Abstract
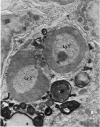
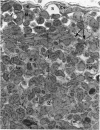
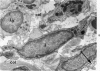
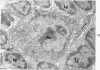
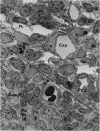
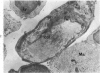
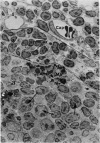
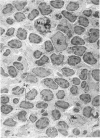
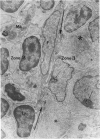
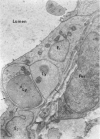
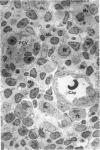
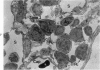
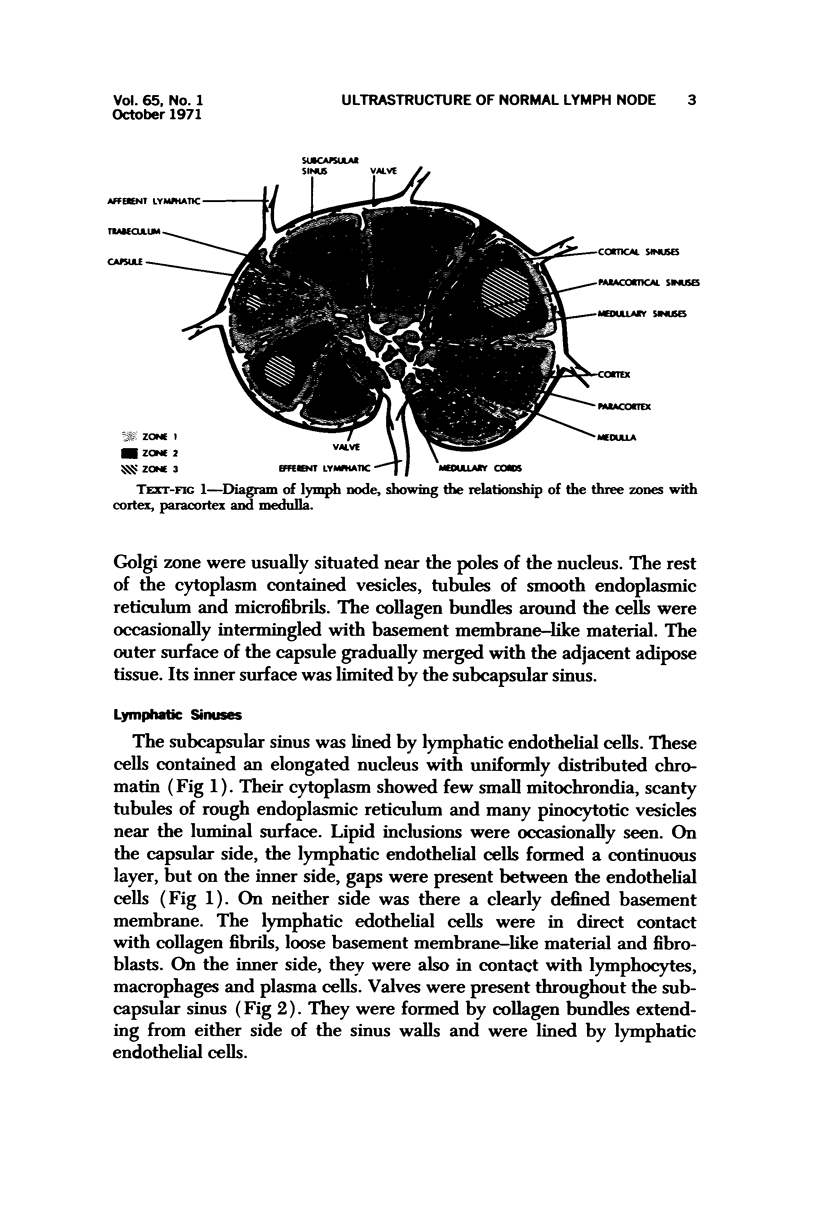
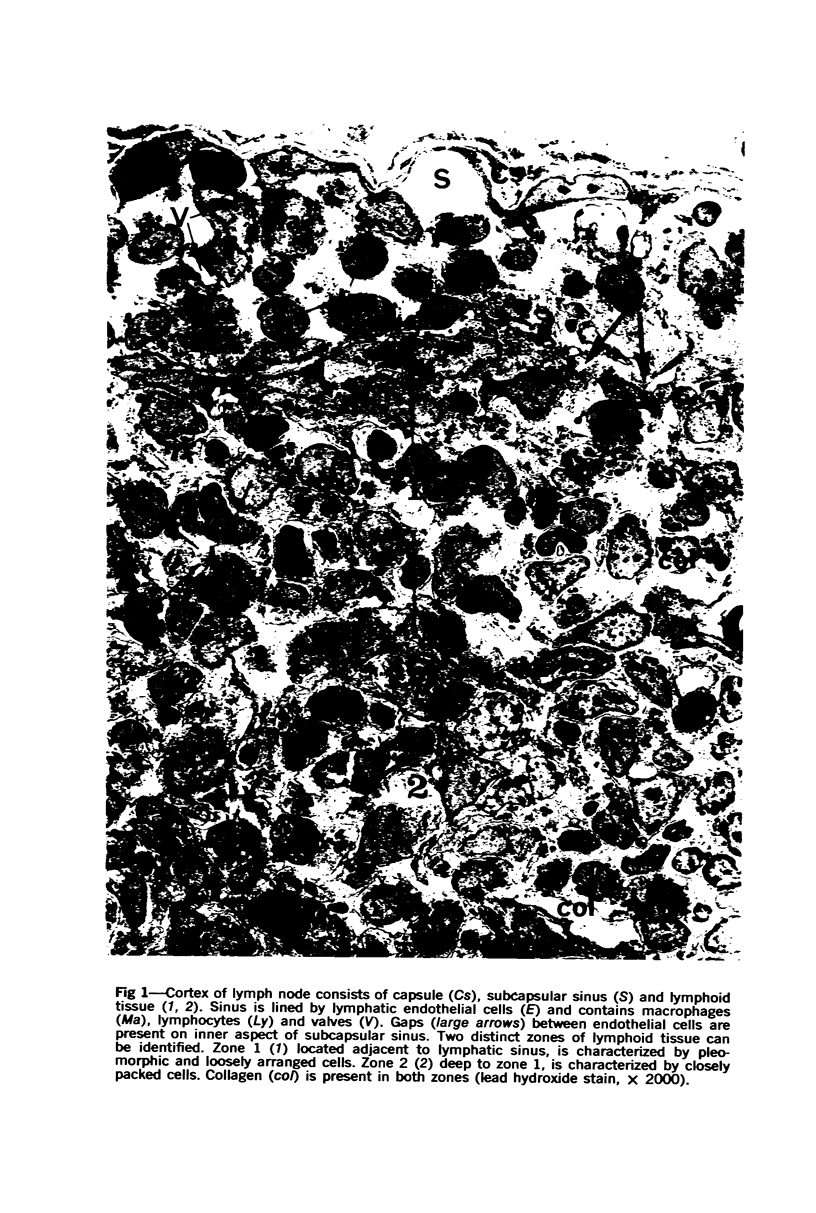
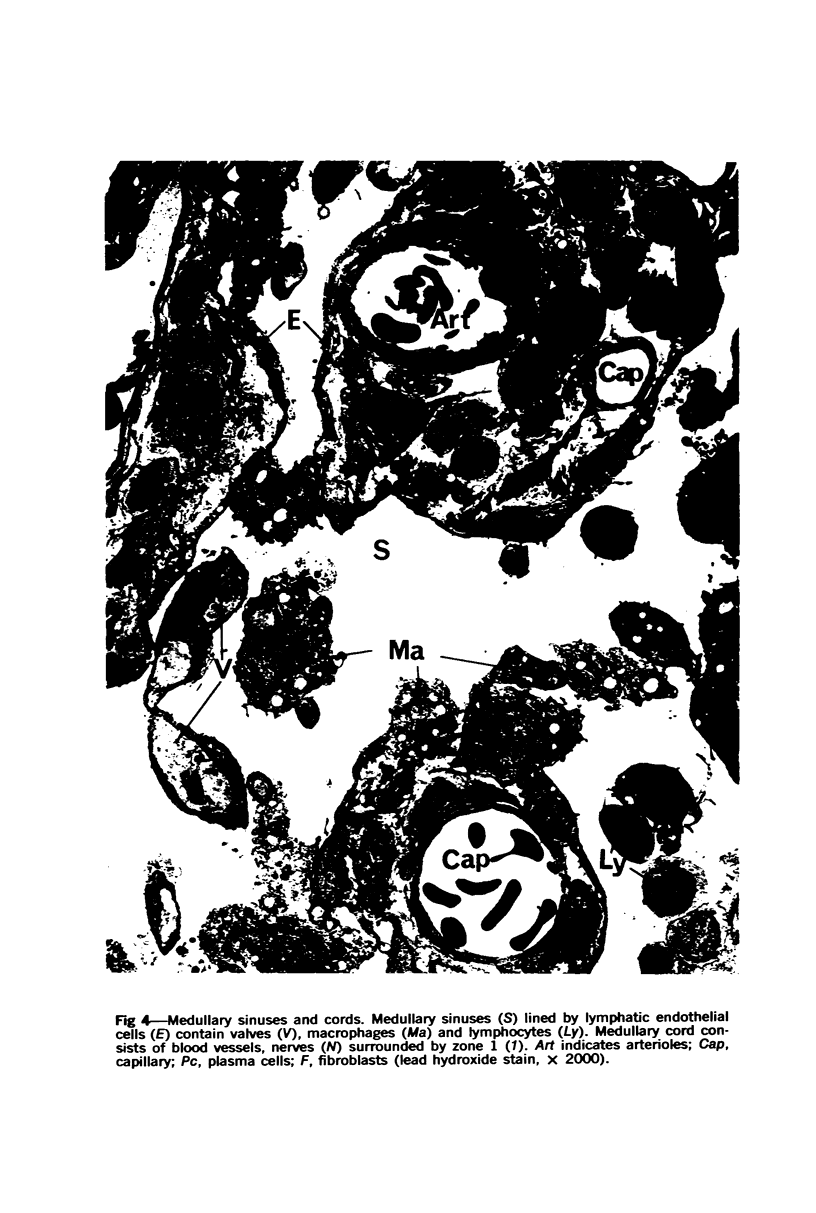
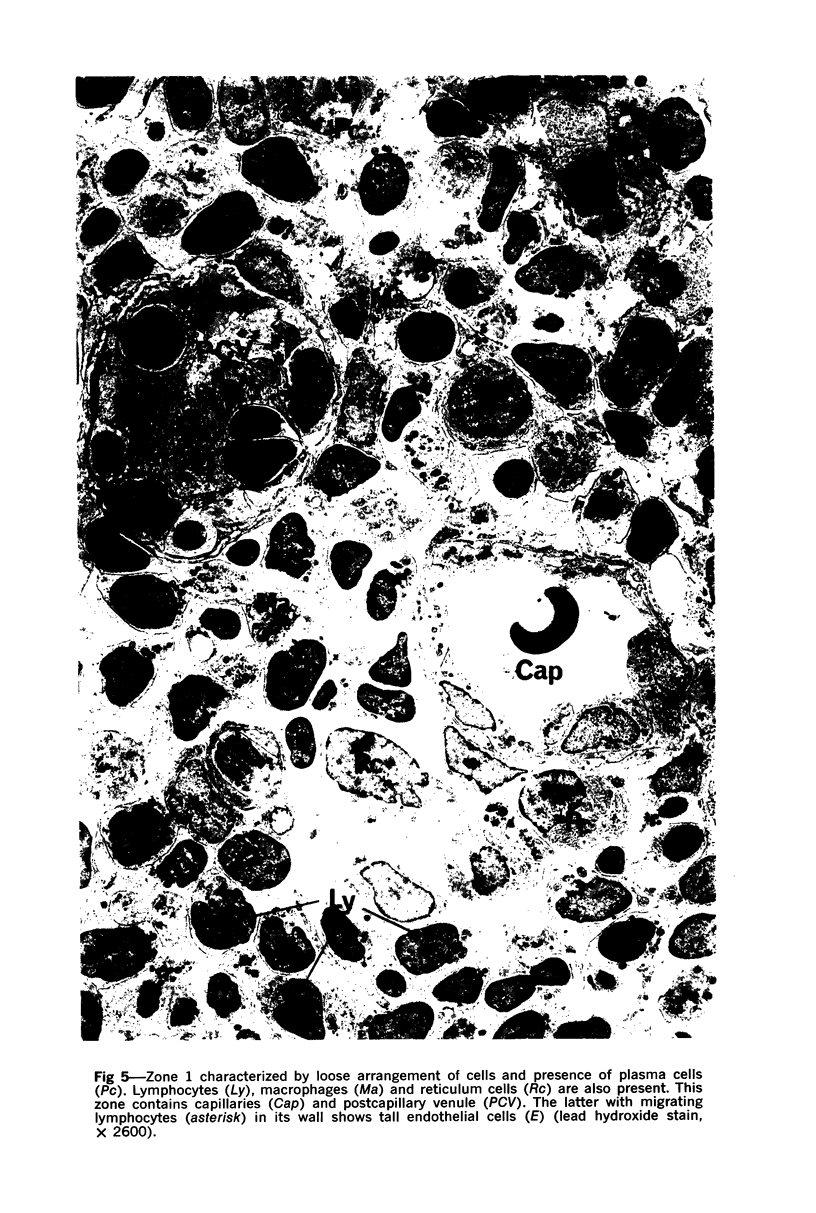
