Abstract
One innate immune response pathway of insects is a serine protease cascade that activates prophenol oxi-dase (pro-PO) in plasma. However, details of this pathway are not well understood, including the number and order of proteases involved. Protease inhibitors from the serpin superfamily appear to regulate the proteases in the pathway. Manduca sexta serpin-4 and serpin-5 suppress pro-PO activation in plasma, apparently by inhibiting proteases upstream of the direct activator of pro-PO. To identify plasma proteases inhibited by these serpins, we used immunoaffinity chromatography with serpin antibodies to isolate serpin-protease complexes that formed after activation of the cascade by exposure of plasma to bacteria or lipopolysaccharide. Covalent complexes of serpin-4 with hemolymph proteases HP-1 and HP-6 appeared in plasma activated by Gram-positive or Gram-negative bacteria, whereas serpin-4 complexes with HP-21 and two unidentified proteases were unique to plasma treated with Gram-positive bacteria. HP-1 and HP-6 were also identified as target proteases of serpin-5, forming covalent complexes after bacterial activation of the cascade. These results suggest that HP-1 and HP-6 may be components of the pro-PO activation pathway, which are activated in response to infection and regulated by serpin-4 and serpin-5. HP-21 and two unidentified proteases may participate in a Gram-positive bacteria-specific branch of the pathway. Several plasma proteins that co-purified with serpin-protease complexes, most notably immulectins and serine protease homologs, are known to be components of the pro-PO activation pathway. Our results suggest that after activation by exposure to bacteria, components of the pro-PO pathway associate to form a large noncovalent complex, which localizes the melanization reaction to the surface of invading microorganisms.
In the hemolymph of insects and crustaceans, microbial infection initiates a serine protease cascade, resulting in proteolytic activation of a prophenol oxidase (pro-PO)1 zymogen (1–4). Activated phenol oxidase (PO) hydroxylates monophenols to o-diphenols and oxidizes o-diphenols to quinones, which can polymerize to form melanin at the injury site or around invading organisms (1, 5). Quinones may also be involved in the production of cytotoxic molecules such as superoxides and hydroxyl radicals that could participate in killing pathogens or parasites (5, 6).
Three pro-PO activating proteases (PAPs) from the tobacco hornworm, Manduca sexta (7–9), are similar to pro-PO-activating enzymes or factors identified from the silkworm, Bombyx mori (10), a beetle, Holotrichia diomphalia (11, 12), and from a crayfish, Pacifastacus leniusculus (13). These enzymes contain one or two clip domains (2) at their amino terminus and a carboxyl-terminal serine protease domain. They are activated by a specific proteolytic cleavage between the clip domain and the protease domain by unknown upstream proteases. For efficient activation of pro-PO, M. sexta PAPs and H. diomphalia PPAF-I require the presence of serine protease homolog(s) (SPHs) that lack proteolytic activity but function as a co-factor (7–9, 11, 14, 15). The SPHs have domain organizations similar to PAPs except that the active site serine residue in the protease-like domain is replaced by glycine. SPHs may also require proteolytic activation of pro-forms to make them functional (14, 16, 17).
Initiation of the pro-PO activation cascade in response to microbial infection is mediated by pattern recognition proteins that recognize pathogen-associated molecular patterns such as lipopolysaccharide (LPS), peptidoglycan, and β-1,3-glucan (1, 18, 19). Two M. sexta C-type lectins (immulectins) bind LPS from Gram-negative bacteria and stimulate pro-PO activation in plasma (20–22). Two β-1,3-glucan recognition proteins have also been characterized from M. sexta (23, 24). They bind to β-1,3-glucan from fungal cell walls and lipoteichoic acid (a cell wall component of Gram-positive bacteria) and stimulate pro-PO activation (23, 24). A peptidoglycan recognition protein (PGRP) that binds to peptidoglycan and initiates pro-PO activation in plasma has been characterized in B. mori (25, 26). PGRPs have also been identified in other insects and arthropods (18, 27–29).
Insect plasma contains serine protease inhibitors, including members of the serpin superfamily, which regulate the pro-PO activation pathway. Serpins are proteins of ~400 amino acid residues, with an exposed reactive center loop near their carboxyl terminus (30–33). Serpins function as suicide-substrate inhibitors by forming stable covalent complexes with proteases after the cleavage of a scissile bond in the reactive center loop (30, 31, 34, 35). The P1 residue located at the amino-terminal side of the scissile bond determines primary specificity of inhibition.
In M. sexta, six serpins have been identified so far (36–41). Serpin-1J, serpin-3, and serpin-6 inhibit PAPs to regulate the last step of the pro-PO activation pathway (9, 40, 41). Two new immune-responsive serpins, serpin-4 and serpin-5, have recently been identified. They are able to inhibit pro-PO activation to different degrees but are not efficient inhibitors of PAPs, indicating that they inhibit serine proteases upstream of PAPs in the activation cascade (43). However, it is still not known how many proteases are involved in the pro-PO activation pathway, how they are regulated, or how microbial components trigger the cascade pathway. In this study, we used M. sexta serpin-4 and serpin-5 to probe functions of proteases in the pro-PO activation pathway.
EXPERIMENTAL PROCEDURES
Insects
M. sexta larvae were reared as described previously (44) from a laboratory colony originally obtained from Carolina Biological Supply.
Immunoaffinity Purification of Serpin-Protease Complexes
Antibody-coupled protein A-Sepharose CL-4B beads (Sigma) were prepared according to Harlow and Lane (45), using rabbit antisera to M. sexta serpin-4 or serpin-5 (43). Hemolymph (20–30 ml) was collected from day 3 fifth instar larvae 24 h after injection with Micrococcus luteus or Escherichia coli (43), and hemocytes were removed by centrifugation at 9000 ✕ g for 15 min at 4 °C. The plasma was warmed to room temperature and adjusted to contain 10 mM diethylthiocarbonate and 1 mM phenylthiourea. Bacteria or lipopolysaccharide (LPS) was then added to the plasma to stimulate activation of protease cascades. Dried M. luteus (Sigma) was added (0.5 µg/µl) to plasma from larvae previously injected with M. luteus. Formaldehyde-killed E. coli XL-1 (1 ✕ 108 cells/ml) or LPS from E. coli 026/B6 (0.01 µg/µl, Sigma) was added to the plasma from larvae induced by E. coli. After incubation for 30 min at room temperature, diisopropyl fluorophosphate (Sigma; final concentration 5 mM) and a protease inhibitor mixture (Sigma, P8849; 1 ml for 30 ml of plasma) were added to inactivate proteases. After 10 min, the mixture was centrifuged at 5000 ✕ g for 15 min at 4 °C.
The supernatant was mixed with 1–2 ml of protein A-Sepharose CL-4B beads coupled with serpin antibody overnight at 4 °C, and the mixture was then packed into a column. The column was washed with 20 volumes of 1 M NaCl and then 10 volumes of 10 mM sodium phosphate, pH 6.5. For purification of serpin-5 complexes from E. coli-treated plasma, these washing steps were replaced with 10 volumes of phosphate-buffered saline (PBS, 4.3 mM Na2PO4, 1.4 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4) and then 10 volumes of 0.5 M NaCl. The columns were eluted with 10 volumes of 100 mM glycine, pH 2.5, 10% ethylene glycol. Fractions (0.5 or 1 ml each, equivalent to 0.5 column volume) were collected into 50 or 100 µl (0.1 fraction volume) of 1 M sodium phosphate, pH 8.0. The fractions were analyzed by SDS-PAGE and immunoblotting (43), using rabbit antisera to the serpins (43) and to M. sexta hemolymph proteases (HPs) HP-1 (AAB94557), HP-2 (AAB94558), HP-6 (AAV91004), HP-8 (AAV91006), HP-9 (AAV91007), HP-10 (AAV91008), HP-12 (AAV91010), HP-13 (AAV91011), HP-14 (AAR29602), HP-15 (AAV91012), HP-16 (AAV91013), HP-17 (AAV91014), HP-18 (AAV91016), HP-19 (AAV91017), HP-21 (AAV91019), and HP-22 (AAV91020) (46, 48). Antisera to M. sexta immulectins (20, 21), pro-PO (49), and serine protease homologs (14) were prepared previously.
Determination of Amino-terminal Sequences
Protein samples were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, stained with 0.025% Coomassie Blue R-250 in 40% methanol, and destained with 50% methanol. The protein bands of interest were excised and subjected to automated Edman degradation sequencing. Serpin-protease complexes were sequenced by the HHMI/Keck Biotechnology Resource Laboratory, Yale University. Other proteins were sequenced by the Biotechnology Microchemical Core Facility, Kansas State University.
