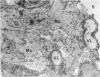Abstract
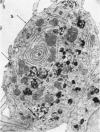
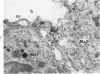
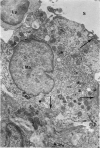
Colloidal carbon, injected intramuscularly, migrates rapidly and selectively to a corresponding lymph node by lymphatics, in which the carbon travels as free particles. In the lymph node, carbon particles are mainly phagocytosed and stored by macrophages, which exhibit morphologic changes in the plasma membrane and the tubules of endoplasmic reticulum. The subsequent migration of these cells results in wide distribution of carbon in the lymph node. Cytoplasmic changes also occur in sinusoidal macrophages, in which no carbon is seen. A possible relation of these macrophages to macrophage migration in lymph node is postulated. The lymphatic endothelial cells, like endothelial cells in any other organ, phagocytose only a small amount of carbon and only after functional overload of the macrophages.
Full text
PDF










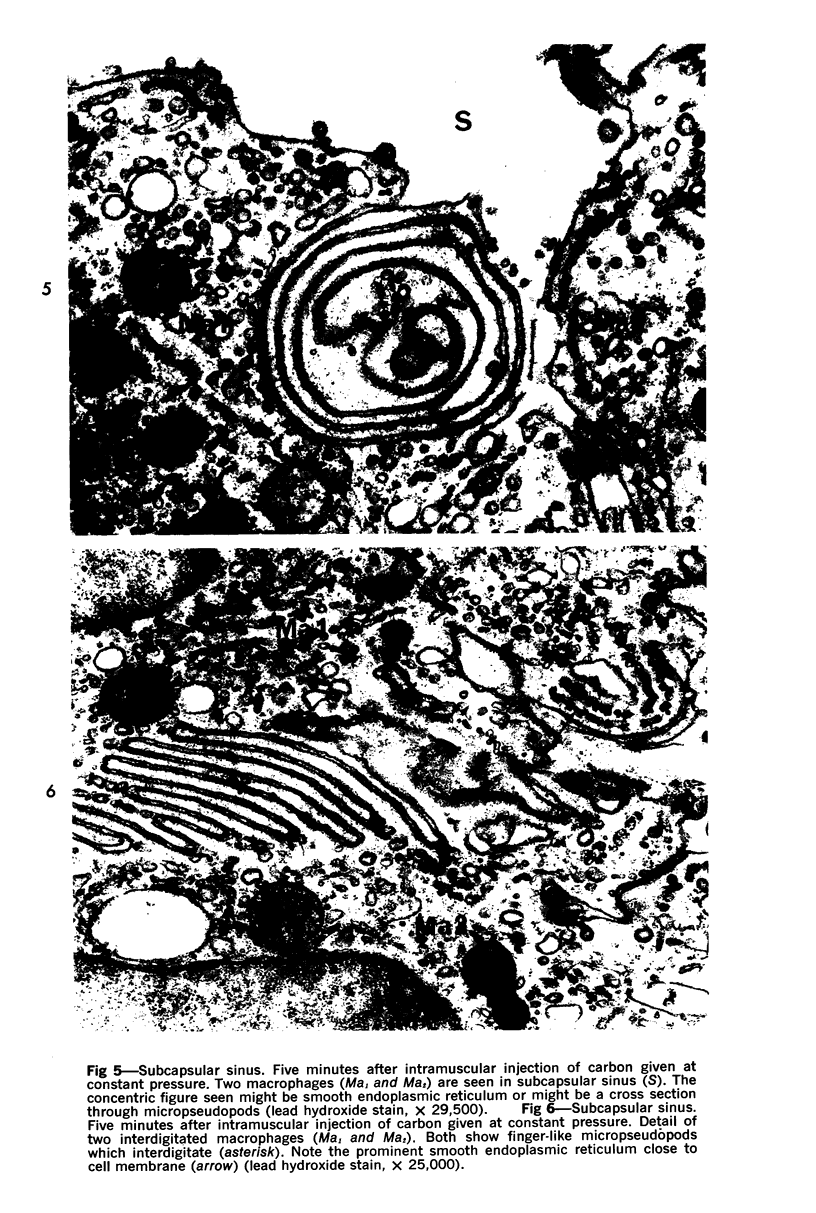
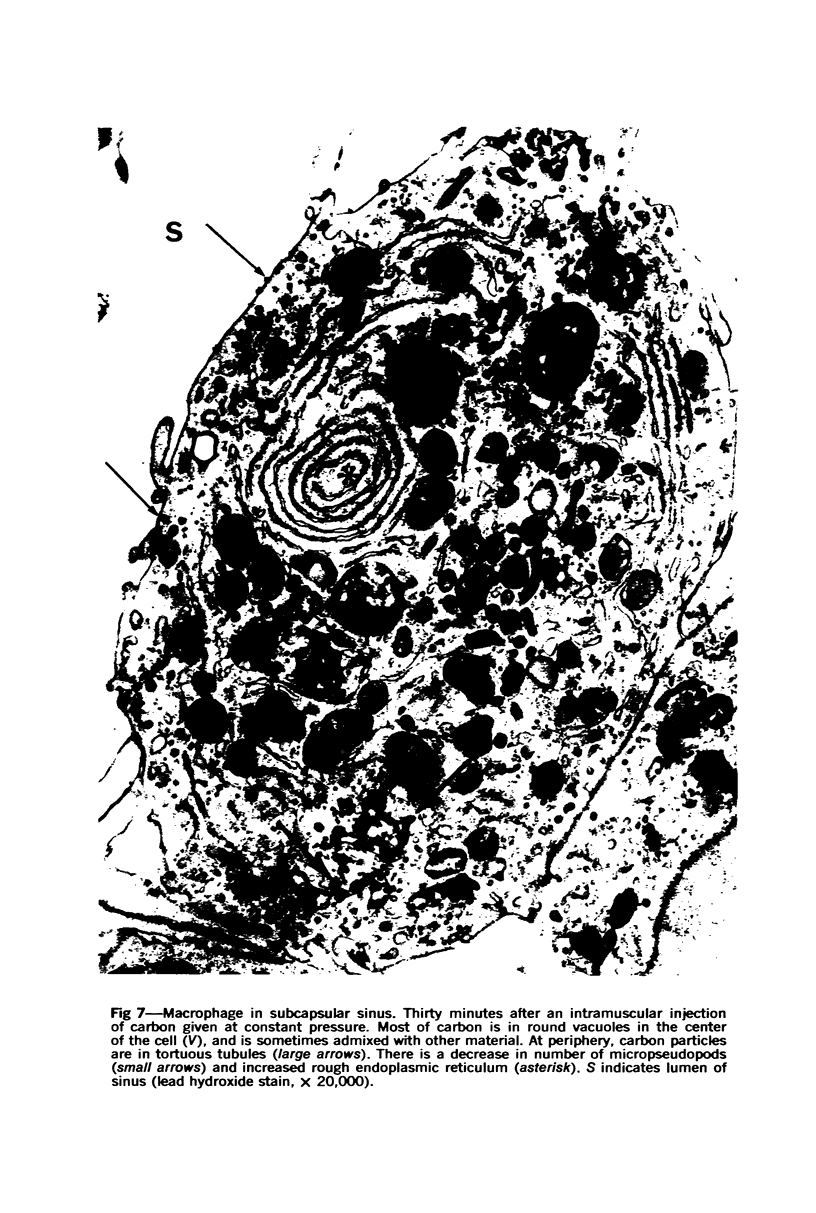
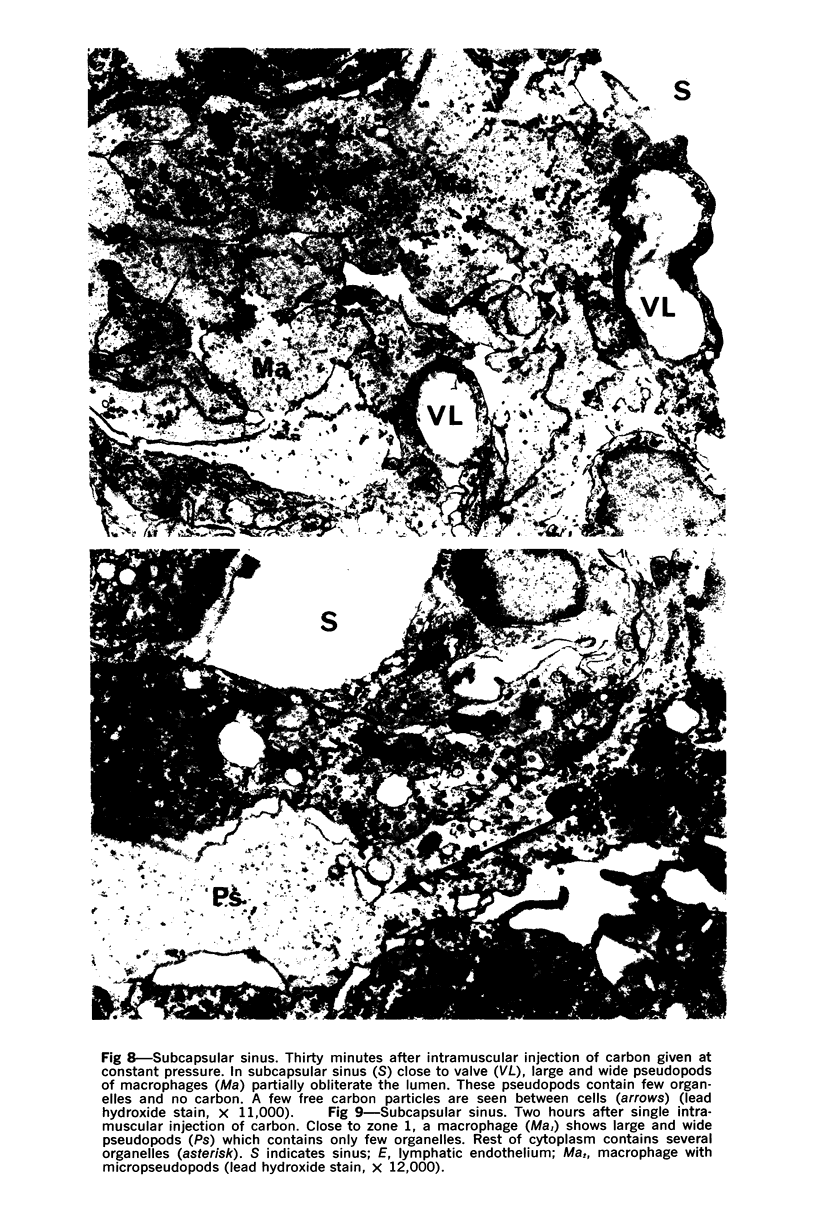
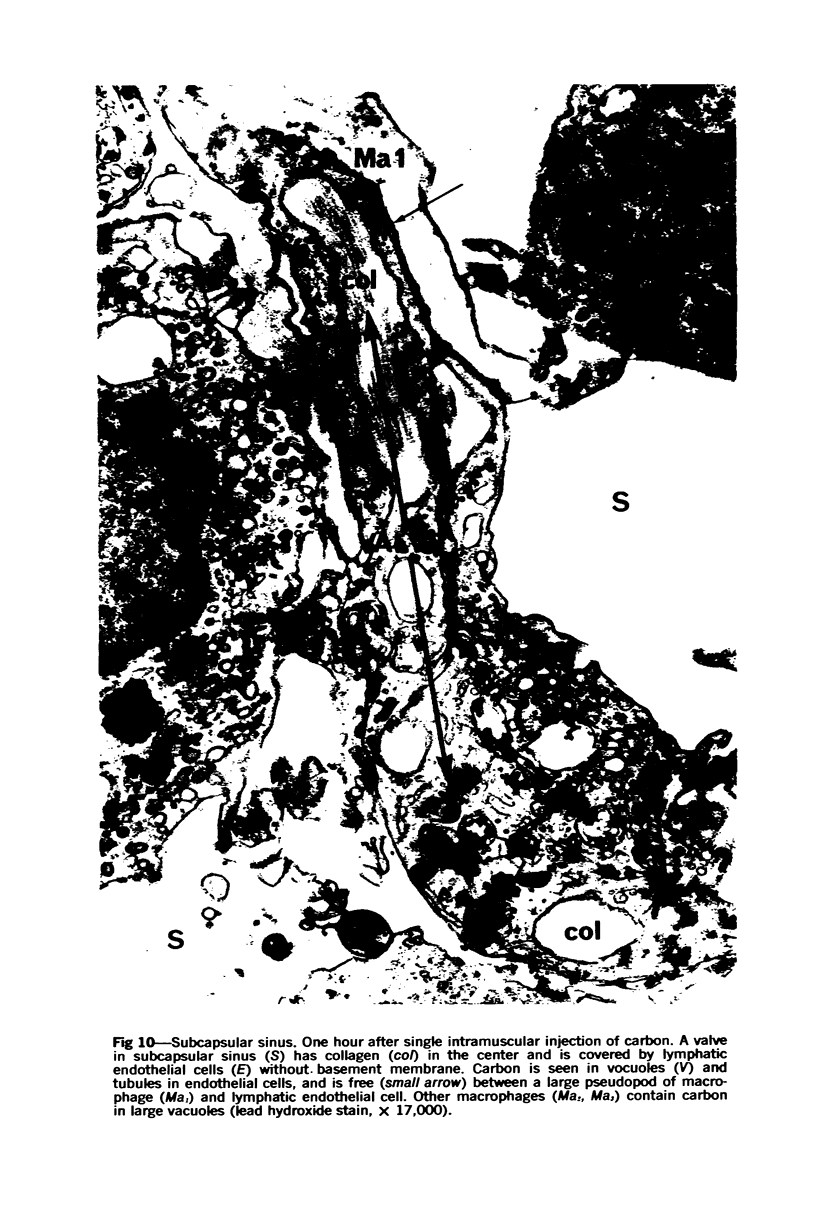
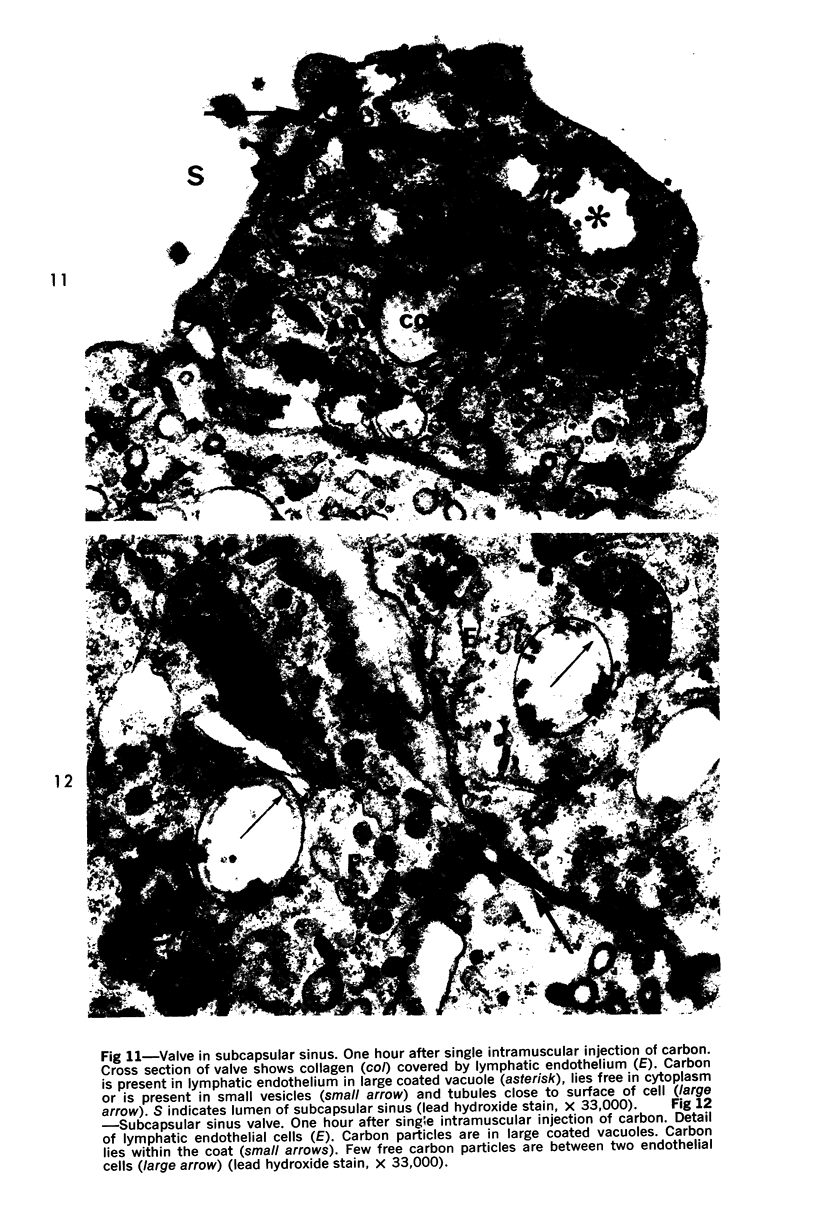
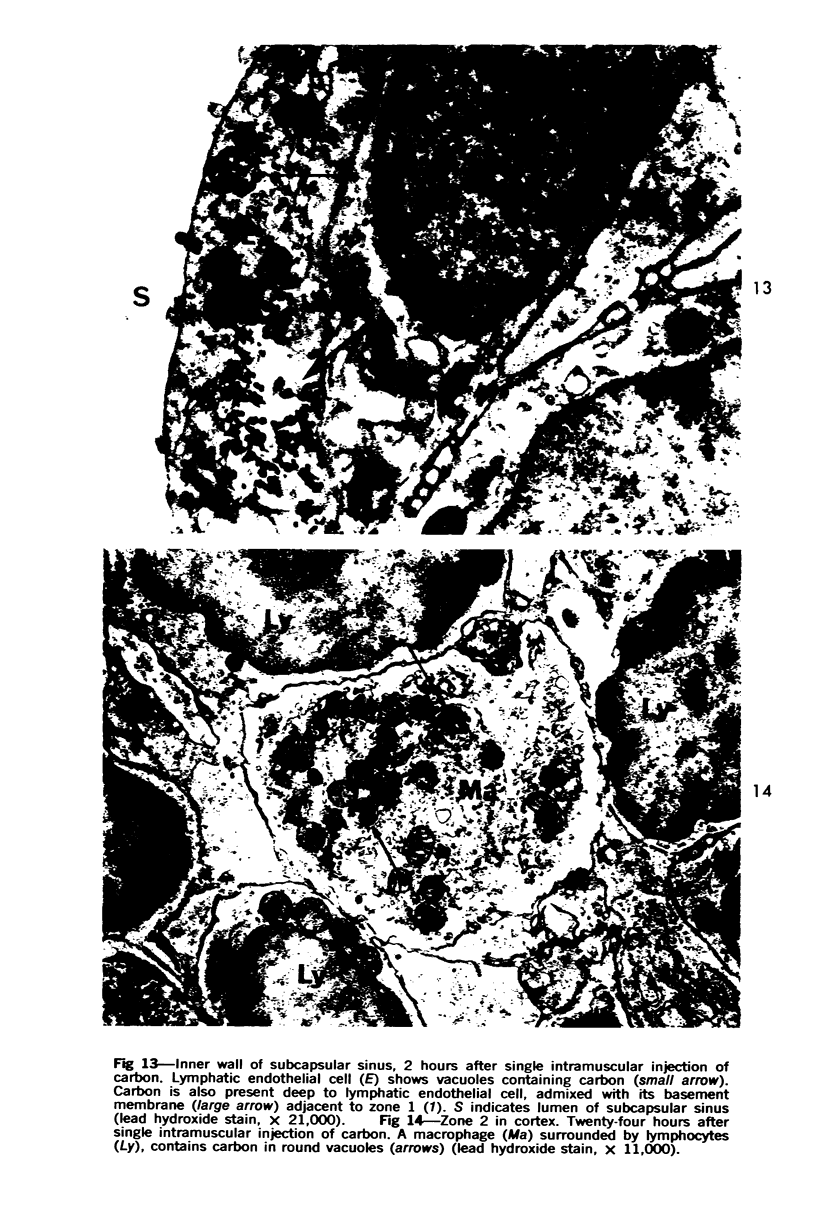
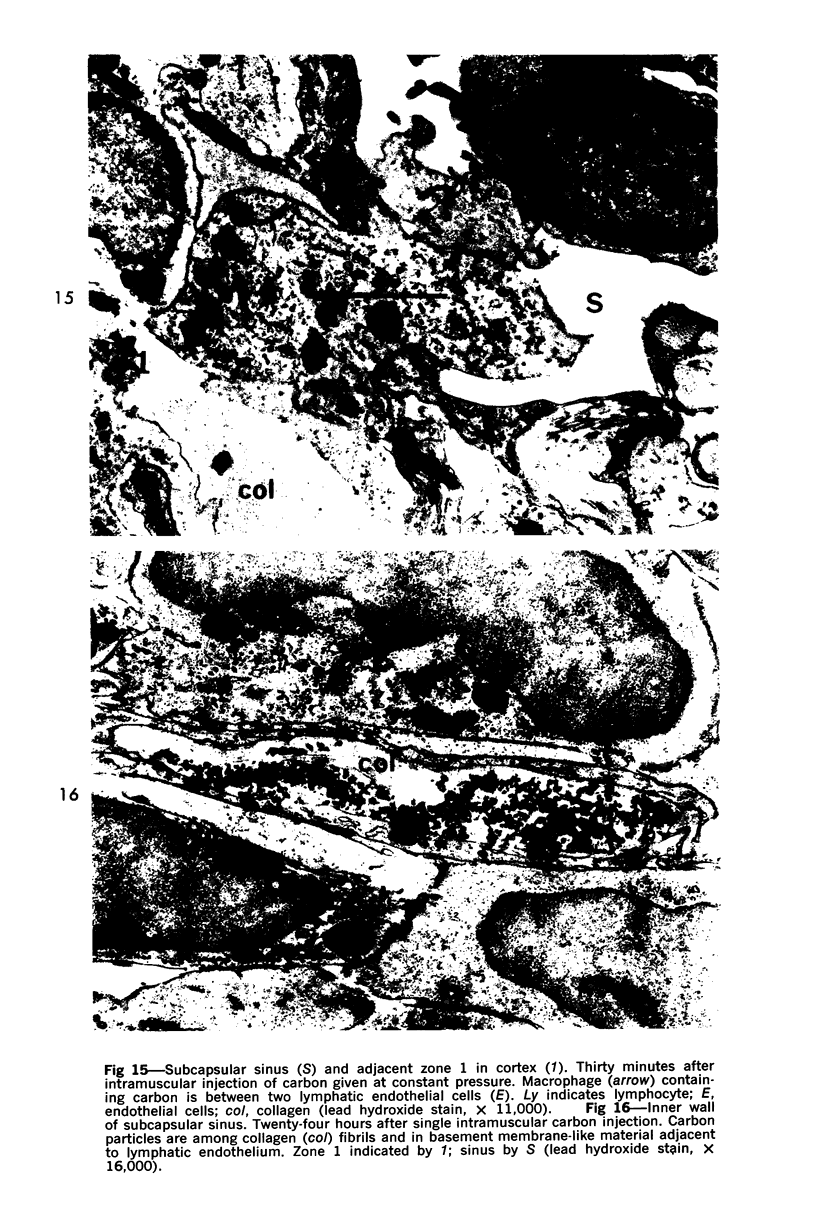