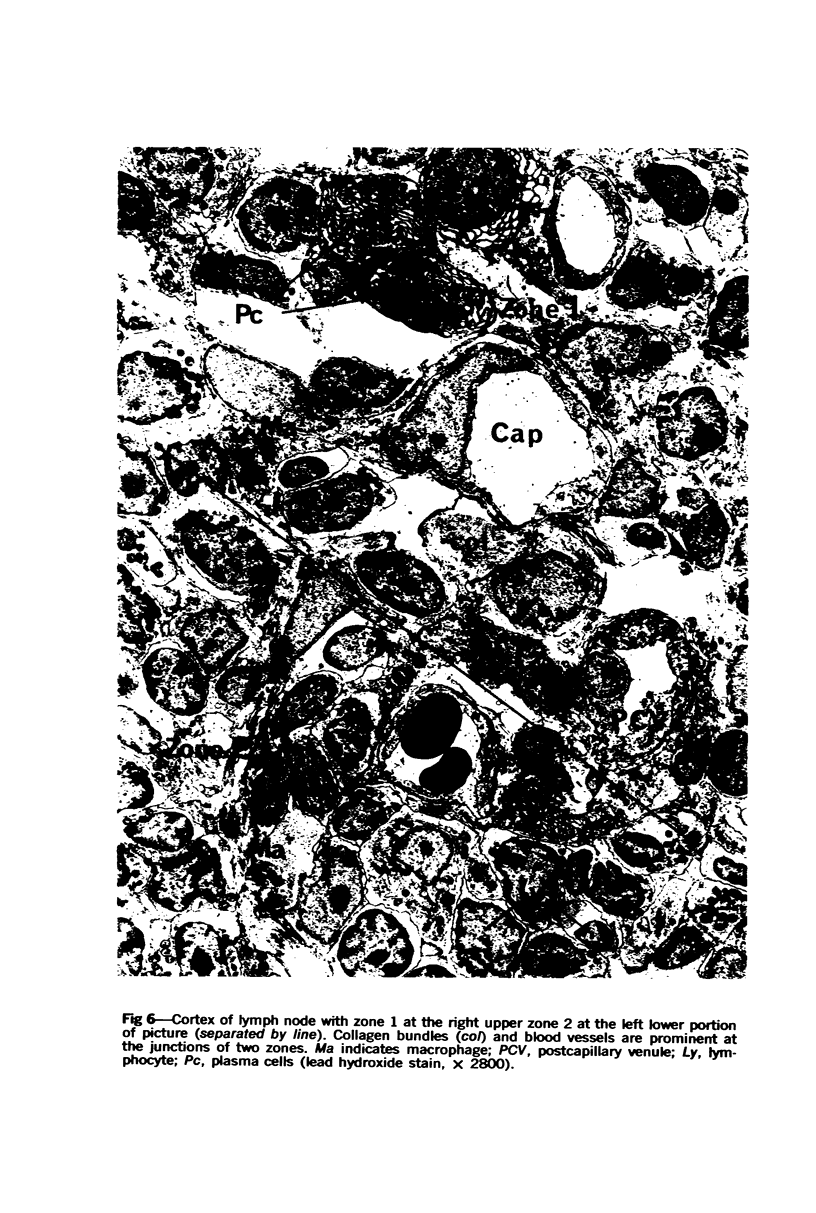
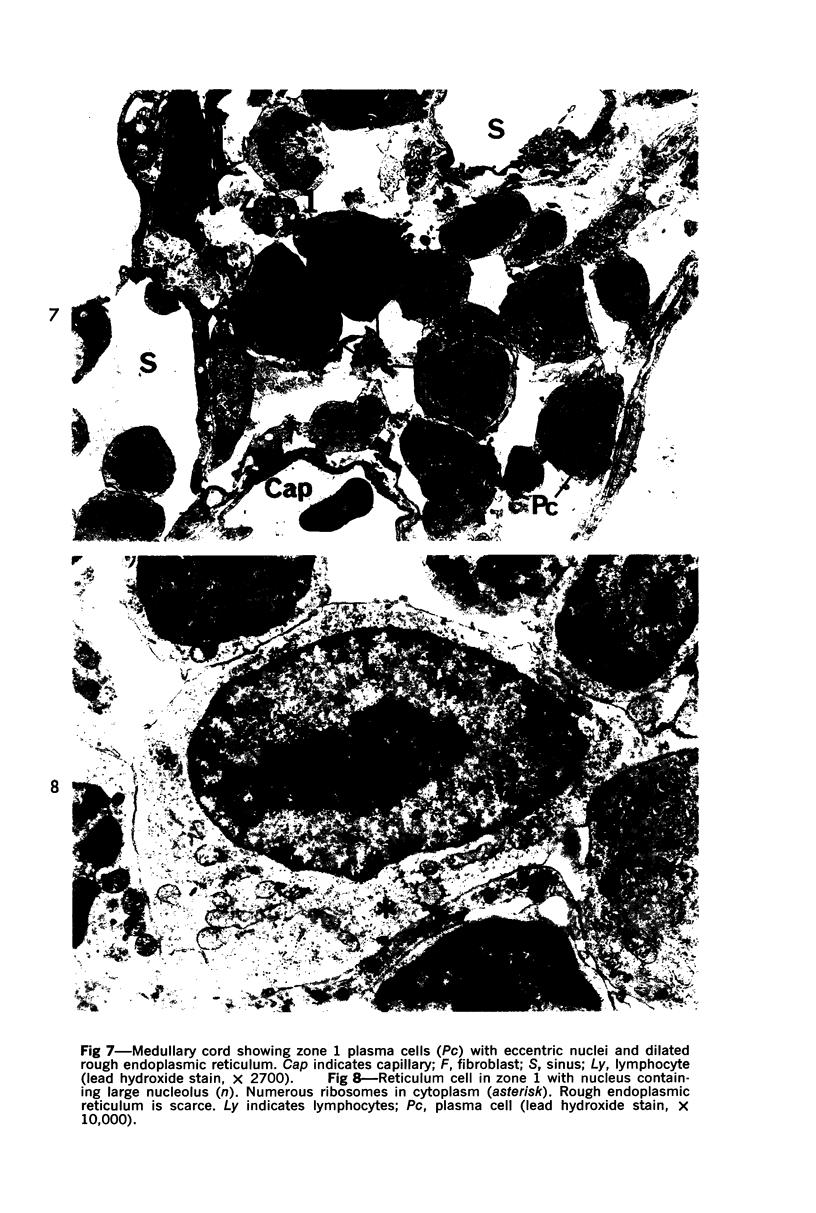
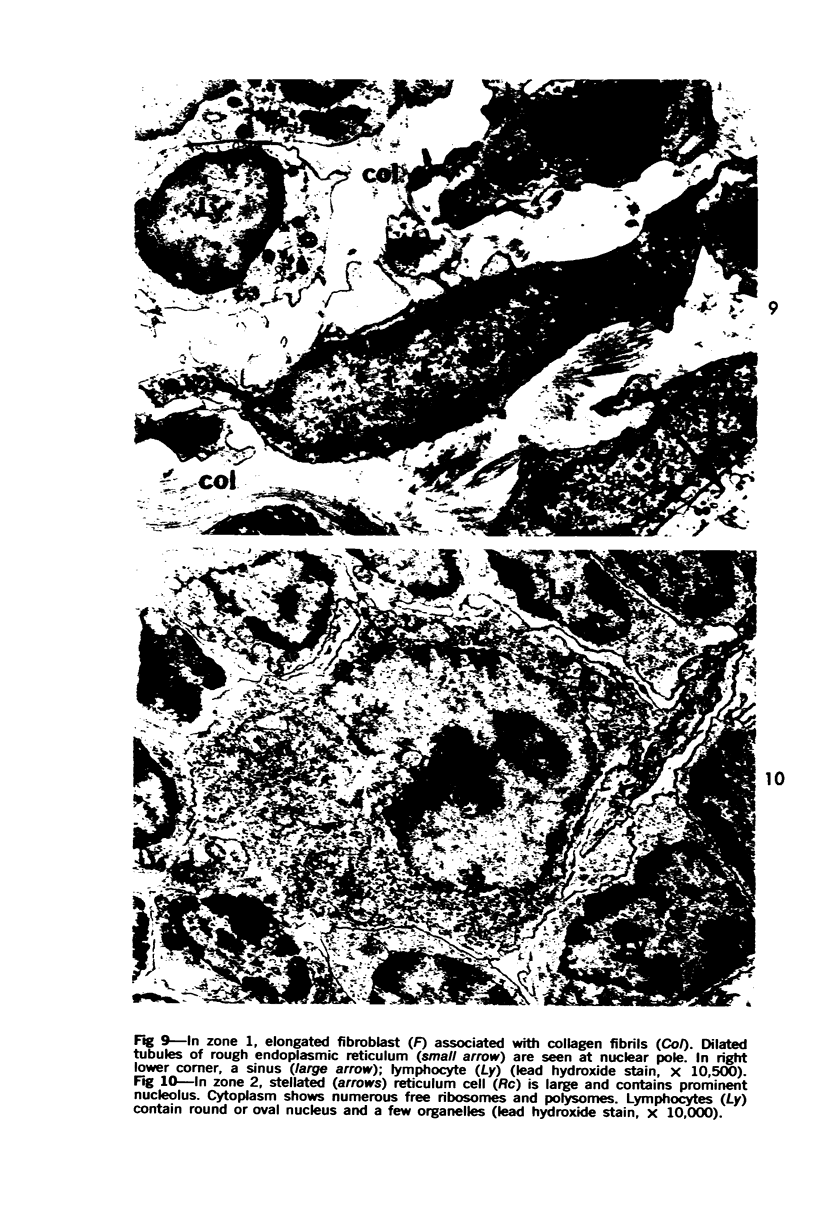
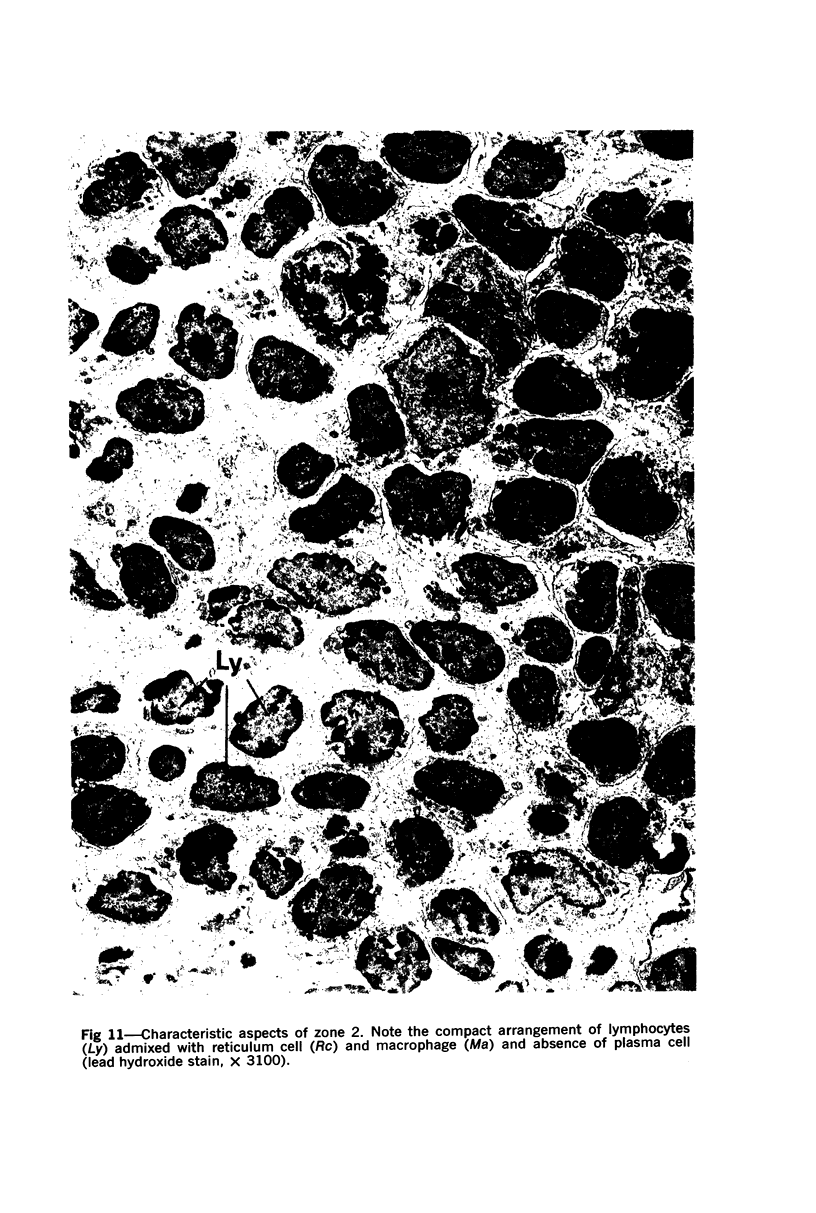
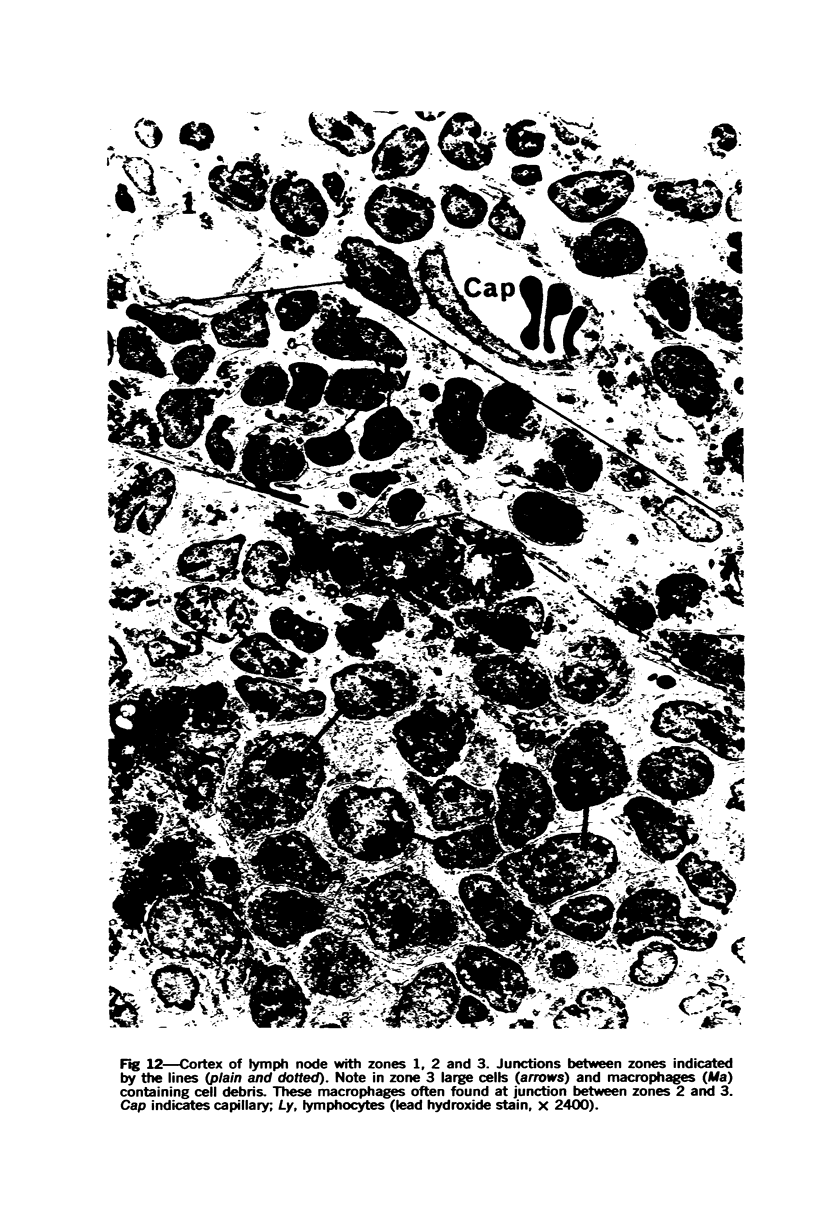
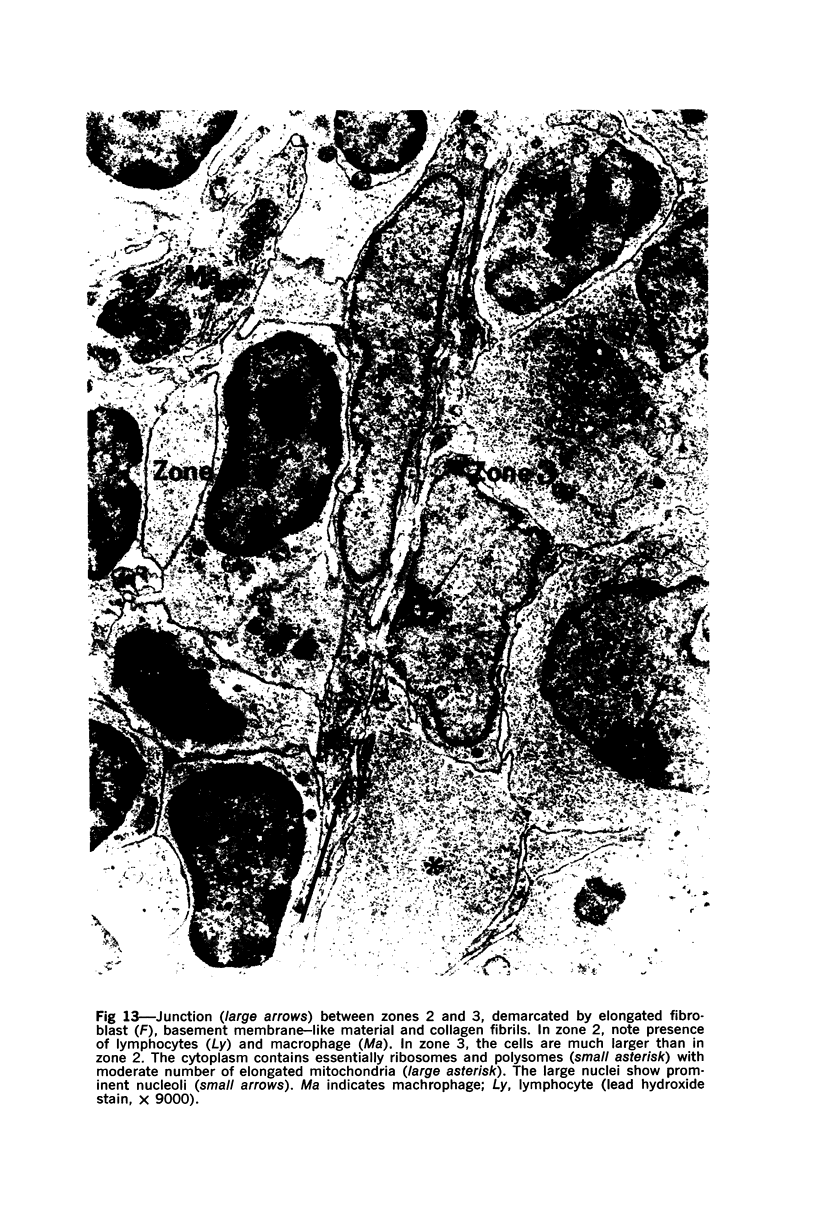
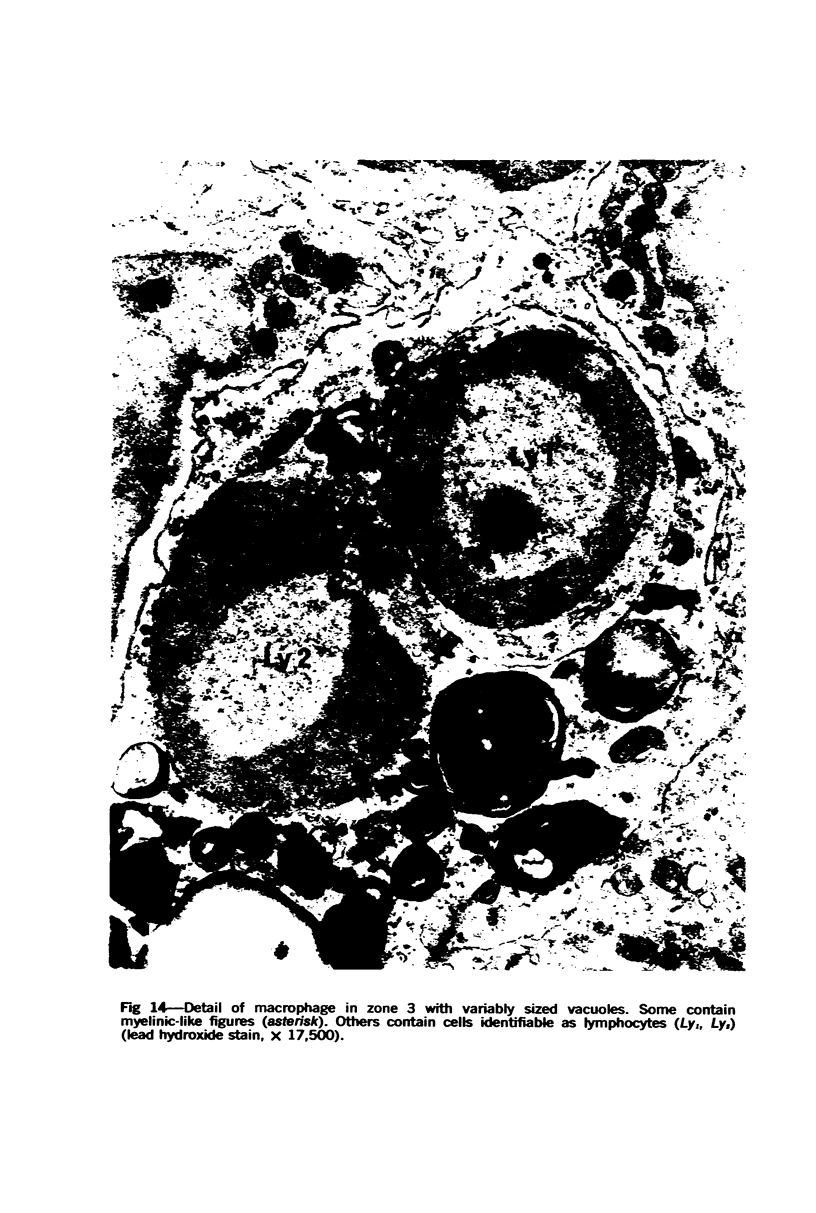
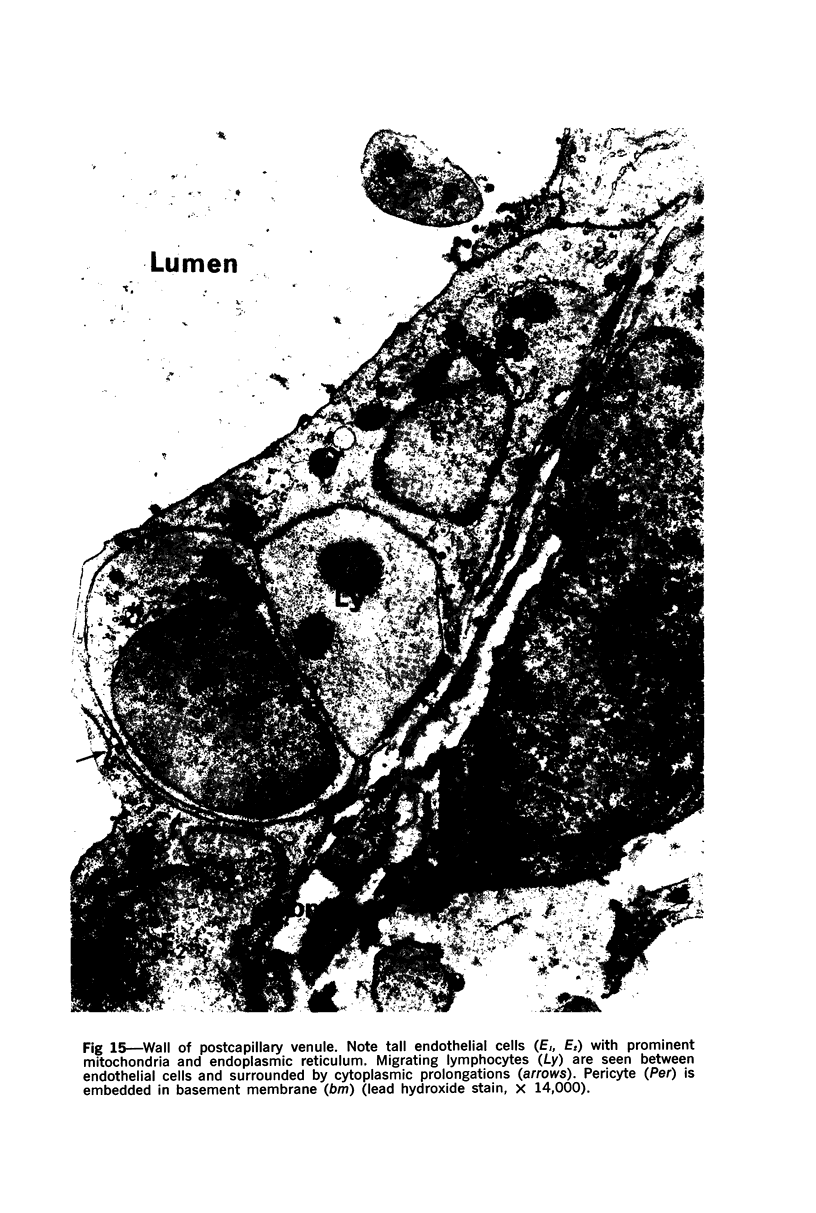
The “normal” lymph node has been studied by electron microscopy. The lymphoid tissue can be divided into three distinct zones. Zone 1 consists of loosely arranged cells surrounding the lymphatic sinuses and blood vessels. This is the only zone in which plasma cells are present. Zone 2 is surrounded by zone 1 and consists of compactly arranged cells in which lymphocytes