Mass Spectrometry Analyses
To identify serpin-4- and serpin-5-protease complexes after separation by SDS-PAGE, the bands were excised, reduced with dithiothreitol, alkylated with iodoacetamide, and then subjected to in-gel digestion with modified l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated porcine trypsin (Promega). The tryptic peptide pools were evaporated to near-dryness and desalted on C18 Ziptips (Millipore). The eluted peptides were analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry in the Proteomics Center, University of Missouri, Columbia. Spectra were acquired in the positive ion delayed extraction reflector mode on a Voyager DEPro mass spectrometer (Applied Biosystems, Inc.). Spectra were calibrated with a six-peptide calibration standard mixture.
The peptide masses were used to search the NCBI nonredundant protein sequence data base with ProFound (prowl.rockefeller.edu) or MASCOT Peptide Mass Fingerprint (www.matrixscience.com) programs. The mass values were also compared with the masses calculated for tryptic peptides derived from M. sexta hemolymph proteases, using the MS-Digest program of ProteinProspector version 4.0.5 (prospector.ucsf.edu).
Inhibition of Pro-HP and Pro-SPH Activation by Serpin-4 or Serpin-5
Samples of 2 µl of plasma were incubated at room temperature for 5 min in the presence or absence of recombinant serpin-4 or serpin-5 (final concentration 0.4 µg/µl), then activated by addition of M. luteus in saline (0.4 µg/µl), and incubated at room temperature for 10 min (43). Samples were then analyzed by SDS-PAGE and immunoblotting with available HP or SPH antibodies.
RESULTS
Identification and Purification of Serpin-Protease Complexes
Serpin-4 and serpin-5 antibodies detected bands in untreated plasma representing intact serpins (~50 kDa) and a minor, slightly smaller protein consistent with cleaved serpin (Fig. 1). After incubation with M. luteus, E. coli, or LPS, the intensity of the 50-kDa band was reduced, and the 45-kDa band increased, suggesting that some intact serpins were converted to the cleaved form. At the same time, higher molecular weight bands (~70 kDa) containing serpin-4 or serpin-5 appeared, consistent with the expected size for serpin-protease complexes (Fig. 1). Apparently, the exposure to bacteria/LPS led to the activation of plasma proteases, which formed covalent complexes with serpin-4 and serpin-5. Similar changes occurred in plasma from naive larvae (data not shown), although all of the serpin bands were less intense due to lower concentration of serpin-4 and serpin-5 in plasma from naive animals. Treatment of plasma with M. luteus consistently stimulated the formation of more intense serpin-4-protease complex bands than did treatment with LPS.
FIG. 1. Formation of serpin-4- and serpin-5-protease complexes in plasma.

As described under “Experimental Procedures,” plasma obtained from larvae 24 h after injection of M. luteus (lanes 1–3) or E. coli (lanes 4–6) were left untreated (lanes 1 and 4) or were incubated for 30 min with sterile water (lane 2), M. luteus (lane 3), LPS (lane 5), or E. coli (lane 6) and subjected to SDS-PAGE/immunoblot analysis using antibodies to serpin-4 (A) or serpin-5 (B).
To identify the proteases inhibited by serpin-4 or serpin-5, we purified the serpin-protease complexes by immunoaffinity chromatography by using the serpin antibodies. Five serpin-4-protease complexes (SPC4-1–5) were isolated from M. luteus-treated plasma (Fig. 2). Two serpin-4-protease complexes, which had the same electrophoretic mobility as SPC4-1 and SPC4-5, were isolated from LPS-treated plasma (Fig. 2). The appearance of the multiple serpin-4-protease complex bands suggests that serpin-4 inhibited several plasma proteases. Two serpin-5-protease complexes (SPC5H and SPC5L) were isolated by immunoaffinity chromatography using serpin-5 antibody (Fig. 3). These two SPCs were present in plasma treated with M. luteus or E. coli.
FIG. 2. Purification and identification of serpin-4-protease complexes from plasma.
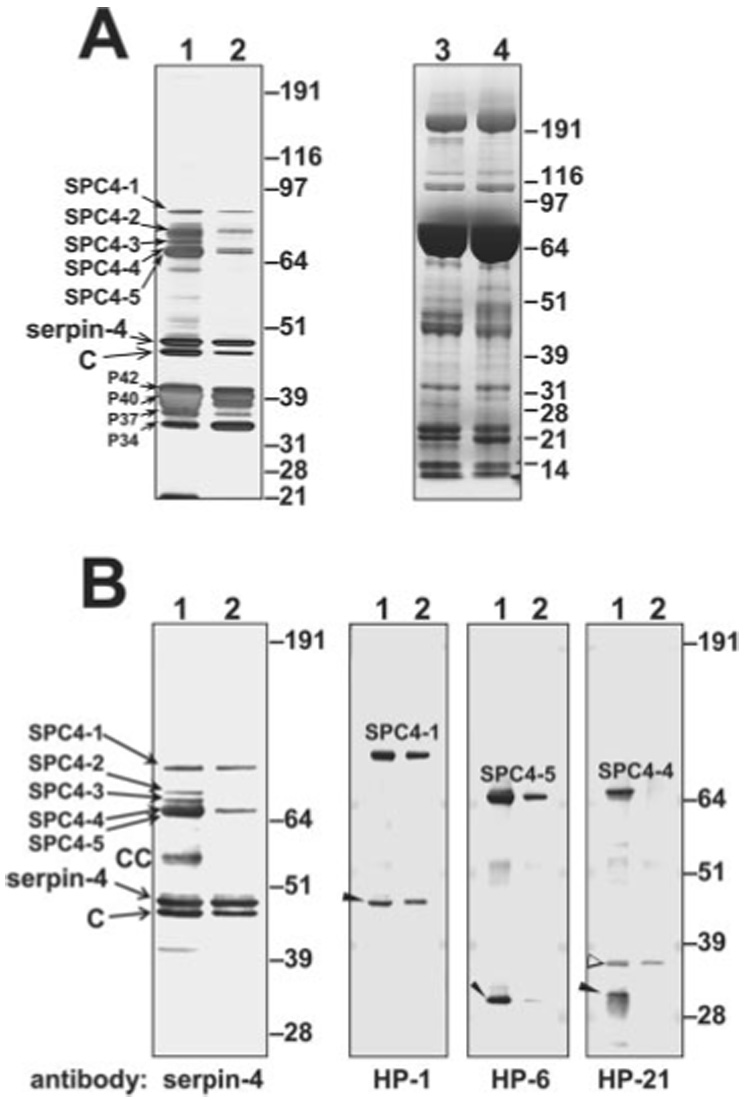
Immunoaffinity-purified serpin-4-protease complexes (SPC4s) were subjected to SDS-PAGE and were detected by silver staining or immunoblotting. Lane 1, serpin-4 and associated proteins isolated from M. luteus-treated plasma; lane 2, serpin-4 and associated proteins isolated from LPS-treated plasma. A, silver-stained gel of purified SPC4 fractions (lanes 1 and 2) and Coomassie Blue-stained gel of whole plasma samples (1 µl) collected 24 h after injection with M. luteus (lane 3) or E. coli (lane 4). B, immunoblots of SPC4 fractions. SPC4-1 to SPC4-5, serpin-4-protease complexes; CC, cleaved complexes; C, cleaved serpin; P42, P40, P37, and P34, co-purified proteins named by their apparent mass. Uncomplexed protease bands are indicated by arrowheads. An open arrowhead denotes a form of HP-21 apparently cleaved at a different site.
FIG. 3. Purification and identification of serpin-5-protease complexes from plasma.
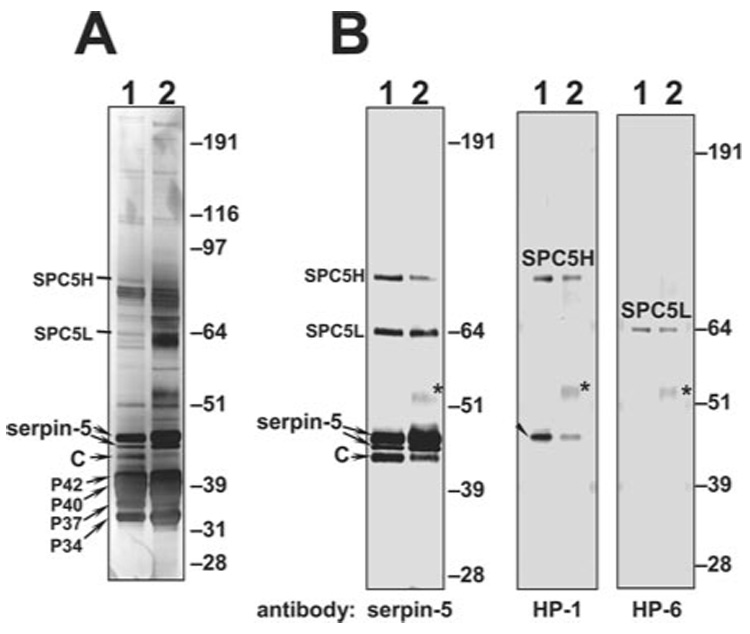
Immunoaffinity-purified serpin-5-protease complexes (SPC5s) were subjected to SDS-PAGE and detected by silver staining or immunoblotting. A, silver-stained gel of purified serpin-5-protease complex (SPC5) fractions. B, immunoblots of SPC5 fractions. Lane 1, SPC5 fraction purified from M. luteus-treated plasma; lane 2, SPC5 fraction purified from E. coli-treated plasma. SPC5H/SPC5L, serpin-5-protease complex of high or low Mr; C, cleaved serpin. An uncomplexed HP-1 band is indicated by an arrowhead. The heavy chain of rabbit IgG is marked with *.
