Abstract
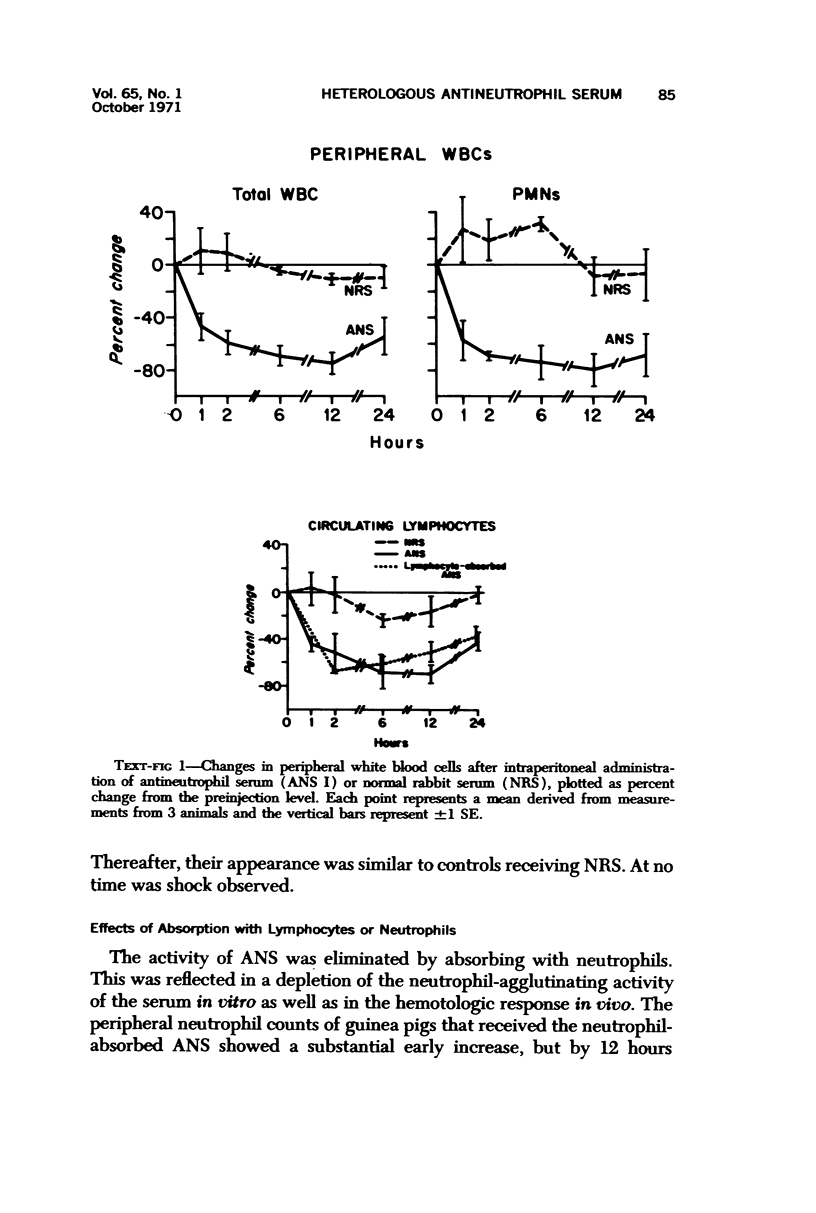
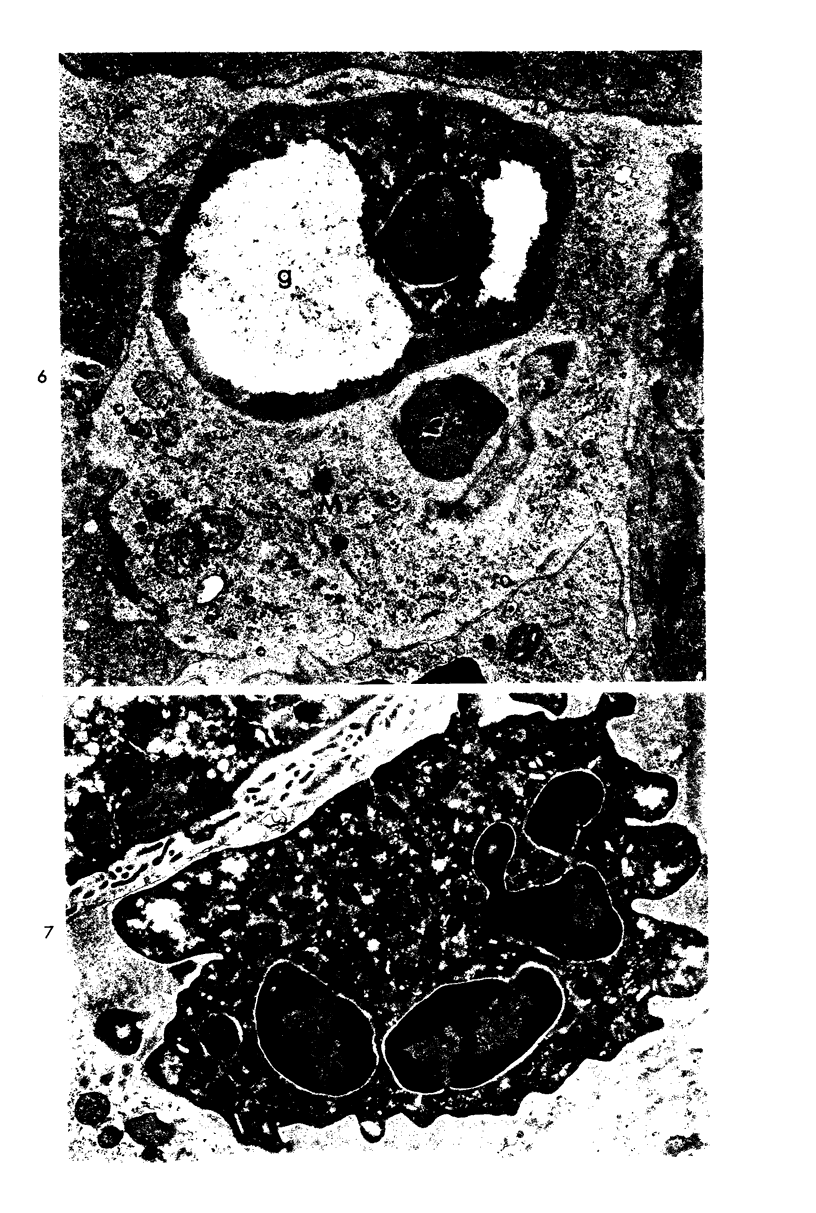
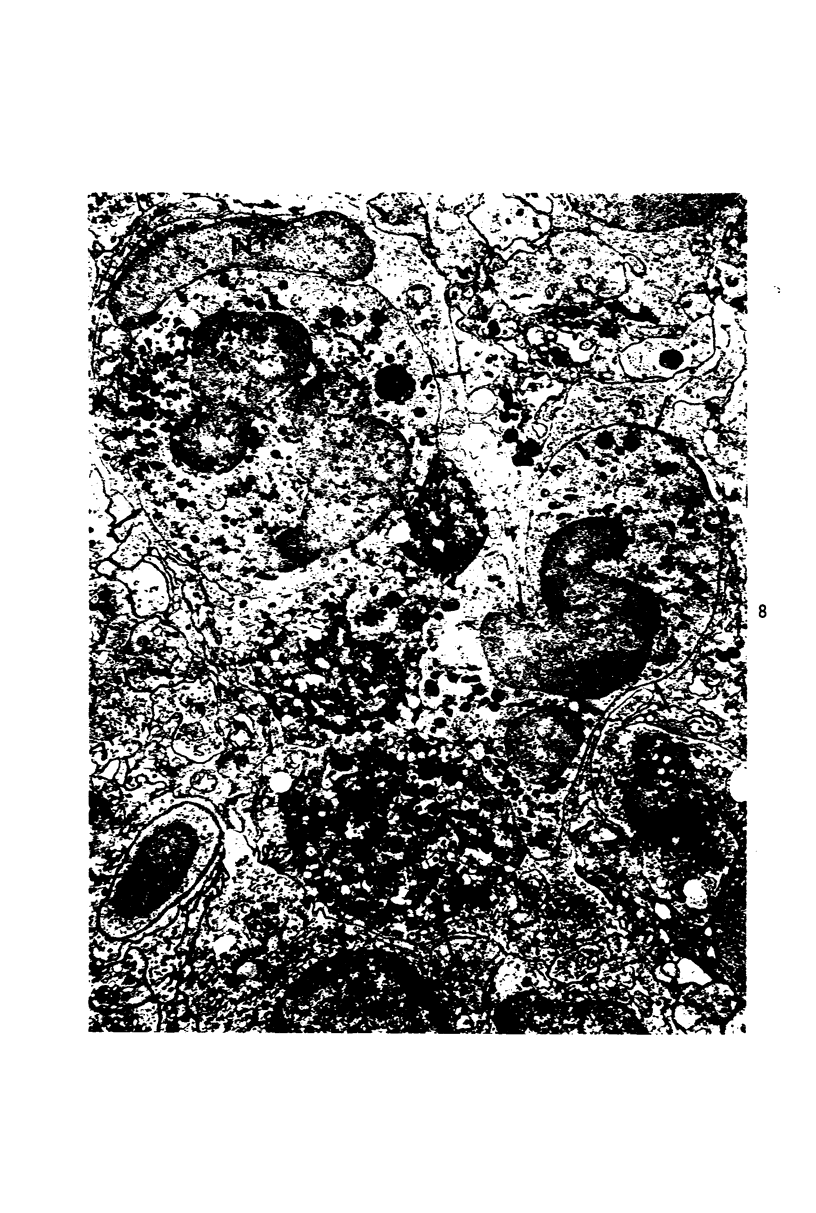
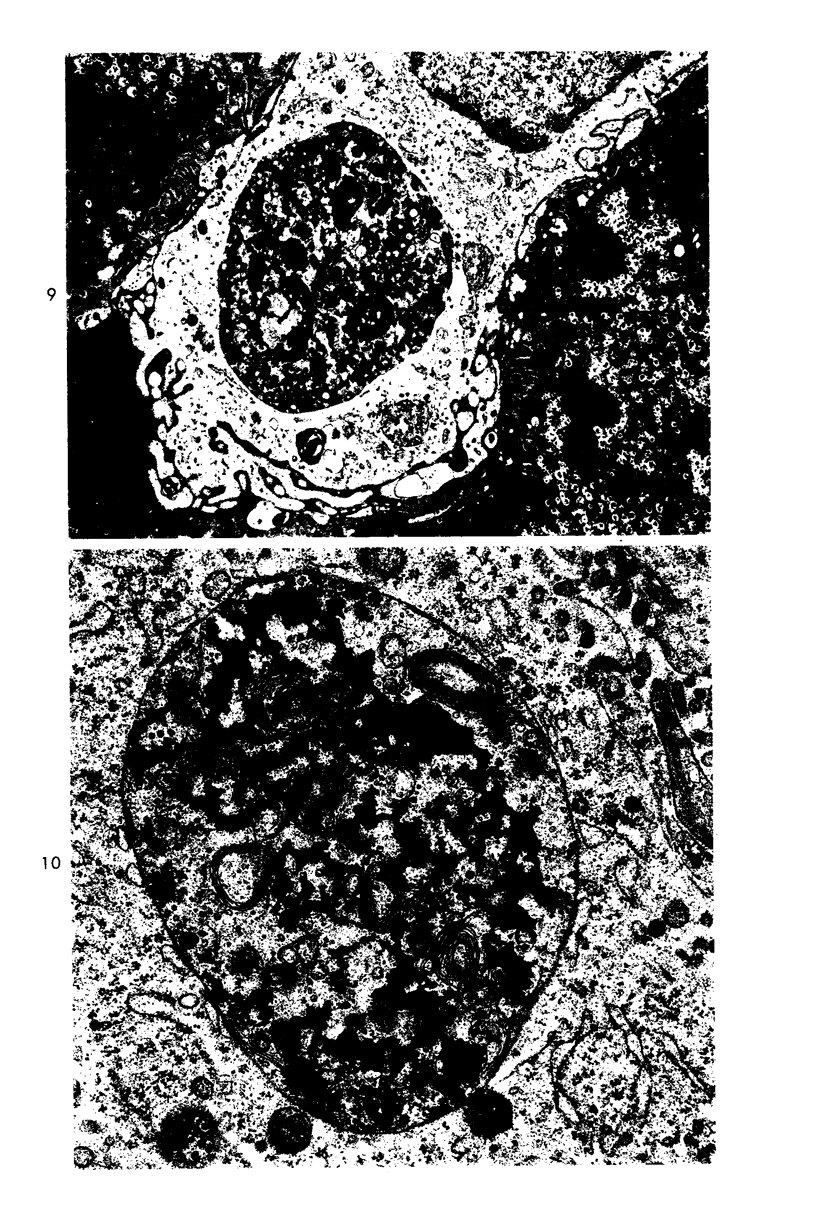
Two pools of rabbit anti-guinea pig neutrophil serum (ANS) were prepared using an intravenous (ANS I) or subcutaneous (ANS II) route of immunization with proteose peptone-stimulated peritoneal exudate neutrophils (PMNs) from albino guinea pigs. In vitro, both pools of ANS contained high titers of agglutinating antibodies to neutrophils and lower titers against lymphocytes and red cells. Agglutinins against all three cell types could be selectively removed by absorption. The in vivo hematologic effects of both the absorbed and unabsorbed antisera were examined after intraperitoneal administration, and the effects of ANS on neutrophils in blood, bone marrow, and peritoneal cavity were examined by light and electron microscopy of spleen, liver, lung, lymph node, buffy coat, bone marrow and pellets of peritoneal cells removed at various time intervals within 24 hours. Injection of either antisera caused a rapid decrease in circulating neutrophils and lymphocytes, which reached their lowest levels within 12 hours. Neutrophils that disappeared from the circulation were sequestered primarily in liver and spleen where they were phagocytized, as morphologically intact cells, by macrophages and then rapidly digested. Immature bone marrow neutrophils as young as early myelocytes were ingested by macrophages in the marrow at 6 hours or later