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W. S. Fine structure of experimentally produced subsynovial carbon granuloma. Nature. 1966 Aug 13;211(5050):771–772. doi: 10.1038/211771a0. [DOI] [PubMed] [Google Scholar]
- BAILLIF R. N. Reaction patterns of the reticuloendothelial system under stimulation. Ann N Y Acad Sci. 1960 Jun 21;88:3–13. [PubMed] [Google Scholar]
- Burke J. S., Simon G. T. Electron microscopy of the spleen. II. Phagocytosis of colloidal carbon. Am J Pathol. 1970 Jan;58(1):157–181. [PMC free article] [PubMed] [Google Scholar]
- CAPPELL D. F., HUTCHISON H. E., HENDRY E. B., CONWAY H. A new carbohydrate-iron haematinic for intramuscular use. Br Med J. 1954 Nov 27;2(4899):1255–1257. doi: 10.1136/bmj.2.4899.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK S. L., Jr The reticulum of lymph nodes in mice studied with the electron microscope. Am J Anat. 1962 May;110:217–257. doi: 10.1002/aja.1001100303. [DOI] [PubMed] [Google Scholar]
- COTRAN R. S. ENDOTHELIAL PHAGOCYTOSIS: AN ELECTRON-MICROSPOPIC STUDY. Exp Mol Pathol. 1965 Apr;28:217–231. doi: 10.1016/0014-4800(65)90034-1. [DOI] [PubMed] [Google Scholar]
- Carr I. The fine structure of the mammalian lymphoreticular system. Int Rev Cytol. 1970;27:283–348. doi: 10.1016/s0074-7696(08)61249-8. [DOI] [PubMed] [Google Scholar]