Other plasma proteins that co-purified with the complexes and serpin-4 or serpin-5 were not recognized by antibodies to the serpins. Based on their apparent masses, we named the most abundant of these proteins P42, P40, P37, and P34 (Fig. 2A and Fig. 3A). The association of these proteins with serpin-4 and serpin-5 was apparently not due to incomplete washing of the columns, because the abundance of these proteins in plasma is much lower than major hemolymph proteins (e.g. hexamerin storage proteins and lipophorin) that did not bind to the antibody columns. In controls using columns coupled with antibodies from preimmune sera, these proteins did not bind (data not shown), further indicating their specific interaction with serpin-4 and serpin-5 or with plasma proteins that interact with the serpins.
Identification of Proteases in Serpin-Protease Complexes
We have cloned cDNAs for 25 M. sexta hemolymph proteases, including three PAPs and other HPs synthesized in fat body or hemocytes (7–9, 46),2 and we prepared antibodies to 16 of these proteases. To test whether these proteases were present in the isolated serpin-4 and serpin-5 complexes described above, we analyzed fractions containing the complexes by immunoblotting, using the HP antibodies. Antibodies to proteases HP-1, HP-6, and HP-21 bound to complex bands SPC4-1, SPC4-5, and SPC4-4, respectively (Fig. 2B). The SPC4-4 band recognized by antibody to HP-21 was present in M. luteus-treated plasma but not in plasma treated with LPS (Fig. 2B), consistent with the result using serpin-4 antibody. Likewise, a 31-kDa band detected by HP-21 antibody (consistent in size with the catalytic domain of active HP-21) was detected only in M. luteus-treated plasma. These results indicate that HP-21 might be involved in a response specific to Gram-positive bacteria. In contrast, the putative catalytic domains of both HP-1 and HP-6 and their complexes with serpin-4 were detected in fractions obtained after treatment with LPS or M. luteus (Fig. 2B). None of the available HP antibodies recognized SPC4-2 or SPC4-3, which were present only in plasma samples activated by treatment with M. luteus. SPC5H (~85 kDa) was recognized by antibodies to serpin-5 and HP-1, whereas SPC5L (~70 kDa) was recognized by serpin-5 and HP-6 antibodies (Fig. 3B). These data suggest that serpin-5 regulates HP-1 and HP-6, which are activated in the presence of Gram-positive or Gram-negative bacteria.
HP-1, HP-6, and HP-21 belong to a family of proteases that contain an amino-terminal clip domain and a serine protease domain, linked by a disulfide bond. They are predicted to be activated by specific cleavage between the clip domain and the protease domain (2). In SDS-PAGE of a serpin-protease complex, it is expected that the catalytic domain of the protease will remain connected to the serpin through a covalent bond between the catalytic serine residue of the protease and the P1 residue of the serpin. The clip domain is released under reducing conditions due to reduction of the disulfide bond that links the clip and protease domains. In Edman degradation analysis of a serpin-protease complex, two amino-terminal sequences can be expected, one for the serpin and one for the catalytic domain of the protease. Amino-terminal sequencing of proteins in SPC4-1, SPC4-4, and SPC4-5 bands isolated from gels showed that all three complexes contained a sequence matching the amino-terminal sequence of serpin-4 (Table I). SPC4-5 also yielded phenylthiohydantoin-derivatives expected from the HP-6 catalytic chain, consistent with the immunoblot result (Fig. 2B) indicating that SPC4-5 is a complex of serpin-4 with HP-6. However, no second sequence was detected in SPC4-1 or SPC4-4, perhaps due to blocking of the amino termini of the proteases.
TABLE I.
Amino-terminal sequencing by automated Edman degradation of serpin-4-protease complexes
| Experimental results |
Expected sequences |
||||
|---|---|---|---|---|---|
| Cycle | SPC4-1 and SPC4-4 | SPC4-5 | Serpin-4 | HP-6 catalytic domain | Phenoloxidase-1 |
| 1 | Asp | Asp, Ile, Phe | Asp | Ile | Phe |
| 2 | Asp | Asp, Leu, Gly | Asp | Leu | Gly |
| 3 | Leu | Leu, Gly, Asn | Leu | Gly | Asn |
| 4 | Pro | Pro, Gly, Glu | Pro | Gly | Glu |
| 5 | Ala | Ala, Glu | Ala | Glu | Ala |
| 6 | Lys | Lys, Glu, Thr | Lys | Glu | Thr |
| 7 | Val | Val, Ala, Lys | Val | Ala | Lys |
| 8 | Arg | Arg, Ser | Arg | Ser | Arg |
| 9 | Asn | Asn, Leu, Ile | Asn | Leu | Ile |
| 10 | Gly | Gly, Gly, Pro | Gly | Gly | Pro |
| 11 | Leu | Leu, Glu, Ile | Leu | Glu | Ile |
| 12 | Thr | Thr, Phe, Arg | Thr | Phe | Arg |
| 13 | Glu | Glu, Pro, Asn | Glu | Pro | Asn |
SPC5H and SPC5L both yielded the amino-terminal sequence of serpin-5 except at residue 10 (Table II), which may be due to glycosylation of Asn10 (43). SPC5L also contained the amino-terminal sequence of the HP-6 catalytic domain (Table II), indicating that SPC5L is a complex of serpin-5 with HP-6, consistent with the immunoblot result (Fig. 3B). No second sequence was detected in SPC5H. As SPC5H and SPC4-1 were both recognized by antibody to HP-1, these results suggested a blocked amino terminus of HP-1 present in the complexes with serpin-4 and serpin-5.
TABLE II.
Amino-terminal sequencing by automated Edman degradation of serpin-5-protease complexes
| Experimental results |
Expected sequences |
|||
|---|---|---|---|---|
| Cycle | SPC5H | SPC5L | Serpin-5 | HP-6 catalytic domain |
| 1 | Asp | Asp, Ile | Asp | Ile |
| 2 | Val | Val, Leu | Val | Leu |
| 3 | Asp | Asp, Gly | Asp | Gly |
| 4 | Phe | Phe, Gly | Phe | Gly |
| 5 | Tyr | Tyr, Glu | Tyr | Glu |
| 6 | Glu | Glu | Glu | Glu |
| 7 | Arg | Arg, Ala | Arg | Ala |
| 8 | Pro | Pro, Ser | Pro | Ser |
| 9 | Arg | Arg, Leu | Arg | Leu |
| 10 | ? | ?, Gly | Asn | Gly |
| 11 | Phe | Phe, Glu | Phe | Glu |
| 12 | Ser | Ser | Ser | Phe |
| 13 | Ile | Ile | Pro | |
As another approach to identify the protease components, we used peptide mass fingerprint analysis to identify protease components in the SPCs. Masses of tryptic peptides derived from all five SPC4s matched well with those of the predicted tryptic peptides of serpin-4, including peptides present only in isoform A or B (Table III) (43), indicating that both forms of serpin-4 are present in plasma and can inhibit the same proteases.
TABLE III. Identification of serpin-4 in SPC4s by MALDI-TOF mass spectrometry.
The mass spectral analysis was performed as described under “Experimental Procedures.” The measured monoisotopic (MH+) masses from SPC4s and their corresponding matching peptide from serpin-4 are shown. The peptides present only in isoform A or B of serpin-4 are indicated. A putative residue modification of a peptide is also indicated. α, one methionine oxidation; β, one cysteine carbamidomethylation by iodoacetamide; γ, one cysteine modification by acrylamide.
