Abstract
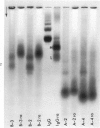
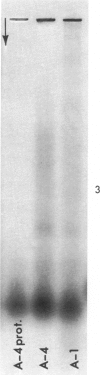
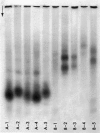
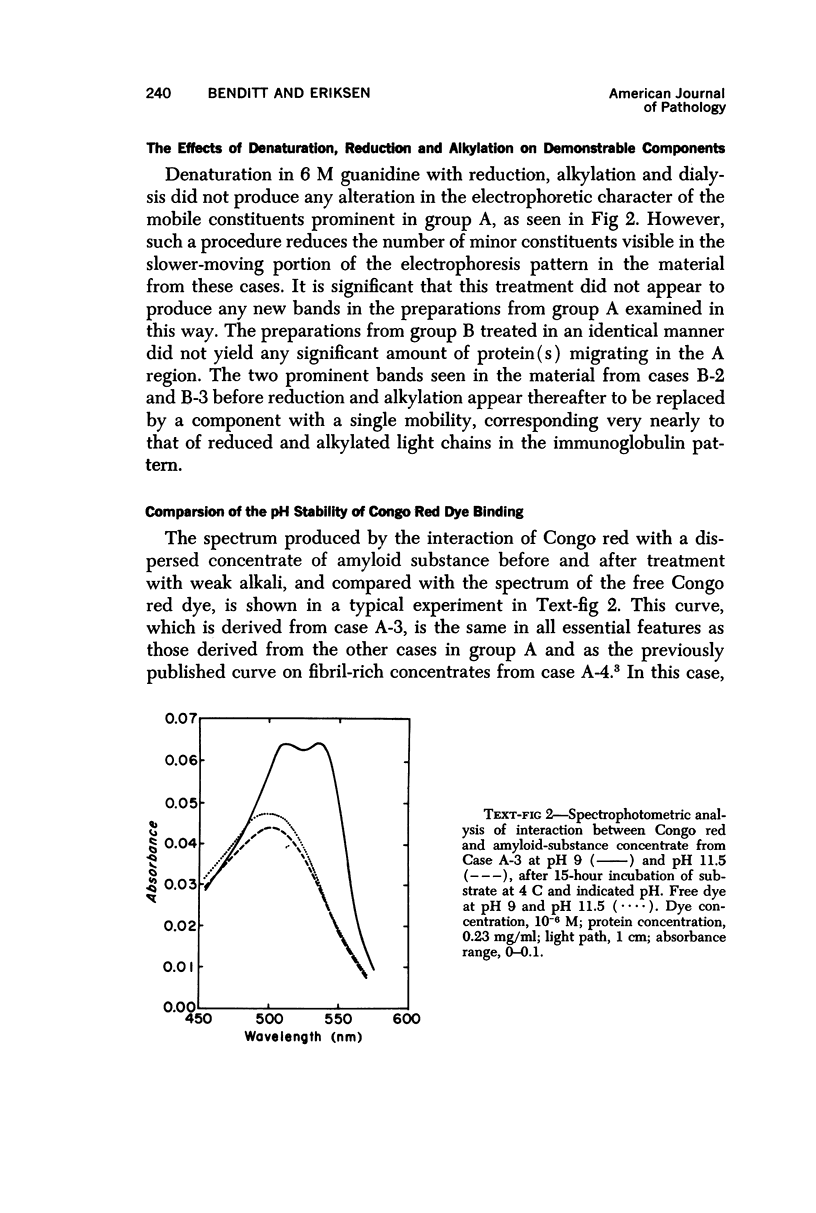
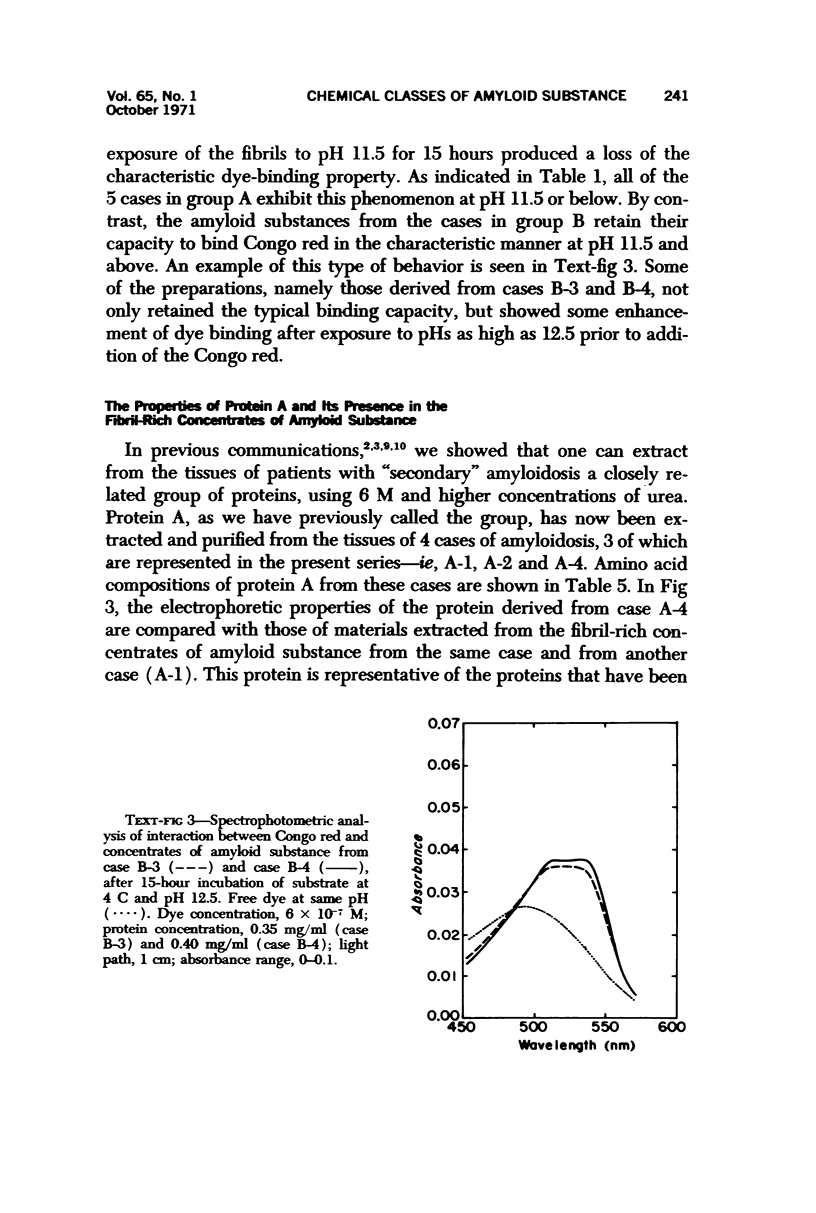
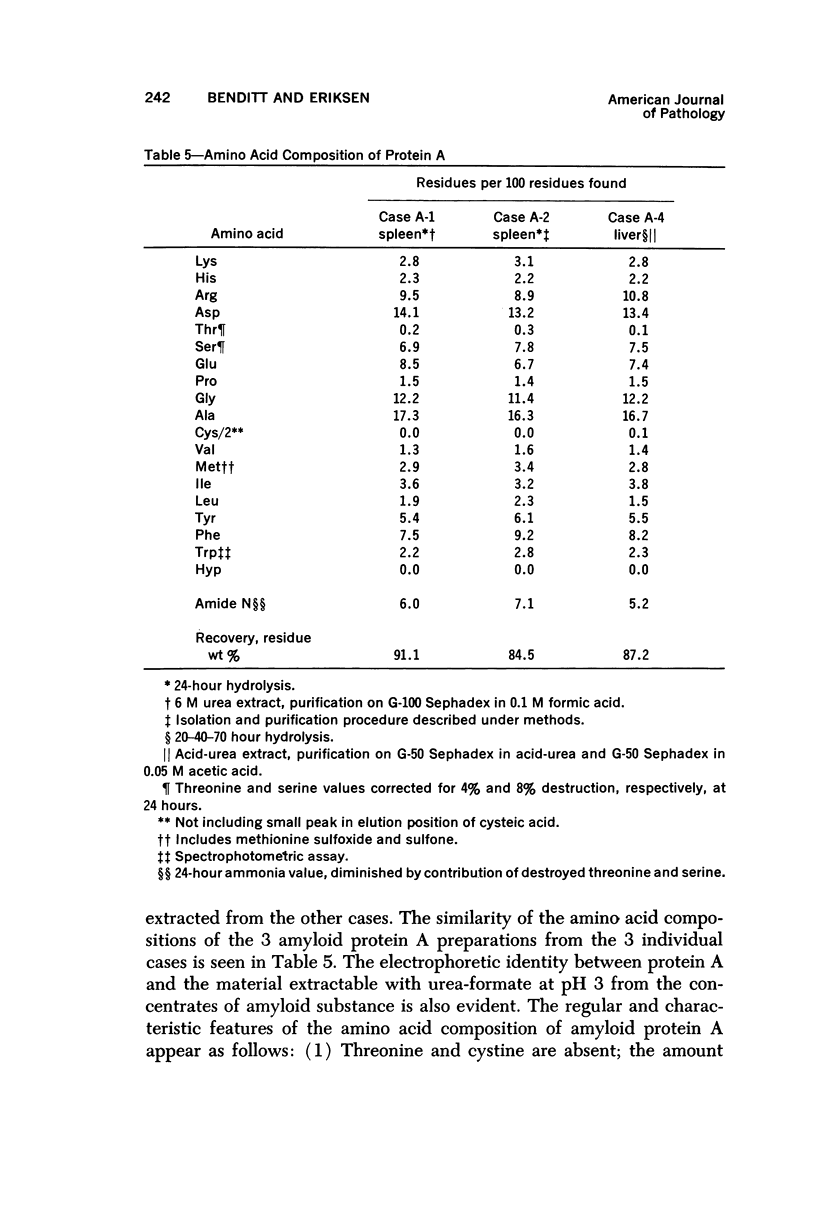
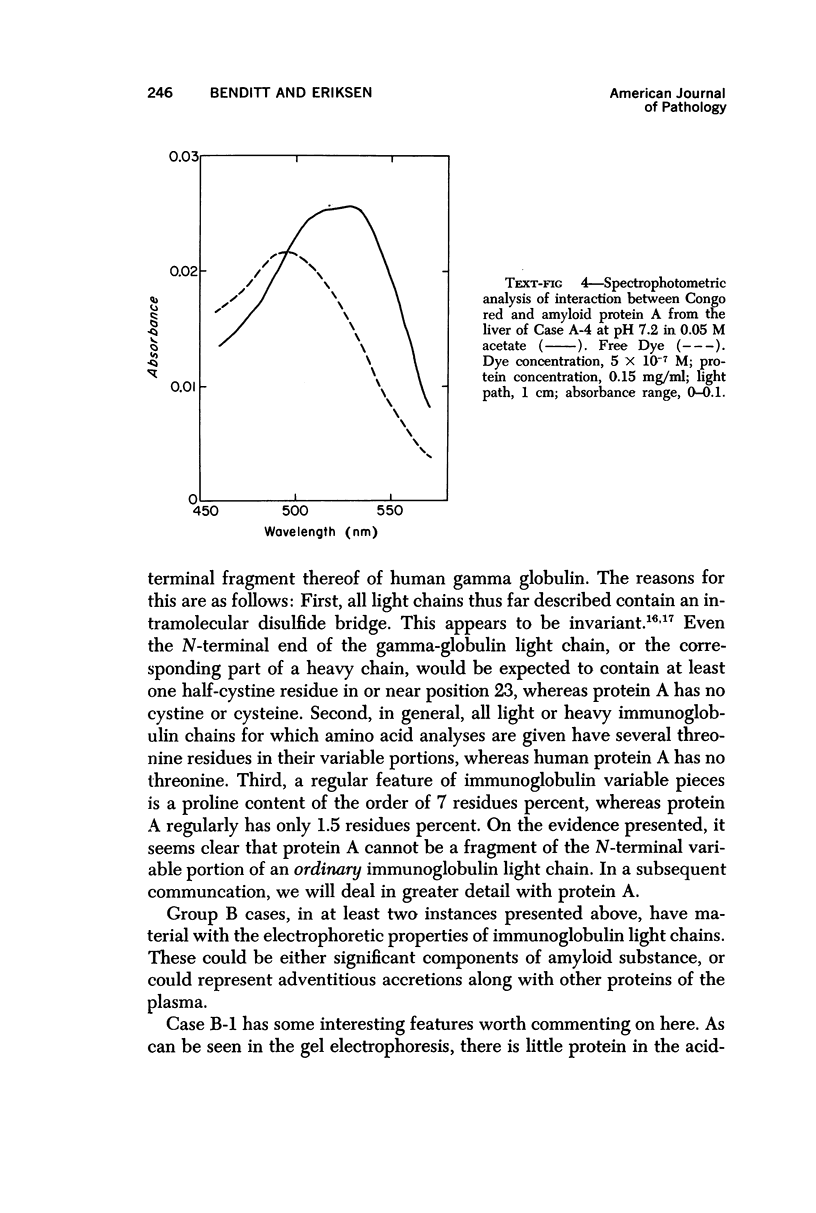
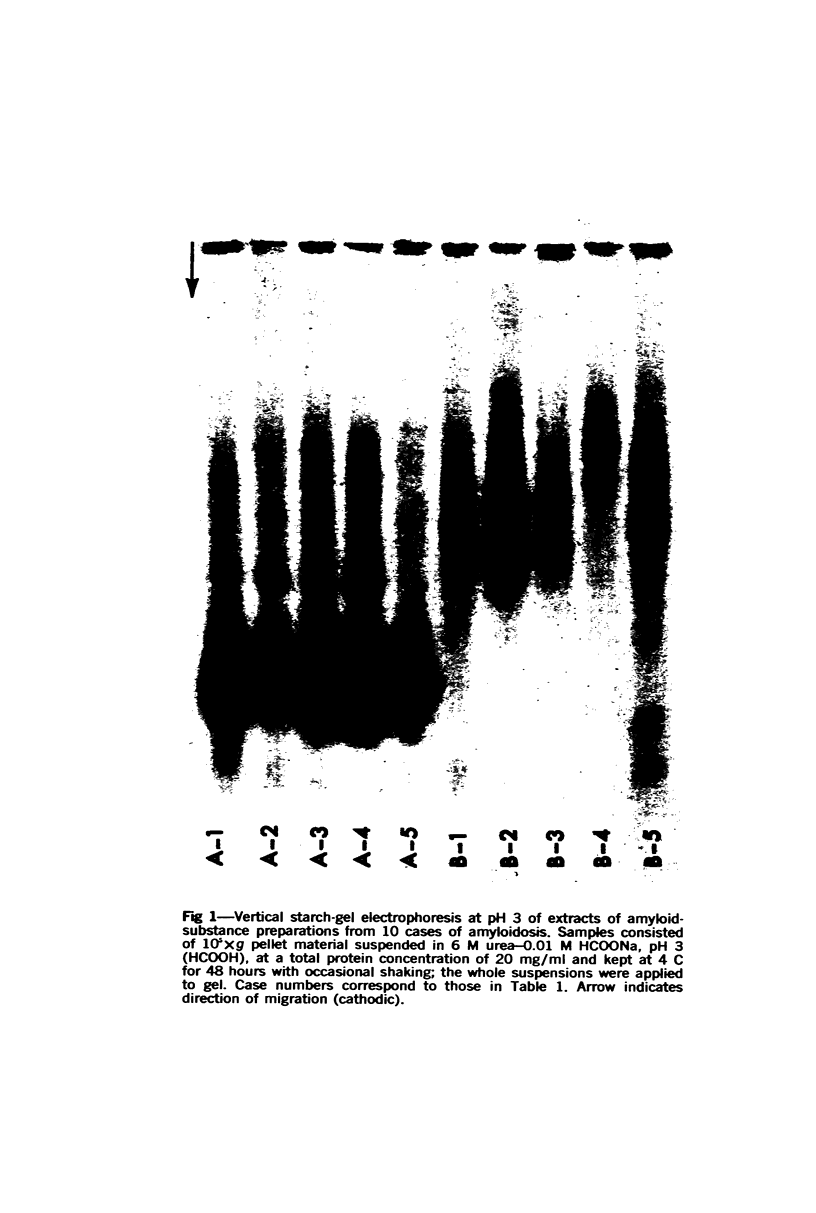
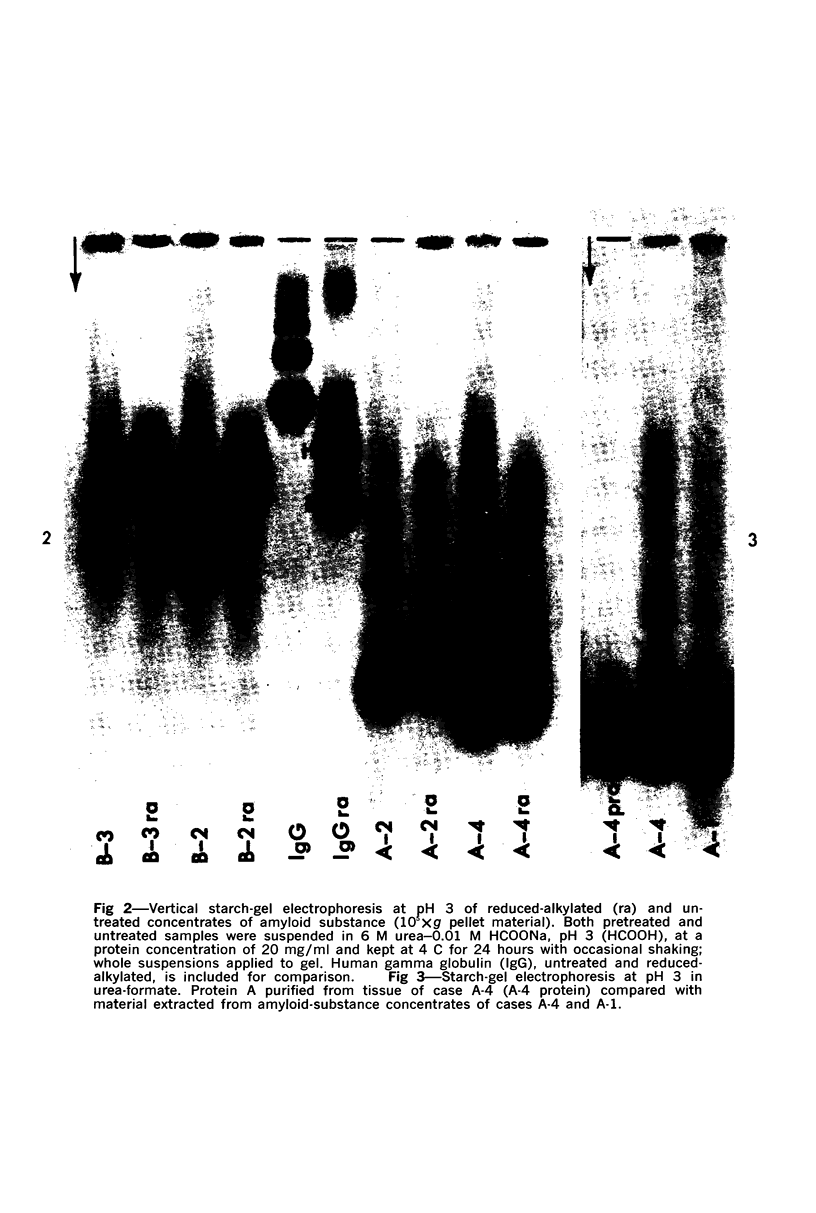
Concentrates of amyloid substance derived from organs of 10 human patients representing a variety of clinical entities were characterized according to their amino acid