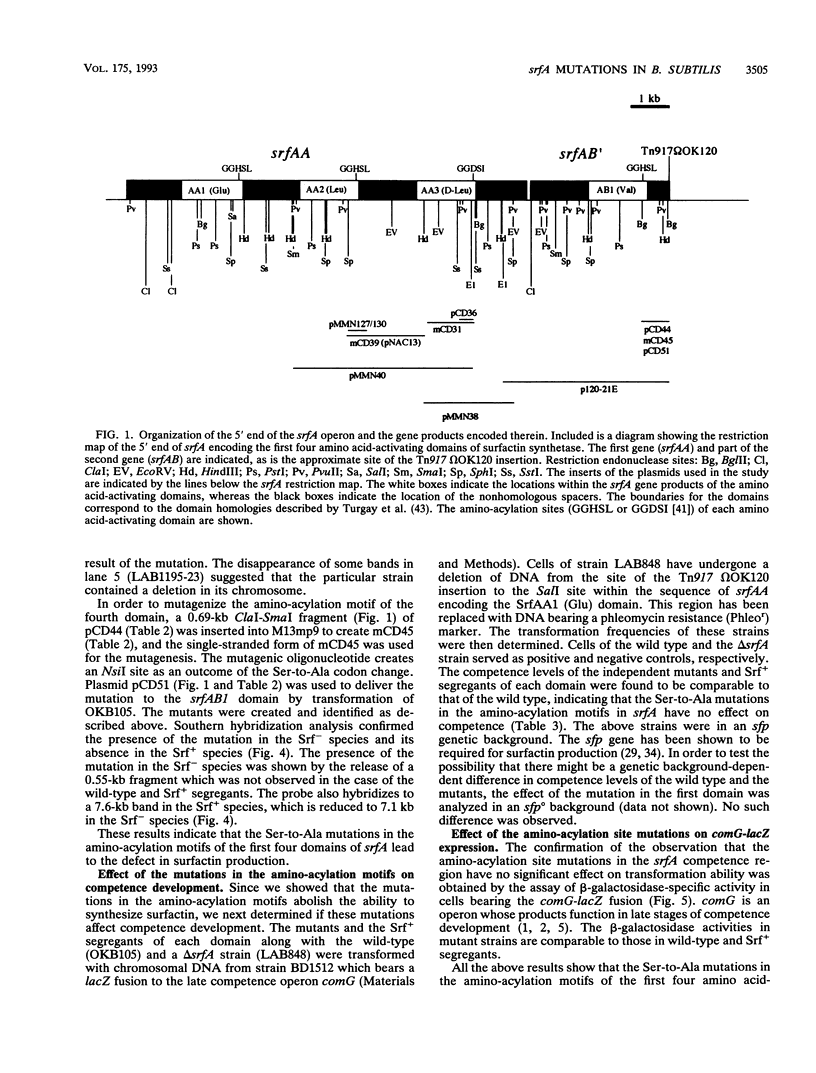
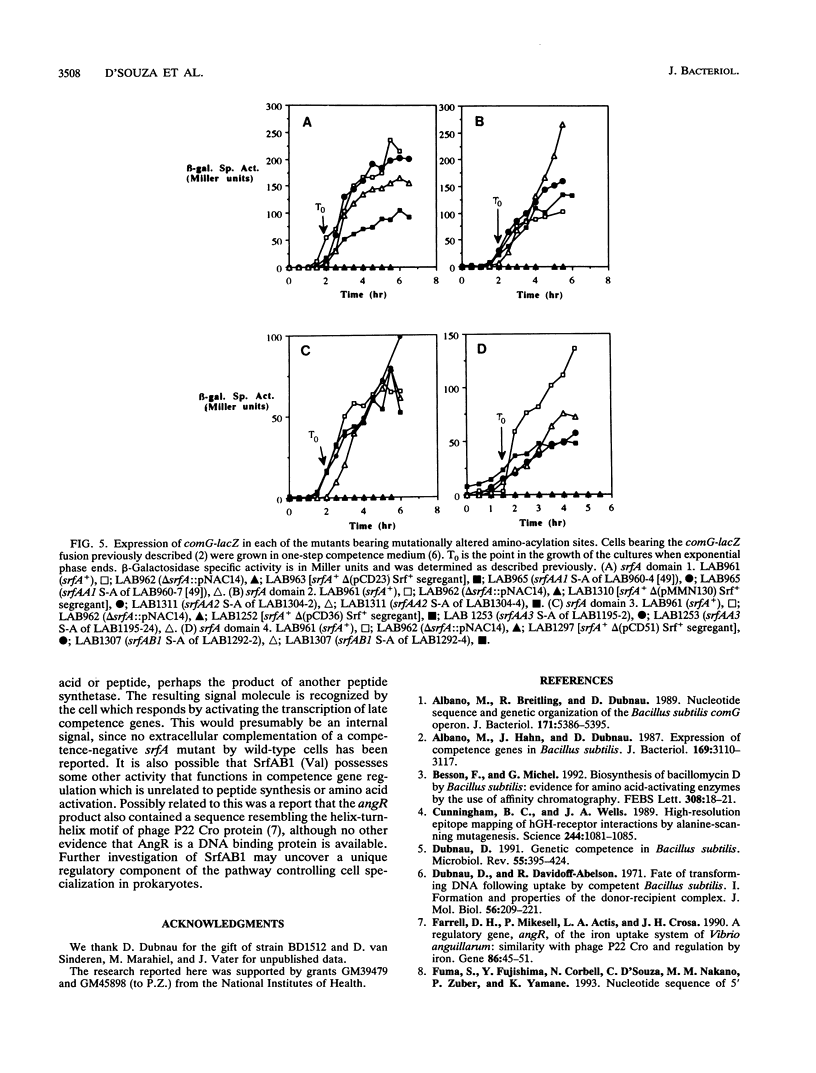
The part of the srfA operon of Bacillus subtilis that contains the region required for competence development is composed of the first four amino acid-activating domains which are responsible for the incorporation of Glu, Leu, D-Leu, and Val into the peptide moiety of the lipopeptide surfactin. Ser-to-Ala substitutions were made in the amino-acylation site of each domain, and their effects on surfactin production and competence development were examined. All of the mutations conferred a surfactin-negative phenotype, supporting the finding that the conserved Ser in the amino-acylation site is required for peptide synthesis. However, none of the mutations affected significantly competence development or the expression of a lacZ fusion to the late competence operon comG. This, coupled with recent findings that only the fourth, Val-activating, domain is required for competence, suggests that some activity, other than amino-acylation and perhaps unrelated to peptide synthesis, possessed by the fourth domain is involved in the role of srfA in regulating competence development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Breitling R., Dubnau D. A. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989 Oct;171(10):5386–5404. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M., Hahn J., Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson F., Michel G. Biosynthesis of bacillomycin D by Bacillus subtilis. Evidence for amino acid-activating enzymes by the use of affinity chromatography. FEBS Lett. 1992 Aug 10;308(1):18–21. doi: 10.1016/0014-5793(92)81040-s. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991 Sep;55(3):395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D. H., Mikesell P., Actis L. A., Crosa J. H. A regulatory gene, angR, of the iron uptake system of Vibrio anguillarum: similarity with phage P22 cro and regulation by iron. Gene. 1990 Jan 31;86(1):45–51. doi: 10.1016/0378-1119(90)90112-5. [DOI] [PubMed] [Google Scholar]
- Fuma S., Fujishima Y., Corbell N., D'Souza C., Nakano M. M., Zuber P., Yamane K. Nucleotide sequence of 5' portion of srfA that contains the region required for competence establishment in Bacillus subtilus. Nucleic Acids Res. 1993 Jan 11;21(1):93–97. doi: 10.1093/nar/21.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Kleinkauf H., Lipmann F. Peptidyl transfers in gramicidin S bisoynthesis from enzyme-bound thioester intermediates. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1335–1342. doi: 10.1073/pnas.63.