| Measured mass (MH+) from SPC4s |
Matched serpin-4 peptides |
Isoform A or B only | |||||
|---|---|---|---|---|---|---|---|
| SPC4-1 | SPC4-2 | SPC4-3 | SPC4-4 | SPC4-5 | Residues | Computed mass (MH+) | |
| 3266.655 | 3266.669 | 3266.739 | 3266.734 | 3266.687 | 29–59 | 3266.702 | |
| 852.443 | 852.436 | 852.441 | 61–67 | 852.469 | |||
| 1087.572 | 1087.581 | 1087.606 | 1087.615 | 1087.587 | 82–90 | 1087.615 | |
| 1203.619 | 1203.612 | 1203.639 | 1203.664 | 1203.634 | 99–108 | 1203.649 | |
| 1244.650 | 1244.654 | 1244.667 | 1244.689 | 1244.656 | 109–119 | 1244.689 | |
| 2752.324 | 2752.318 | 2752.374 | 2752.388 | 2752.334 | 120–143 | 2752.350 | A |
| 2768.321 | 2768.368 | 2768.374 | 2768.401 | 2768.335 | 120–143 | 2768.345 | Aβ, B |
| 1448.697 | 1448.679 | 1448.703 | 1448.717 | 1448.692 | 131–143 | 1448.702 | A |
| 1464.700 | 1464.680 | 131–143 | 1464.697 | B | |||
| 1186.618 | 1186.564 | 1186.584 | 1186.578 | 167–176 | 1186.655 | ||
| 1202.596 | 1202.594 | 1202.615 | 1202.640 | 1202.623 | 167–176 | 1202.650 | β |
| 1081.464 | 1081.453 | 1081.460 | 1081.489 | 1081.465 | 191–199 | 1081.478 | A |
| 1199.582 | 1199.616 | 1199.637 | 191–200 | 1199.563 | Bβ | ||
| 1354.643 | 1354.640 | 1354.666 | 1354.687 | 1354.635 | 200–210 | 1354.661 | |
| 1226.552 | 1226.539 | 1226.567 | 1226.594 | 1226.558 | 201–210 | 1226.566 | |
| 1242.543 | 1242.535 | 1242.554 | 1242.582 | 1242.554 | 201–210 | 1242.561 | β |
| 1139.550 | 1139.554 | 1139.586 | 1139.537 | 211–219 | 1139.567 | A | |
| 1127.508 | 1127.501 | 1127.535 | 1127.566 | 1127.510 | 211–219 | 1127.531 | B |
| 1155.532 | 1155.492 | 1155.510 | 1155.501 | 1155.446 | 211–219 | 1155.562 | Aβ |
| 1276.634 | 1276.624 | 1276.643 | 1276.668 | 1276.643 | 225–235 | 1276.654 | |
| 1549.682 | 1549.667 | 1549.698 | 1549.722 | 1549.689 | 258–269 | 1549.700 | |
| 1565.681 | 1565.656 | 1565.675 | 1565.700 | 1565.691 | 258–269 | 1565.695 | β |
| 2028.860 | 2028.851 | 2028.894 | 2028.920 | 2028.879 | 270–286 | 2028.886 | α |
| 2042.864 | 2042.871 | 2042.911 | 2042.941 | 2042.895 | 270–286 | 2042.902 | γ |
| 2793.352 | 2793.286 | 2793.343 | 2793.387 | 289–313 | 2793.344 | ||
| 2409.155 | 2409.125 | 2409.188 | 2409.212 | 2409.171 | 333–355 | 2409.179 | |
The experimental peptide masses of SPC4-1 covered 57% of the sequence of the catalytic domain of HP-1 (46) (Fig. 4). A typical mass spectrum of tryptic peptides of SPC4-1 shows the correspondence between the experimental masses and the predicted peptide masses from both HP-1 and serpin-4 (Fig. 5). Tryptic peptides from SPC4-5 matched those predicted from HP-6, covering 61% of the HP-6 catalytic domain (Fig. 6). Peptides derived from SPC4-4 matched those predicted for HP-21, covering 47% of the catalytic domain sequence (Fig. 7). Peptides from SPC5L had masses matching those predicted from serpin-5 (46% coverage) and the catalytic domain of HP-6 (64% coverage) (Fig. 8). These results, consistent with the immunoblot and sequencing data, indicate that serpin-4 formed complexes with HP-1, HP-21, and HP-6 and that SPC5L is a complex of serpin-5 and HP-6. Peptide mass fingerprints of SPC4-2 or SPC4-3 did not match any known M. sexta proteases. For SPC5H, the number of experimentally detected tryptic peptides was too small for identification of its protease component.
FIG. 4. Identification of HP-1 in SPC4-1 by MALDI-TOF mass spectrometry.
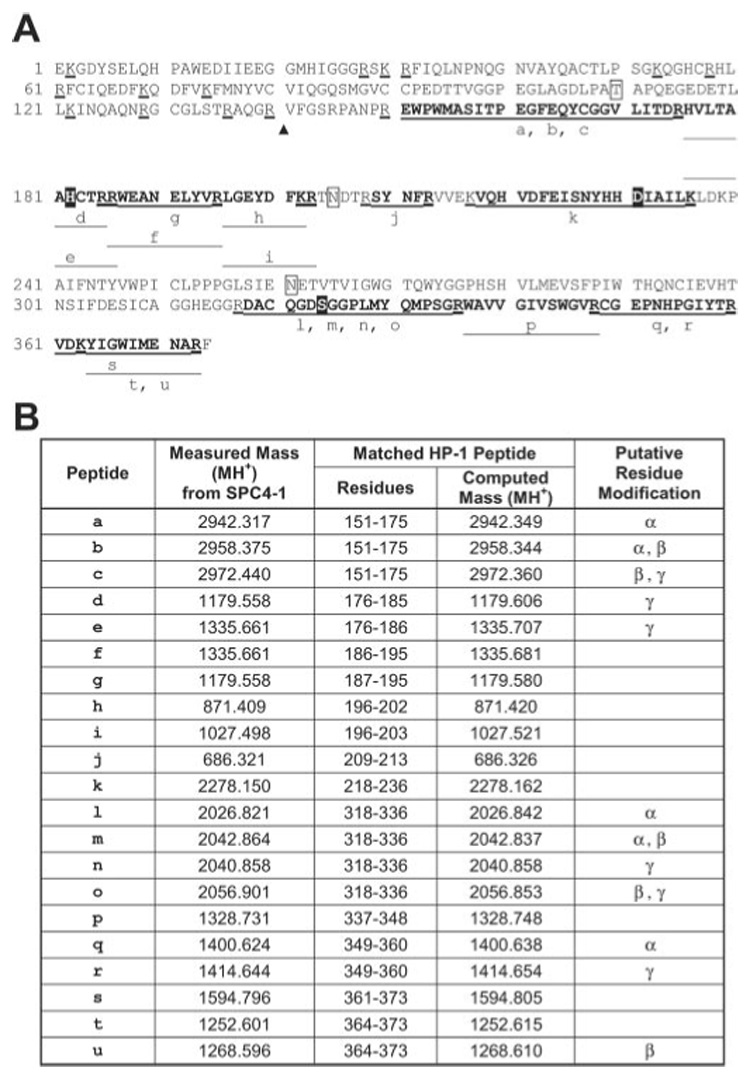
A, the amino acid sequence of mature HP-1 (GenBankTM number AAB94557). In the sequence, the potential N-glycosylation sites (N) and O-glycosylation site (T) are boxed. The predicted cleavage site between the clip domain and catalytic domain is indicated by a triangle. The predicted catalytic triad residues (H, D, and S) are in solid black boxes. Trypsin cleavage sites have a thick underline. The tryptic peptides for which the matching masses were found from mass spectral analysis are indicated by lines below the sequence. The lowercase letters indicate the matching masses, which are listed in B. B,, the measured monoisotopic (MH+) masses from SPC4-1 and the corresponding matching peptide from HP-1. The peptides with putative residue modifications are indicated. α, one cysteine carbamidomethylation by iodoacetamide; β, one methionine oxidation; γ, one cysteine modification by acrylamide.
FIG. 5. A representative spectrum of MALDI-TOF mass spectrometry analysis of tryptic peptides from SPC4-1.
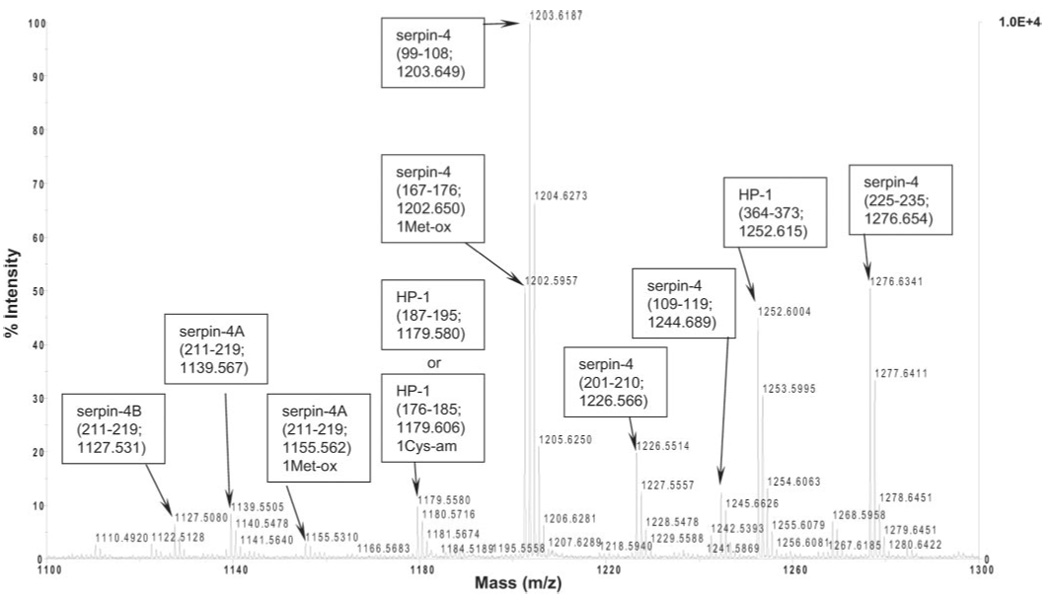
Monoisotopic peaks matching either serpin-4 or HP-1 peptides are labeled. In parentheses, peptide residues are followed by the theoretical monoisotopic peptide mass. 1Met-ox indicates a predicted mass with one methionine oxidation, and 1Cys-am indicates a mass with one cysteine modification by acrylamide.