compositions, their electrophoretic constituents mobile in urea-starch gel at pH 3 and their stability with respect to the binding of Congo red in the pH interval 9-12.5. The analyses revealed the existence of two major classes of amyloid substance. Material from one class, embracing those cases that had a chronic inflammatory disease as the principal associated process (type A amyloidosis), had a similar amino acid composition and was distinguished by the presence in the amyloid substance of a large amount of an electrophoretically well-defined group of low-molecular-weight proteins with an unusual amino acid composition (amyloid protein A); type A amyloid substance lost the typical binding of Congo red dye at pH 11.5 or lower. Concentrates of amyloid substance in the other class (type B amyloidosis), represented by a case of multiple myeloma, 3 other cases of neoplastic disease and a case of primary cardiac amyloidosis, were distinguished by the presence of an electrophoretically more heterogeneous group of protein constituents with different mobilities and with apparently higher molecular weights than that of protein A, by an amino acid composition that is clearly different from that of the first class, and by retention of the typical Congo red-binding property at pH 12 or higher. The major constituent, protein A, of amyloid substances of the first class is clearly different from ordinary fragments of immunoglobulins in size, electrophoretic mobility, and amino acid composition.
Full text
PDF



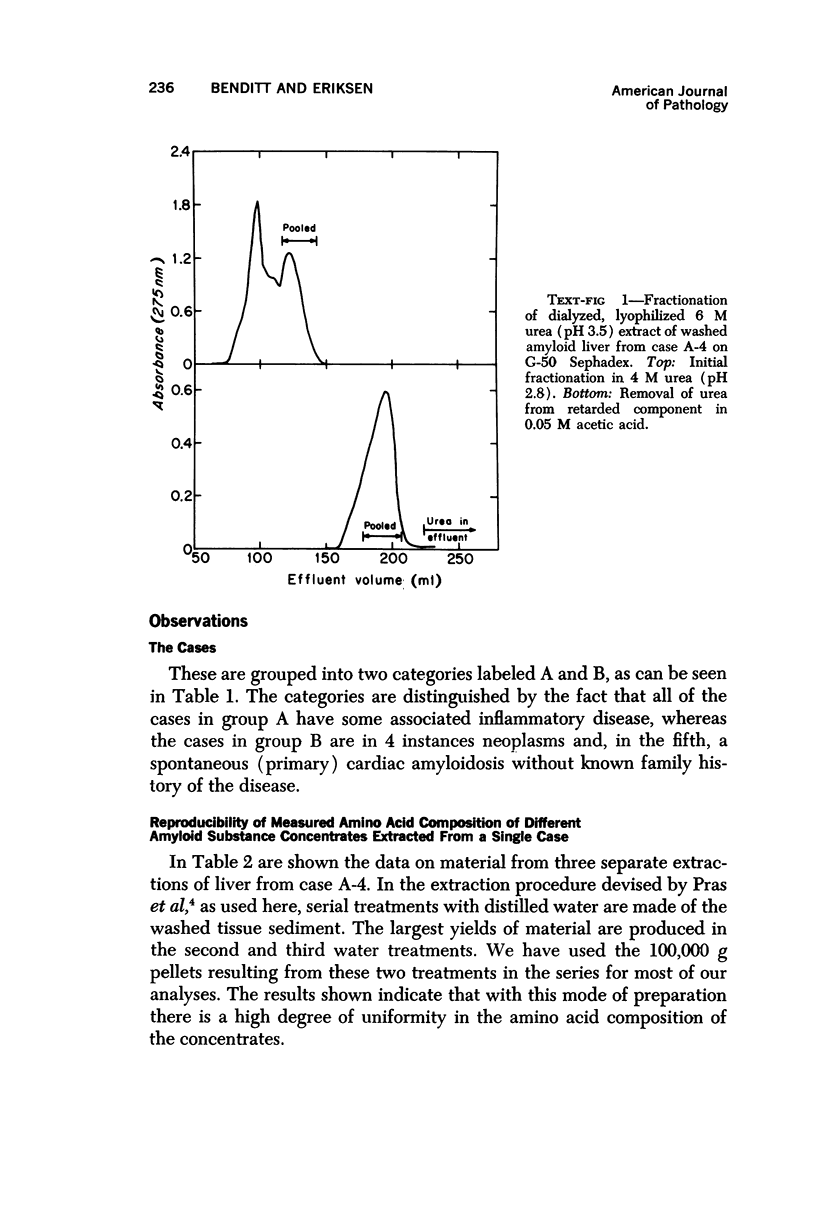
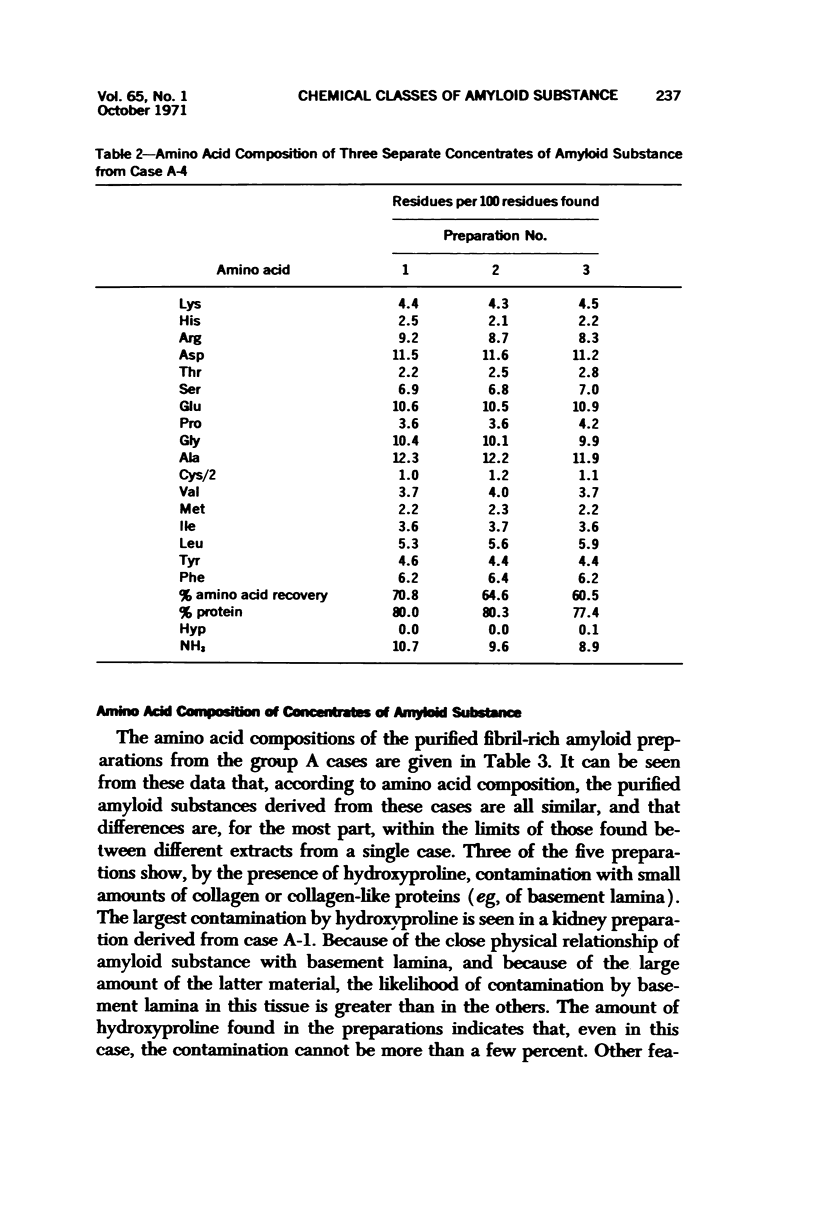
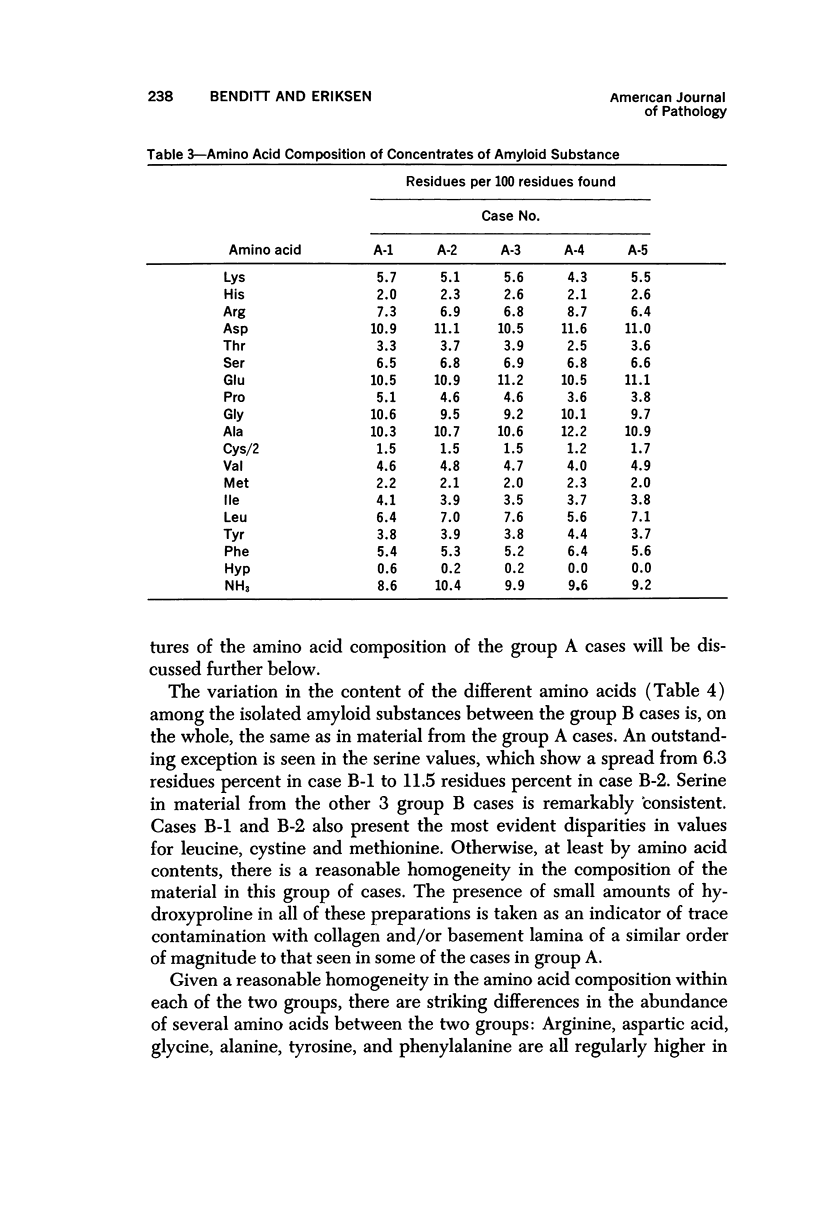