- Fisher B., Fisher E. R. Barrier function of lymph node to tumor cells and erythrocytes. I. Normal nodes. Cancer. 1967 Nov;20(11):1907–1913. doi: 10.1002/1097-0142(196711)20:11<1907::aid-cncr2820201117>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Givan K. F., Turnbull C., Jézéquel A. M. Pepsin digestion of virus particles in canine hepatitis using epon-embedded material. J Histochem Cytochem. 1967 Aug;15(11):688–694. doi: 10.1177/15.11.688. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Electron microscopic study of the differentiation of mouse peritoneal macrophages stimulated by Corynebacterium ovis infection. Lab Invest. 1969 Oct;21(4):309–315. [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARRER H. E. Electron microscopic study of the phagocytosis process in lung. J Biophys Biochem Cytol. 1960 Apr;7:357–366. doi: 10.1083/jcb.7.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA M., IMAI Y. Mechanism of phagocytosis of the reticulo-endothelial cells. Tohoku J Exp Med. 1962 Mar 25;76:161–178. doi: 10.1620/tjem.76.161. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S. N. Lymphadenopathy due to intramuscular iron. J Indian Med Assoc. 1966 May 16;46(10):564–564. [PubMed] [Google Scholar]
- Nopajaroonsri C., Luk S. C., Simon G. T. Ultrastructure of the normal lymph node. Am J Pathol. 1971 Oct;65(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- SUWA K. ELECTRON MICROSCOPE STUDIES ON THE LYMPH NODES OF RABBITS VITALLY STAINED BY REPEATED INTRAVENOUS OR SUBCUTANEOUS INJECTIONS OF ACID AND BASIC DYES WITH SPECIAL REFERENCE TO THE DIRECTION OF THE PASSAGE OF VITAL DYES IN THE NODES. Acta Med Okayama. 1962 Dec;16:SUPPL–SUPPL:151. [PubMed] [Google Scholar]
- WELLENSIEK H. J., COONS A. H. STUDIES ON ANTIBODY PRODUCTION. IX. THE CELLULAR LOCALIZATION OF ANTIGEN MOLECULES (FERRITIN) IN THE SECONDARY RESPONSE. J Exp Med. 1964 Apr 1;119:685–696. doi: 10.1084/jem.119.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT C. S., DODD M. C. Phagocytosis. Ann N Y Acad Sci. 1955 Mar 24;59(5):945–950. doi: 10.1111/j.1749-6632.1955.tb45993.x. [DOI] [PubMed] [Google Scholar]