FIG. 6. Identification of HP-6 in SPC4-5 by MALDI-TOF mass spectrometry.

A, amino acid sequence of the predicted mature HP-6 (GenBankTM number AAV91004). B, the measured monoisotopic (MH+) masses of tryptic peptides from SPC4-5 and the corresponding matching peptide from HP-6. The HP-6 sequence and the peptides are labeled as in Fig. 4 except that the sequence obtained from amino-terminal sequencing of SPC4-5 is indicated with double underlines.
FIG. 7. Identification of HP-21 in SPC4-4 by MALDI-TOF mass spectrometry.

A, amino acid sequence of the predicted mature HP-21 (GenBankTM accession number AAV91019). B, the measured monoisotopic (MH+) masses of tryptic peptides from SPC4-4 and the corresponding matching peptide from HP-21. The HP-21 sequence and the peptides are labeled as in Fig. 4.
FIG. 8. Identification of HP-6 in SPC5L by MALDI-TOF mass spectrometry.
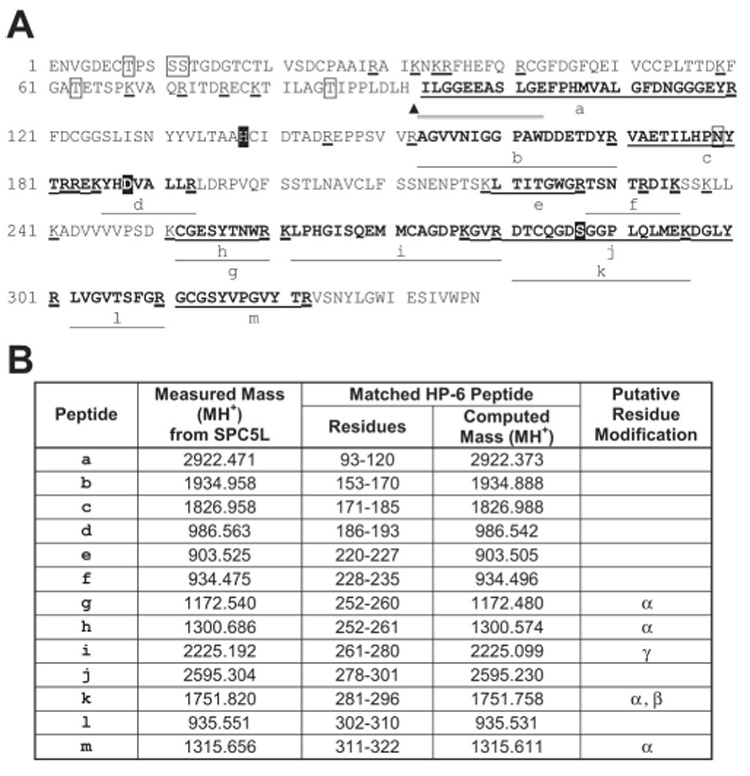
A, amino acid sequence of the predicted mature HP-6 (GenBankTM accession number AAV91004). B, the measured monoisotopic (MH+) masses of tryptic peptides from SPC5L and their corresponding matching peptide from HP-6. The HP-6 sequence and the peptides are shown the same as in Fig. 4 except that the sequence obtained from amino-terminal sequencing of SPC5L is indicated with double underlines.
Identification of Proteins Co-purified with SPCs
P40, P42, and an ~38-kDa band were recognized by antibody to immulectin-2 (IML-2), and they had apparent molecular masses similar to two isoforms of IML-2 present in M. sexta plasma (21) (Fig. 9). P40 and P42 had identical amino-terminal sequences (Table IV) that matched exactly that of IML-2 (21). These results indicate that P40 and P42 are two isoforms of IML-2 identified previously. The 38-kDa band recognized by the IML-2 antibody may be another isoform or a cleaved form of IML-2. P34 and P37, which were recognized by antiserum to IML-3 (Fig. 9), had identical amino-terminal sequences (Table IV) that matched the sequence of IML-3 predicted from its cDNA (GenBankTM accession number AAV41236), except at residue 6, with Ser in P34 and P37 and Ala predicted for IML-3. Thus, P34 and P37 appear to be isoforms of IML-3. These C-type lectins (IML-2 and IML-3) that co-purified with the serpin-protease complexes were even more abundant than the serpins themselves in the fractions that bound to the serpin-antibody columns (Fig. 2A and Fig. 3A).
FIG. 9. Identification of proteins co-purified with SPC4s (A) or SPC5s (B).
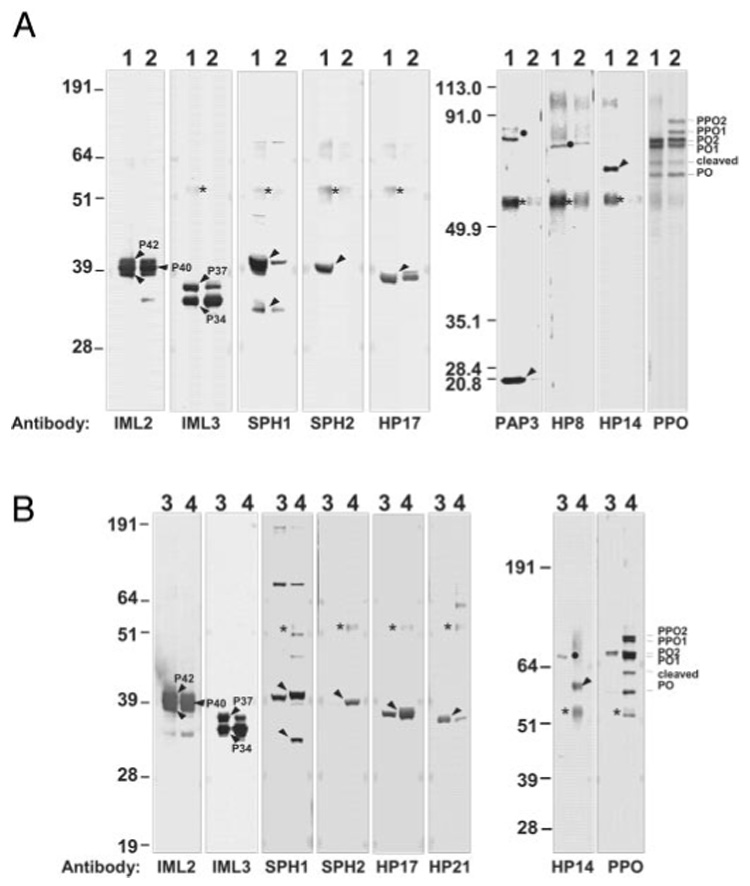
Proteins eluted from the antibody columns were screened by immunoblot analysis by using antibodies to various M. sexta plasma proteins. Lanes 1 and 2, serpin-4 and associated proteins from M. luteus- and LPS-treated plasma, respectively; lanes 3 and 4, serpin-5 and associated proteins from M. luteus- and E. coli-treated plasma, respectively. The amount of samples loaded for immulectin-2 detection was 1:10 of the others. Bands corresponding to each co-purified protein are indicated by arrowheads, and putative serpin-protease complexes are denoted by ●. The heavy chain of rabbit IgG is marked *.
TABLE IV.