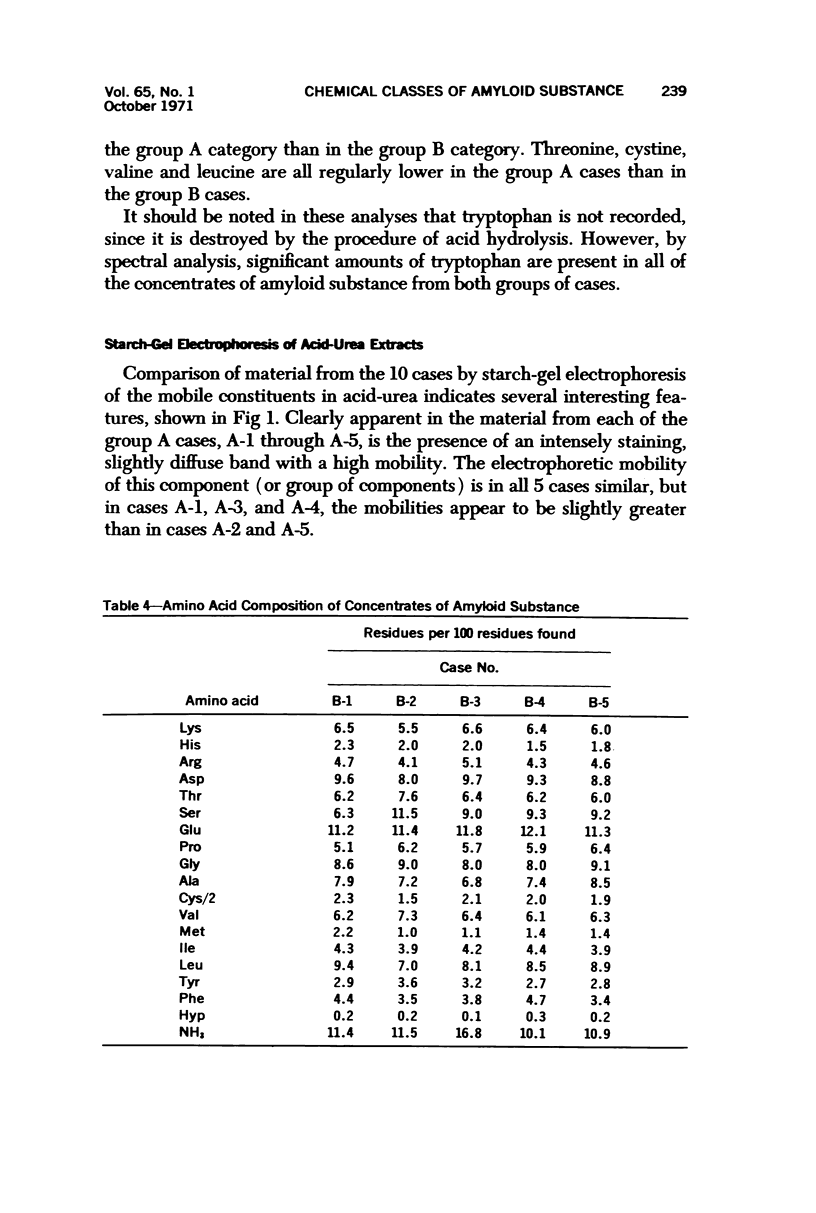







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDITT E. P., ERIKSEN N. AMYLOID. II. STARCH GEL ELECTROPHORETIC ANALYSIS OF SOME PROTEINS EXTRACTED FROM AMYLOID. Arch Pathol. 1964 Oct;78:325–330. [PubMed] [Google Scholar]
- BENDITT E. P., LAGUNOFF D., ERIKSEN N., ISERI O. A. Amyloid. Extraction and preliminary characterization of some proteins. Arch Pathol. 1962 Oct;74:323–330. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid. 3. A protein related to the subunit structure of human