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Kleinkauf H., Lipmann F. The activation of amino acids for biosynthesis of gramicidin S. Proc Natl Acad Sci U S A. 1968 May;60(1):269–276. doi: 10.1073/pnas.60.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J., Dubnau D. Growth stage signal transduction and the requirements for srfA induction in development of competence. J Bacteriol. 1991 Nov;173(22):7275–7282. doi: 10.1128/jb.173.22.7275-7282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Kanda M., Kurotsu T., Miura S., Yamada Y., Saito Y. Absence of pantothenic acid in gramicidin S synthetase 2 obtained from some mutants of Bacillus brevis. J Biochem. 1981 Aug;90(2):439–447. doi: 10.1093/oxfordjournals.jbchem.a133491. [DOI] [PubMed] [Google Scholar]
- Hori K., Yamamoto Y., Tokita K., Saito F., Kurotsu T., Kanda M., Okamura K., Furuyama J., Saito Y. The nucleotide sequence for a proline-activating domain of gramicidin S synthetase 2 gene from Bacillus brevis. J Biochem. 1991 Jul;110(1):111–119. doi: 10.1093/oxfordjournals.jbchem.a123528. [DOI] [PubMed] [Google Scholar]
- Kanda M., Hori K., Kurotsu T., Miura S., Yamada Y., Saito Y. Sulfhydryl groups related to the catalytic activity of gramicidin S synthetase 1 of Bacillus brevis. J Biochem. 1981 Sep;90(3):765–771. doi: 10.1093/oxfordjournals.jbchem.a133531. [DOI] [PubMed] [Google Scholar]
- Katz E., Demain A. L. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977 Jun;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Nonribosomal biosynthesis of peptide antibiotics. Eur J Biochem. 1990 Aug 28;192(1):1–15. doi: 10.1111/j.1432-1033.1990.tb19188.x. [DOI] [PubMed] [Google Scholar]
- Kluge B., Vater J., Salnikow J., Eckart K. Studies on the biosynthesis of surfactin, a lipopeptide antibiotic from Bacillus subtilis ATCC 21332. FEBS Lett. 1988 Apr 11;231(1):107–110. doi: 10.1016/0014-5793(88)80712-9. [DOI] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Krause M., Marahiel M. A., von Döhren H., Kleinkauf H. Molecular cloning of an ornithine-activating fragment of the gramicidin S synthetase 2 gene from Bacillus brevis and its expression in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1120–1125. doi: 10.1128/jb.162.3.1120-1125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krätzschmar J., Krause M., Marahiel M. A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989 Oct;171(10):5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotsu T., Hori K., Kanda M., Saito Y. Characterization and location of the L-proline activating fragment from the multifunctional gramicidin S synthetase 2. J Biochem. 1991 May;109(5):763–769. doi: 10.1093/oxfordjournals.jbchem.a123454. [DOI] [PubMed] [Google Scholar]