Amino-terminal sequencing by automated Edman degradation of major proteins co-purified with SPC5s
| Cycle | Experimental P34 and P37 | Expected immulectin-3 | Experimental P40 and P42 | Expected immulectin-2 |
|---|---|---|---|---|
| 1 | Ser | Ser | Asn | Asn |
| 2 | Asn | Asn | His | His |
| 3 | Val | Val | Val | Val |
| 4 | Phe | Phe | Asn | Asn |
| 5 | Arg | Arg | Phe | Phe |
| 6 | Ser | Ala | Arg | Arg |
| 7 | Asp | Asp | ? | Cys |
| 8 | Tyr | Tyr | Asp | Asp |
| 9 | Glu | Glu | Tyr | Tyr |
| 10 | Tyr | Tyr | Lys | Lys |
| 11 | His | His | Tyr | Tyr |
| 12 | Ala | Ala | Leu | Leu |
| 13 | Ser | Ser | Asp | Asp |
| 14 | Ala | Ala | Val | Val |
| 15 | Gly | Gly | ||
| 16 | Gly | Gly |
Immunoblot analysis using available antibodies to other M. sexta hemolymph proteins detected other components that co-eluted with the SPCs. Based on antibody reactivity and size, we identified SPH-1 and SPH-2, pro-PO-1 and pro-PO-2, PO-1 and PO-2, as well as five hemolymph proteases (PAP-3, HP-8, HP-14, HP-17, and HP-21) (Fig. 9). SPH-1 and SPH-2 were detected in both types of serpin-4-protease complex fractions, although they were more abundant in the fraction from Gram-positive bacterial treatment. In the serpin-5-protease complex fractions, SPH-2 was more abundant in the sample obtained from plasma treated with E. coli than in the samples exposed to M. luteus. HP-8, HP-17, immulectin-2, immulectin-3, and activated POs were present in similar amounts in both serpin-4-protease complex fractions. PAP-3 and HP-14 were detected only in the fraction from Gram-positive bacterial treatment. PAP-3 and HP-8 antibodies recognized bands of the expected sizes for their complexes with serpins (but these bands were not recognized by serpin-4 antibodies), indicating that they were activated in response to microbial infection and then inhibited by serpins other than serpin-4. Pro-PO was present in the serpin-4-protease complex fraction from Gram-negative bacterial treatment. No specific antibody-labeled bands were detected in the serpin-4-protease complex fractions using antibodies against PAP-1, PAP-2, HP-2, HP-10, HP-12, HP-13, HP-15, HP-16, HP-18, HP-19, HP-22, SPH-3, immulectin-1, peptidoglycan recognition protein, or serpin-5 (data not shown).
Slightly different results were obtained in analysis of the proteins co-purified with serpin-5-protease complexes. HP-14 antibody detected a band of ~67 kDa (perhaps an HP-14-serpin complex) and a band at ~43 kDa (consistent with the predicted size of the HP-14 pro-domain region; see Ref. 47) in the fraction purified from M. luteus-treated plasma (Fig. 9B). HP-14 antibody detected a band of ~58 kDa in the serpin-5-protease complex fraction purified from E. coli-treated plasma, which was also present in the serpin-4-protease complex fraction (Fig. 9A). HP-21 antibody detected a band of ~36 kDa (Fig. 9B), which was also present in the serpin-4-protease complex fractions (Fig. 2B). Specific antibody-labeled bands were not detected in serpin-5-protease complex fractions when using antibodies to PAP-1, PAP-2, HP-2, HP-8, HP-10, HP-12, HP-13, HP-15, HP-16, HP-18, HP-19, HP-22, SPH-3, immulectin-1, peptidoglycan recognition protein, or serpin-4.
Immunoblot analysis indicated PO-1 and PO-2 were present in the complex fraction and co-migrated with SPC4-5 and SPC4-4, respectively in SDS-PAGE (Fig. 9A). Edman degradation of SPC4-5 yielded a third amino-terminal sequence Phe-Gly-Asn-Glu-Ala-Thr-Lys-Arg-Ile-Pro-Ile-Pro-Ile-Arg-Asn (Table I), identical to residues 52–66 of pro-PO-1. The proteolytic activation site of pro-PO-1 is located between Arg51 and Phe52 (8). Molecular masses of 30 tryptic peptides from SPC4-4 and SPC4-5 matched well with those predicted from PO-2 and PO-1 (Table V). These results confirmed the co-purification of Pos (as well as pro-POs) with the serpin-4-protease complexes.
TABLE V. Matching tryptic peptides for PO-1 and PO-2 co-purified with SPC4s.
The serpin-4-protease complex (SPC4) bands were cut out and subjected to in-gel trypsin digestion. The peptides extracted were analyzed by MALDI-TOF mass spectrometry. The experimental peptide masses matching peptides of PO-1 and PO-2 were obtained from co-migrating SPC4-5 and SPC4-4 bands, respectively. The numbering of peptide residues of PO-1 (GenBankTM accession number AAC05796) and PO-2 (accession number AAC37243) refers to the pro-forms. The peptides with putative residue modifications are indicated by superscript greek as follows: α, one cysteine carbamidomethylation by iodoacetamide; β, one methionine oxidation.
| Measured mass (MH+) from SPC4-5 band | Matched PO-1 peptide |
Measured mass (MH+) from SPC4-4 band | Matched PO-2 peptide |
||
|---|---|---|---|---|---|
| Residues | Computed mass (MH+) | Residues | Computed mass (MH+) | ||
| 2923.355 | 96–119α, β, β | 2923.346 | 2367.231 | 123–141α | 2367.174 |
| 1871.793 | 105–119α, α | 1871.827 | 1311.691 | 160–170 | 1311.670 |
| 2174.046 | 177–195 | 2174.055 | 708.408 | 202–206 | 708.383 |
| 2857.330 | 201–223 | 2857.360 | 1909.011 | 237–251 | 1908.991 |
| 1910.959 | 231–245 | 1910.970 | 1752.887 | 238–251 | 1752.890 |
| 2843.411 | 334–359 | 2843.417 | 1897.928 | 267–281 | 1897.913 |
| 2673.154 | 360–382 | 2673.169 | 1155.501 | 290–299 | 1155.585 |
| 1749.795 | 383–398 | 1749.805 | 1729.843 | 305–319 | 1729.854 |
| 1417.741 | 450–461 | 1417.744 | 1802.871 | 385–400 | 1802.864 |
| 3220.482 | 475–502 | 3220.549 | 844.420 | 401–406 | 844.399 |
| 1591.820 | 407–419 | 1591.791 | |||
| 1324.719 | 482–491 | 1324.703 | |||
| 1199.637 | 519–528 | 1199.617 | |||
| 1503.846 | 537–550 | 1503.817 | |||
| 1870.934 | 551–566 | 1870.919 | |||
| 1087.615 | 567–576 | 1087.538 | |||
| 2621.256 | 577–598α, α | 2621.222 | |||
| 2408.124 | 578–598α | 2408.099 | |||
| 1630.748 | 627–640 | 1630.728 | |||
| 1127.566 | 682–691 | 1127.570 | |||
Inhibition of Pro-HP and Pro-SPH Activation
To investigate further how serpins regulate plasma protease cascades, we tested whether activation of HPs and SPH cofactors could be inhibited by serpin-4 or serpin-5 (Fig. 10). In larval plasma, a band representing a zymogen or pro-form was detected for HP-1, HP-6, HP-8, HP-14, SPH-1, and SPH-2. For HP-8, another lower molecular weight band may represent a species resulting from an initial proteolytic cleavage that does not cause activation. After incubation with a microbial elicitor, the bands representing pro-forms disappeared (HP-6 and HP-8) or slightly decreased (SPH-1and SPH-2), and a new band representing the catalytic domain (HP-6 and HP-8) or protease-like domain (SPH-1 and SPH-2) appeared. For HP-6, a higher molecular weight band, probably representing a complex of activated HP-6 with a serpin, was also observed. These results suggest the proteolytic activation of HP-6, HP-8, SPH-1, and SPH-2, coincident with pro-PO activation. When plasma was incubated with recombinant serpin-4 prior to M. luteus treatment, the bands for HP-6 and HP-8 zymogens did not decrease and their catalytic domains did not appear. Similarly, the bands representing protease-like domains of SPH-1 and SPH-2 were significantly decreased in intensity. These results suggest that serpin-4 blocked the proteolytic activation of HP-6, HP-8, SPH-1, and SPH-2. Recombinant serpin-5 also decreased HP-6 and HP-8 processing but was less effective than serpin-4. The appearance of a more intense HP-6-serpin complex band in the presence of serpin-5 also suggested that serpin-5 did not fully prevent HP-6 activation and that once activated HP-6 formed a complex with serpin-5. The processing of pro-SPH-1 and pro-SPH-2 did not appear to be affected significantly by serpin-5.
FIG. 10. Inhibition of pro-HP and pro-SPH activation by serpin-4 or serpin-5.