after ANS. Neutrophils that were phagocytized were apparently opsonized by ANS since there was no ultrastructural evidence of neutrophil lysis in blood or bone marrow after ANS treatment. However, both lysed and ingested neutrophils were observed in the peritoneal cavity. Absorption of ANS with neutrophils removed the ability of the serum to produce neutropenia. However, absorption of ANS with lymphocytes did not alter the lymphopenia produced by the antiserum. The fate of lymphocytes leaving the peripheral circulation was not apparent. Lymphocytes did not accumulate in liver or spleen sinusoids and were not ingested by macrophages in these organs, as were the neutrophils. There was no evidence of paracortical depletion or extensive phagocytosis of lymphocytes in lymph nodes after ANS, as other investigators have reported after administration of antilymphocyte serum.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew H. D. The effect of heterologous anti-lymphocytic serum on the small lymphocyte population of rats. J Exp Med. 1968 Jul 1;128(1):111–119. doi: 10.1084/jem.128.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. D., Wood S., Jr Intravascular thrombosis and leukocyte destruction in vivo by heterologous antilymphocyte serum. Fed Proc. 1970 Jan-Feb;29(1):145–149. [PubMed] [Google Scholar]
- BENNETT B., OLD L. J., BOYSE E. A. Opsonization of cells by isoantibody in vitro. Nature. 1963 Apr 6;198:10–12. doi: 10.1038/198010a0. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Origin of granules in polymorphonuclear leukocytes. Two types derived from opposite faces of the Golgi complex in developing granulocytes. J Cell Biol. 1966 Feb;28(2):277–301. doi: 10.1083/jcb.28.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAJANO A., MAUREA C. Sieri anticellule ed antiorgano; ricerche sperimentali. II. Haematologica. 1950;34(10):1133–1179. [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett N. B., Schwarz M. R., Tyler R. W., Perkins W. D. Observations relative to the mechanism of action of antilymphocyte serum. Fed Proc. 1970 Jan-Feb;29(1):212–219. [PubMed] [Google Scholar]
- FINCH S. C., CAJANO A., ROSS J. F. Leukocyte response to leukocyte nuclear and cytoplasmic antisera. J Lab Clin Med. 1955 Dec;46(6):871–878. [PubMed] [Google Scholar]
- FINCH S. C., ROSS J. F., EBAUGH F. G., Jr Immunologic mechanisms of leukocyte abnormalities. J Lab Clin Med. 1953 Oct;42(4):555–569. [PubMed] [Google Scholar]
- Glick A. D., Horn R. G., Collins R. D., Bryant R. E. An electron microscopic study of human polymorphonuclear leukocyte injury induced by rabbit antiserum. Exp Mol Pathol. 1970 Jun;12(3):275–285. doi: 10.1016/0014-4800(70)90059-6. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. Origin of blood platelets. Nature. 1955 Jul 2;176(4470):38–38. doi: 10.1038/176038a0. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. The mechanism of Arthus reactions. II. The role of polymorphonuclear leucocytes and platelets in reversed passive reactions in the guinea-pig. Br J Exp Pathol. 1955 Jun;36(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by antiplatelet antibody in the presence and absence of complement. J Immunol. 1970 Apr;104(4):924–934. [PubMed] [Google Scholar]