- Lipmann F. Bacterial production of antibiotic polypeptides by thiol-linked synthesis on protein templates. Adv Microb Physiol. 1980;21:227–266. doi: 10.1016/s0065-2911(08)60357-4. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Kleckner N. Mismatch repair mutations of Escherichia coli K12 enhance transposon excision. Genetics. 1985 Jan;109(1):3–19. doi: 10.1093/genetics/109.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahiel M. A. Multidomain enzymes involved in peptide synthesis. FEBS Lett. 1992 Jul 27;307(1):40–43. doi: 10.1016/0014-5793(92)80898-q. [DOI] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Corbell N., Besson J., Zuber P. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol Gen Genet. 1992 Mar;232(2):313–321. doi: 10.1007/BF00280011. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Magnuson R., Myers A., Curry J., Grossman A. D., Zuber P. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991 Mar;173(5):1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Marahiel M. A., Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Xia L. A., Zuber P. Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis. J Bacteriol. 1991 Sep;173(17):5487–5493. doi: 10.1128/jb.173.17.5487-5493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol. 1989 Oct;171(10):5347–5353. doi: 10.1128/jb.171.10.5347-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Molecular biology of antibiotic production in Bacillus. Crit Rev Biotechnol. 1990;10(3):223–240. doi: 10.3109/07388559009038209. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Mutational analysis of the regulatory region of the srfA operon in Bacillus subtilis. J Bacteriol. 1993 May;175(10):3188–3191. doi: 10.1128/jb.175.10.3188-3191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. The primary role of comA in establishment of the competent state in Bacillus subtilis is to activate expression of srfA. J Bacteriol. 1991 Nov;173(22):7269–7274. doi: 10.1128/jb.173.22.7269-7274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet B., Ehrlich S. D. In vitro genetic labeling of Bacillus subtilis cryptic plasmid pHV400. Plasmid. 1979 Jan;2(1):48–58. doi: 10.1016/0147-619x(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Revill W. P., Leadlay P. F. Cloning, characterization, and high-level expression in Escherichia coli of the Saccharopolyspora erythraea gene encoding an acyl carrier protein potentially involved in fatty acid biosynthesis. J Bacteriol. 