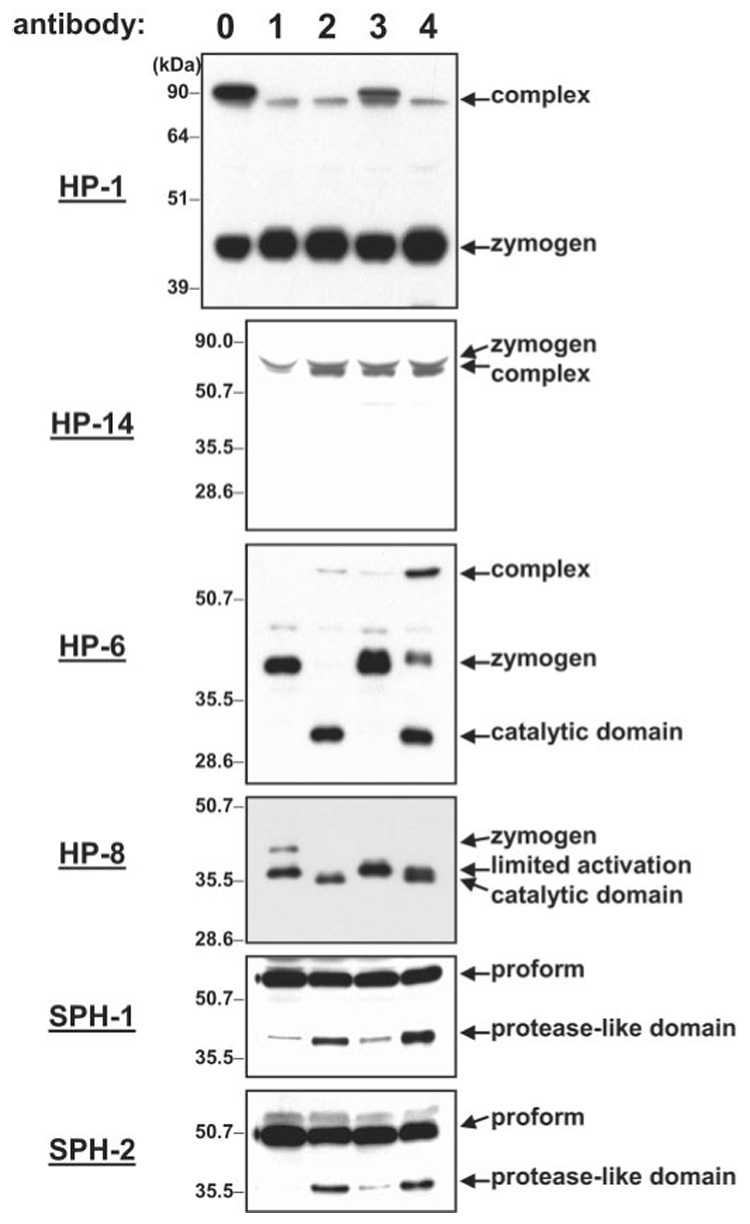
Samples of naive plasma were treated as described under “Experimental Procedures” and analyzed by immunoblotting with the indicated antiserum. Naive plasma was incubated with recombinant serpin-4 and then treated with saline (lane 0) or M. luteus (lane 3); naive plasma incubated with buffer and then treated with saline (lane 1) or M. luteus (lane 2); naive plasma incubated with recombinant serpin-5 and then treated with M. luteus (lane 4). The equivalent of 1 µl of plasma was loaded in each lane. The bands of pro-HPs, pro-SPHs, catalytic/protease-like domain, and putative serpin complexes are indicated by arrows.
In contrast to HP-6 and HP-8, proteolytic activation of HP-1 and HP-14 was unaffected by recombinant serpin-4 or serpin-5. After microbial activation of the plasma, bands representing the catalytic domain and pro-domain of HP-14 were not observed, but a protein band of the size expected for a serpin complex with the catalytic domain of HP-14, detected by HP-14 antibody, became more abundant (Fig. 10). Supplementation with recombinant serpin-4 or serpin-5 had no effect on intensity of this band. Likewise, no cleavage products were observed for HP-1 after incubation of plasma with the bacteria, and a more intense band probably representing HP-1-serpin complex was observed when activation occurred in the presence of recombinant serpin-4, suggesting that active HP-1 was inhibited by serpin-4. Because a similar intensity of HP-1-serpin complex appeared in plasma mixed with recombinant serpin-4 but not incubated with bacteria, HP-1 may have been active without microbial stimulation of a protease pathway.
DISCUSSION
In this study, we used two recently identified M. sexta serpins as probes to detect proteases that are activated upon exposure of plasma to bacteria. Synthesis of serpin-4 and serpin-5 is up-regulated when larvae are infected with bacteria, and these two serpins can block the microbe-stimulated pro-PO activation cascade at a step upstream of the terminal proteases, PAPs (43). The M. sexta pro-PO activation pathway appears to be regulated by serpins at several different steps. The PAPs are efficiently inhibited by serpin-3 and serpin-1J (9, 40, 41), but the pro-PO activation cascade has not yet been characterized sufficiently (in M. sexta or any other insect species) for predicting which proteases are upstream of the PAPs and might be inhibited by serpin-4 or serpin-5. We know that a relatively large number of serine proteases, many containing regulatory clip domains, are present in M. sexta plasma, but their functions have not yet been identified (2, 3, 7–9, 46).3 In this work, we used available antibodies and cDNA sequence information from our collection of M. sexta hemolymph proteases to identify the proteases inhibited by serpin-4 and serpin-5. This approach made it possible to identify specific serpin-protease interactions in the natural mixture of proteases and serpins in plasma, without the need for the purified proteases. The availability of a large amount of plasma from M. sexta, a large caterpillar, contributed to the feasibility of this strategy.
Serpin-4 formed complexes with three proteases, HP-1, HP-6, and HP-21. HP-1 and HP-6 were also identified in complexes with serpin-5. These are all clip domain serine proteases (2) with one amino-terminal clip domain and a carboxyl-terminal catalytic domain. Reaction of HP-1 and HP-6 with serpin-4 and serpin-5 in plasma after treatment with Gram-positive or Gram-negative bacteria indicates that HP-1 and HP-6 are activated in response to both types of infection. These results, combined with the previous finding that recombinant serpin-4 or serpin-5 added to plasma can block pro-PO activation (43), suggest that HP-1, HP-6, or perhaps both function upstream of PAPs in the pro-PO activation pathway. The apparently redundant function of serpin-4 and serpin-5 may indicate that regulation of HP-1 and HP-6 is essential for preventing the inappropriate activation of the pro-PO cascade. However, serpin-4 also inhibits at least three additional proteases in plasma and thus may also function in regulating other steps in the pro-PO pathway or proteases involved in other innate immune responses.
With M. luteus as an elicitor, recombinant serpin-5 only partially blocked pro-PO activation in plasma, whereas serpin-4 and the PAP inhibitors serpin-1J and serpin-3 can completely inhibit the activation pathway (40, 41, 43). Serpin-4 and serpin-5 both inhibit HP-1 and HP-6, but serpin-4 also inhibits HP-21 and two proteases that have not yet been characterized. These observations are consistent with an hypothesis that the pro-PO cascade is a branched pathway, with one branch not including HP-1 or HP-6 and therefore not inhibited by serpin-5 (Fig. 11). Complexes of serpin-4 with HP-21 and the two unknown proteases were not detected in plasma activated by E. coli or LPS, suggesting that these proteases may function in a branch of the activation pathway that is stimulated specifically by Gram-positive bacteria. Activation of a protease cascade pathway through branches specific for fungi and Gram-negative bacteria is known for the hemolymph coagulation pathway of horseshoe crabs (47). When recombinant, active HP-1, HP-6, and HP-21 become available, it will be possible to study further their functions and the relative efficiency of serpin-4 and serpin-5 as inhibitors of these proteases.
FIG. 11. A model for the M. sexta pro-PO activation pathway and its regulation by serpins.

Arrows indicate activation of downstream components or steps. Curved arrows represent autoactivation of initiating proteases. Dashed arrows indicate potentially more than one step. Arrows labeled with “?” indicate steps that have not been experimentally verified. Regulation of proteases by serpins is indicated. Two unidentified serpins that regulate HP-14 and HP-8 are labeled as X and Y, respectively.
Proteins that co-purified with serpin-4- and serpin-5-protease complexes may be components of noncovalent complexes that form during activation of the pro-PO cascade, in a manner similar to the complement system. Some HPs that were associated with the SPCs (PAP-3, HP-8, HP-14, and HP-17) were not in the form of a covalent complex with serpin-4 or serpin-5, and thus may have co-purified because of other types of interactions. The co-occurrence of HP-1, HP-6, HP-8, and HP-17 with SPCs after M. luteus or E. coli treatment may indicate that they are components of a protease cascade common to the response to Gram-positive and Gram-negative bacterial infection. HP-14 is a large protein with multiple regulatory domains, which can auto-activate in the presence of peptidoglycan and then stimulate pro-PO activation in plasma (48). Thus, HP-14 is apparently a protease that can initiate the pro-PO activation cascade.
Among other proteins that co-purified with the serpin-protease complexes were immulectins, serine protease homologs, pro-PO, and PO, all known to be components of the pro-PO activation system. A large amount of IML-2 and IML-3 co-purified with serpin-protease complexes. IML-2 binds to LPS and stimulates activation of the pro-PO cascade (21, 22). The presence of IML-2 also in a complex stimulated by Gram-positive bacteria suggests that IML-2 might also bind to a cell wall component of Gram-positive bacteria. Alternatively, the immulectins might function as linkers in protein interactions that bring components of the pro-PO activation pathway together even in the absence of LPS binding. The SPHs are clip domain protease homologs, in which the active site serine of the catalytic domain is replaced by glycine (14). They lack protease activity but are required for efficient activation of pro-PO by purified PAPs (7–9, 14, 50). The SPHs bind to IML-2, pro-PO, and PAPs (14) and form a high molecular weight complex with pro-PO and PAPs in vitro (50), which presents the possibility that a complex of proteases (including some associated with serpins) is held together through their interactions with linking SPH and IML molecules. The formation of such a noncovalent complex, anchored to bacteria by immulectins or other M. sexta plasma proteins that bind to microbial polysaccharides (51), might localize pro-PO activation to the microbial surface. Some components of the pro-PO activation pathway might weakly associate in the hemolymph as a multimolecular complex in the absence of microbial activation. Such initiation complexes in mammalian plasma participate in the classical and the mannan-binding lectin pathways of complement activation (52–54).