predominate. Zone 3 (germinal center) appears only after antigenic stimulation. It is characterized by large, ribosome-rich cells and macrophages containing phagocytosed lymphocytes. These zones are arranged with their longest diameters pointing towards the hilus. Zone 1 is the longest and extends across the cortex, paracortex and medulla. Zone 2 spans across cortex and paracortex. Zone 3 usually is confined to the cortex. Our preliminary studies indicate that zone 1 is mainly bursal dependent, zone 2 is mainly thymic dependent and zone 3 is bursal dependent.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler F. L., Fishman M., Dray S. Antibody formation initiated in vitro. 3. Antibody formation and allotypic specificity directed by ribonucleic acid from peritoneal exudate cells. J Immunol. 1966 Oct;97(4):554–558. [PubMed] [Google Scholar]
- BERNHARD W., HAGUENAU F., LEPLUS R. Coupes ultrafines d'éléments sanguins et de ganglions lymphatiques étudiées au microscope électronique. Rev Hematol. 1955;10(2):267-82; discussion, 324-44. [PubMed] [Google Scholar]
- BRAUNSTEINER H., FELLINGER K., PAKESCH F. Demonstration of a cytoplasmic structure in plasma cells. Blood. 1953 Oct;8(10):916–922. [PubMed] [Google Scholar]
- Burke J. S., Simon G. T. Electron microscopy of the spleen. I. Anatomy and microcirculation. Am J Pathol. 1970 Jan;58(1):127–155. [PMC free article] [PubMed] [Google Scholar]