amyloid fibrils. Proc Natl Acad Sci U S A. 1966 Feb;55(2):308–316. doi: 10.1073/pnas.55.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Berglund C. Congo red dichroism with dispersed amyloid fibrils, an extrinsic cotton effect. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1044–1051. doi: 10.1073/pnas.66.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar L., Cohen A. S., Skinner M. M. Characterization of the amyloid fibril as a cross-beta protein. Proc Soc Exp Biol Med. 1969 Sep;131(4):1373–1375. doi: 10.3181/00379727-131-34110. [DOI] [PubMed] [Google Scholar]
- Cohen A. S. Amyloidosis. N Engl J Med. 1967 Sep 7;277(10):522–contd. doi: 10.1056/NEJM196709072771006. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Harada M., Isersky C., Cuatrecasas P., Page D., Bladen H. A., Eanes E. D., Keiser H. R., Glenner G. G. Human amyloid protein: chemical variability and homogeneity. J Histochem Cytochem. 1971 Jan;19(1):1–15. doi: 10.1177/19.1.1. [DOI] [PubMed] [Google Scholar]
- Hunter G. Colour Standards for use in the Determination of Iminazoles. Biochem J. 1925;19(1):42–46. doi: 10.1042/bj0190042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Jones D., Hansen R. An accelerated step-gradient procedure for the automatic analysis of collagen hydrolyzates. Anal Biochem. 1970 Oct;37(2):293–297. doi: 10.1016/0003-2697(70)90051-5. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. N., Hartmann W. H. Familial amyloid-producing medullary thyroid carcinoma and pheochromocytoma. A distinct genetic entity. Ann Intern Med. 1965 Dec;63(6):1027–1039. doi: 10.7326/0003-4819-63-6-1027. [DOI] [PubMed] [Google Scholar]