1991 Jul;173(14):4379–4385. doi: 10.1128/jb.173.14.4379-4385.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F., Sakaitani M., Drueckhammer D., Reichert J., Walsh C. T. Biosynthesis of the Escherichia coli siderophore enterobactin: sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991 Mar 19;30(11):2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- Schlumbohm W., Stein T., Ullrich C., Vater J., Krause M., Marahiel M. A., Kruft V., Wittmann-Liebold B. An active serine is involved in covalent substrate amino acid binding at each reaction center of gramicidin S synthetase. J Biol Chem. 1991 Dec 5;266(34):23135–23141. [PubMed] [Google Scholar]
- Staab J. F., Elkins M. F., Earhart C. F. Nucleotide sequence of the Escherichia coli entE gene. FEMS Microbiol Lett. 1989 May;50(1-2):15–19. doi: 10.1016/0378-1097(89)90450-3. [DOI] [PubMed] [Google Scholar]
- Turgay K., Krause M., Marahiel M. A. Four homologous domains in the primary structure of GrsB are related to domains in a superfamily of adenylate-forming enzymes. Mol Microbiol. 1992 Feb;6(4):529–546. doi: 10.1111/j.1365-2958.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Ullrich C., Kluge B., Palacz Z., Vater J. Cell-free biosynthesis of surfactin, a cyclic lipopeptide produced by Bacillus subtilis. Biochemistry. 1991 Jul 2;30(26):6503–6508. doi: 10.1021/bi00240a022. [DOI] [PubMed] [Google Scholar]
- Vining L. C. Functions of secondary metabolites. Annu Rev Microbiol. 1990;44:395–427. doi: 10.1146/annurev.mi.44.100190.002143. [DOI] [PubMed] [Google Scholar]
- Weckermann R., Fürbass R., Marahiel M. A. Complete nucleotide sequence of the tycA gene coding the tyrocidine synthetase 1 from Bacillus brevis. Nucleic Acids Res. 1988 Dec 23;16(24):11841–11841. doi: 10.1093/nar/16.24.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yansura D. G., Henner D. J. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Healy J., Carter H. L., 3rd, Cutting S., Moran C. P., Jr, Losick R. Mutation changing the specificity of an RNA polymerase sigma factor. J Mol Biol. 1989 Apr 20;206(4):605–614. doi: 10.1016/0022-2836(89)90569-x. [DOI] [PubMed] [Google Scholar]
- Zuber P. Non-ribosomal peptide synthesis. Curr Opin Cell Biol. 1991 Dec;3(6):1046–1050. doi: 10.1016/0955-0674(91)90127-k. [DOI] [PubMed] [Google Scholar]
- van Sinderen D., Withoff S., Boels H., Venema G. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis. Mol Gen Genet. 1990 Dec;224(3):396–404. doi: 10.1007/BF00262434. [DOI] [PubMed] [Google Scholar]