- Burke J. S., Simon G. T. Electron microscopy of the spleen. II. Phagocytosis of colloidal carbon. Am J Pathol. 1970 Jan;58(1):157–181. [PMC free article] [PubMed] [Google Scholar]
- CLARK S. L., Jr The reticulum of lymph nodes in mice studied with the electron microscope. Am J Anat. 1962 May;110:217–257. doi: 10.1002/aja.1001100303. [DOI] [PubMed] [Google Scholar]
- GALL E. A. The cytological identity and interrelation of mesenchymal cells of lymphoid tissue. Ann N Y Acad Sci. 1958 Sep 5;73(1):120–130. doi: 10.1111/j.1749-6632.1959.tb40796.x. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Givan K. F., Turnbull C., Jézéquel A. M. Pepsin digestion of virus particles in canine hepatitis using epon-embedded material. J Histochem Cytochem. 1967 Aug;15(11):688–694. doi: 10.1177/15.11.688. [DOI] [PubMed] [Google Scholar]
- HAN S. S. The ultrastructure of the mesenteric lymph node of the rat. Am J Anat. 1961 Sep;109:183–225. doi: 10.1002/aja.1001090208. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOE R. E. ELECTRON MICROSCOPIC APPEARANCE OF THE PARENCHYMA OF LYMPH NODES. Am J Anat. 1964 Mar;114:341–369. doi: 10.1002/aja.1001140211. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF LYMPHOID TISSUE. Exp Mol Pathol. 1964 Dec;90:546–568. doi: 10.1016/0014-4800(64)90034-6. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF THE LYMPHOID TISSUE DURING ANTIBODY FORMATION. Exp Mol Pathol. 1965 Apr;28:155–188. doi: 10.1016/0014-4800(65)90031-6. [DOI] [PubMed] [Google Scholar]
- Mori M., Ishi Y., Onoé T. Studies on the germinal center. I. Ultrastructural study of germinal centers in human lymph nodes with correspondence to the zonal differentiation. J Reticuloendothel Soc. 1969 Apr;6(2):140–157. [PubMed] [Google Scholar]
- Nopajaroonsri C., Simon G. T. Phagocytosis of colloidal carbon in a lymph node. Am J Pathol. 1971 Oct;65(1):25–42. [PMC free article] [PubMed] [Google Scholar]
- OORT J., TURK J. L. A HISTOLOGICAL AND AUTORADIOGRAPHIC STUDY OF LYMPH NODES DURING THE DEVELOPMENT OF CONTACT SENSITIVITY IN THE GUINEA-PIG. Br J Exp Pathol. 1965 Apr;46:147–154. [PMC free article] [PubMed] [Google Scholar]
- SCHOENBERG M. D., MUMAW V. R., MOORE R. D., WEISBERGER A. S. CYTOPLASMIC INTERACTION BETWEEN MACROPHAGES AND LYMPHOCYTIC CELLS IN ANTIBODY SYNTHESIS. Science. 1964 Feb 28;143(3609):964–965. doi: 10.1126/science.143.3609.964. [DOI] [PubMed] [Google Scholar]
- WEISS L. A study of the structure of splenic sinuses in man and in the albino rat with the light microscope and the electron microscope. J Biophys Biochem Cytol. 1957 Jul 25;3(4):599–610. doi: 10.1083/jcb.3.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]