- KRITZMAN J., MCCARTHY J. S. EFFECTS OF HETEROLOGOUS ANTILEUKOCYTE SERUM ON LEUKOCYTE METABOLISM. J Immunol. 1964 Feb;92:299–304. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. S., Craddock C. G., Jr, Campbell T. N. Antineutrophilic serum, its use in studies of white blood cell dynamics. J Lab Clin Med. 1967 Jan;69(1):88–101. [PubMed] [Google Scholar]
- MIESCHER P. The antigenic constituents of the neutrophilic leukocyte with special reference to the L. E. phenomenon. Vox Sang. 1957 Jul;2(3):145–158. doi: 10.1111/j.1423-0410.1957.tb03450.x. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOESCHLIN S., MEYER H., ISRAELS L. G., TARR-GLOOR E. Experimental agranulocytosis. Its production through leukocyte agglutination by antileukocytic serum. Acta Haematol. 1954 Feb;11(2):73–94. doi: 10.1159/000204538. [DOI] [PubMed] [Google Scholar]
- Martin W. J., Miller J. F. Cell to cell interaction in the immune response. IV. Site of action of antilymphocyte globulin. J Exp Med. 1968 Oct 1;128(4):855–874. doi: 10.1084/jem.128.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATT H. M., MALONEY M. A., JACKSON E. M. Recovery of blood neutrophils after acute peripheral depletion. Am J Physiol. 1957 Mar;188(3):585–592. doi: 10.1152/ajplegacy.1957.188.3.585. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- STUART A. E. Properties of antileucocytic serum. Br J Exp Pathol. 1962 Dec;43:614–620. [PMC free article] [PubMed] [Google Scholar]
- Shigeno N., Hämmerling U., Arpels C., Boyse E. A., Old L. J. Preparation of lymphocyte-specific antibody from anti-lymphocyte serum. Lancet. 1968 Aug 10;2(7563):320–323. doi: 10.1016/s0140-6736(68)90530-8. [DOI] [PubMed] [Google Scholar]
- Taub R. N., Lance E. M. Histopathological effects in mice of heterologous antilymphocyte serum. J Exp Med. 1968 Dec 1;128(6):1281–1307. doi: 10.1084/jem.128.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Willoughby D. A. Central and peripheral effects of anti-lymphocyte sera. Lancet. 1967 Feb 4;1(7484):249–251. doi: 10.1016/s0140-6736(67)91307-4. [DOI] [PubMed] [Google Scholar]
- Tyler R. W., Everett N. B., Schwarz M. R. Effect of antilymphocytic serum on rat lymphocytes. J Immunol. 1969 Jan;102(1):179–186. [PubMed] [Google Scholar]
- Unanue E. R. Properties and some uses of anti-macrophage antibodies. Nature. 1968 Apr 6;218(5136):36–38. doi: 10.1038/218036a0. [DOI] [PubMed] [Google Scholar]
- Valentine M. D., Bloch K. J., Austen K. F. Mechanisms of immunologic injury of rat peritoneal mast cells. 3. Cytotoxic histamine release. J Immunol. 1967 Jul;99(1):98–110. [PubMed] [Google Scholar]
- WAKSMAN B. H., ARBOUYS S., ARNASON B. G. The use of specific "lymphocyte" antisera to inhibit hypersensitive reactions of the "delayed" type. J Exp Med. 1961 Dec 1;114:997–1022. doi: 10.1084/jem.114.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]