Based on the observations from this study and from previous studies, we propose a model for pro-PO activation and serpin regulation in M. sexta (Fig. 11). At the end of the pathway, active PAPs interact with SPH-1 and SPH-2 to cleave pro-PO at Arg51 and generate active PO (7–9, 14). The active PAPs are regulated by serpin-1J and serpin-3 (9, 40). Serpin-4 and serpin-5 block pro-PO activation (43) by inhibiting HP-1 and HP-6, which are upstream of the PAPs. Serpin-4 and serpin-5 do not directly inhibit HP-8, but they block its activation, suggesting that HP-8 is downstream of HP-1 and HP-6. Among the cloned M. sexta HPs, HP-8 has a sequence at its predicted activation site (Asp-Arg-Ile-Val) most similar to the reactive center loop sequences of serpin-4 (Asn-Arg-Ile-Gly) and serpin-5 (Asp-Arg-Ile-Ser), all with Arg-Ile at the P1–P1′ scissile bond. Therefore, we speculate that HP-1 or HP-6, which are inhibited by serpin-4 and serpin-5, may activate HP-8.
In addition to inhibiting active HP-6, serpin-4 blocks activation of the HP-6 zymogen, indicating that serpin-4 inhibits a protease upstream of HP-6 in the activation pathway. A candidate for such a protease is HP-21, which was identified in a serpin-protease complex with serpin-4 after activation by Gram-positive bacteria. Thus, if HP-21 is involved in activation of HP-6, some other unidentified protease must lead to activation of HP-6 in the response to Gram-negative bacteria. Among the serine proteases of D. melanogaster (55), HP-6 is most similar (36% identity) to Persephone, a plasma clip domain protease involved in a response to fungal infection, resulting in synthesis of an antifungal peptide (56). Furthermore, both HP-6 and Persephone have an unusual His residue at the P1 position of their putative activation sites. If HP-6 and Persephone are orthologs, they might have similar functions in pro-PO activation and induced synthesis of antimicrobial peptides.
At the beginning of the pathway, microbial cell wall components bind to microbial pattern recognition proteins (PGRPs, β-1,3-glucan recognition proteins, and immulectins) known to stimulate pro-PO activation (51). HP-14 interacts with peptidoglycan from Gram-positive bacteria and auto-activates (48), leading directly or indirectly to activation of HP-21. A Gram-positive bacteria-specific branch involving HP-21 and two unidentified target proteases of serpin-4 are probably upstream of the components of the pathway common to activation by Gram-positive and Gram-negative bacteria, including HP-1, HP-6, HP-8, and HP-17. A Gram-negative bacteria-specific branch also exists upstream in the pathway, involving a different initiation complex comprising pattern recognition proteins and unknown proteases. HP-21 might directly or indirectly activate HP-1, HP-6, or HP-17. HP-6 or HP-1 may activate HP-8. HP-8 is a candidate for an activator of PAPs. Finally, pro-PO is activated by PAPs in the presence of SPHs 1 and 2. The SPHs must also be activated by specific proteolytic cleavage, which is blocked by serpin-4. These proteins associate to form a large noncovalent complex on the surface of microbial aggregates through their interactions with immulectins and SPHs. Melanization occurs locally, and the activation cascade is limited in time by inhibition of the proteases by serpins.
Acknowledgment
We thank Xiao-Qiang Yu for providing antibodies to immulectins and SPHs.
Footnotes
This work was supported by National Institutes of Health Grants GM41247 (to M. R. K.) and GM58634 (to H. J.). This is contribution 05-217-J from the Kansas Agricultural Experiment Station.
The abbreviations used are: pro-PO, prophenol oxidase; PO, phenol oxidase; HP, hemolymph protease; PAP, pro-PO-activating protease; SPHs, serine protease homologs; LPS, lipopolysaccharide; IML, immulectin; PGRP, peptidoglycan recognition protein; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; SPC, serpin-protease complexes.
H. Jiang, Y. Wang, and M. R. Kanost, unpublished results.
Y. Wang, M. R. Kanost, and H. Jiang, unpublished results.
REFERENCES
- 1.Ashida M, Brey PT. In: Molecular Mechanisms of Immune Responses in Insects. Brey PT, Hultmark D, editors. London: Chapman & Hall Ltd.; 1997. pp. 135–172. [Google Scholar]
- 2.Jiang H, Kanost MR. Insect Biochem. Mol. Biol. 2000;30:95–105. doi: 10.1016/s0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 3.Kanost MR, Jiang H, Wang Y, Yu X-Q, Ma C, Zhu Y. Adv. Exp. Med. Biol. 2001;484:319–328. doi: 10.1007/978-1-4615-1291-2_32. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Wang Y, Kanost MR. Adv. Exp. Med. Biol. 2001;484:313–317. doi: 10.1007/978-1-4615-1291-2_31. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie JP, Kanost MR, Trenczek T. Annu. Rev. Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 6.Nappi AJ, Vass E. Adv. Exp. Med. Biol. 2001;484:329–348. doi: 10.1007/978-1-4615-1291-2_33. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Wang Y, Kanost MR. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12220–12225. doi: 10.1073/pnas.95.21.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Wang Y, Yu X-Q, Kanost MR. J. Biol. Chem. 2003;278:3552–3561. doi: 10.1074/jbc.M205743200. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Wang Y, Yu X-Q, Zhu Y, Kanost MR. Insect Biochem. Mol. Biol. 2003;33:1049–1060. doi: 10.1016/s0965-1748(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 10.Satoh D, Horij A, Ochiai M, Ashida M. J. Biol. Chem. 1999;274:7441–7453. doi: 10.1074/jbc.274.11.7441. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Kwon TH, Hyun JH, Choi JS, Kawabata S-I, Iwanaga S, Lee BL. Eur. J. Biochem. 1998;254:50–57. doi: 10.1046/j.1432-1327.1998.2540050.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SY, Cho MY, Hyun JH, Lee KM, Homma K-I, Natori S, Kawabata S-I, Iawanaga S, Lee BL. Eur. J. Biochem. 1998;257:615–621. doi: 10.1046/j.1432-1327.1998.2570615.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Lee SY, Cerenius L, Soderhall K. Eur. J. Biochem. 2001;268:895–902. doi: 10.1046/j.1432-1327.2001.01945.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu X-Q, Jiang H, Wang Y, Kanost MR. Insect Biochem. Mol. Biol. 2003;33:197–208. doi: 10.1016/s0965-1748(02)00191-1. [DOI] [PubMed] [Google Scholar]
- 15.Kwon TH, Kim MS, Choi HW, Joo CH, Cho MY, Lee BL. Eur. J. Biochem. 2000;267:6188–6196. doi: 10.1046/j.1432-1327.2000.01695.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee KY, Zhang R, Kim MS, Park JW, Park HY, Kawabata S, Lee BL. Eur. J. Biochem. 2002;269:4375–4383. doi: 10.1046/j.1432-1033.2002.03155.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Baek MJ, Lee MH, Park JW, Lee SY, Soderhall K, Lee BL. J. Biol. Chem. 2002;277:39999–40004. doi: 10.1074/jbc.M205508200. [DOI] [PubMed] [Google Scholar]
- 18.Yu X-Q, Zhu Y-F, Ma C, Fabrick JA, Kanost MR. Insect Biochem. Mol. Biol. 2002;32:1287–1293. doi: 10.1016/s0965-1748(02)00091-7. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R, Janeway CA. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 20.Yu X-Q, Gan H, Kanost MR. Insect Biochem. Mol. Biol. 1999;29:585–597. doi: 10.1016/s0965-1748(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 21.Yu X-Q, Kanost MR. J. Biol. Chem. 2000;275:37373–37381. doi: 10.1074/jbc.M003021200. [DOI] [PubMed] [Google Scholar]
- 22.Yu X-Q, Kanost MR. Dev. Comp. Immunol. 2003;27:189–196. doi: 10.1016/s0145-305x(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 23.Ma C, Kanost MR. J. Biol. Chem. 2000;275:7505–7514. doi: 10.1074/jbc.275.11.7505. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Ma C, Lu Z-Q, Kanost MR. Insect Biochem. Mol. Biol. 2004;34:89–100. doi: 10.1016/j.ibmb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida H, Kinoshita K, Ashida M. J. Biol. Chem. 1996;271:13854–13860. doi: 10.1074/jbc.271.23.13854. [DOI] [PubMed] [Google Scholar]
- 26.Ochiai M, Ashida M. J. Biol. Chem. 1999;274:11854–11858. doi: 10.1074/jbc.274.17.11854. [DOI] [PubMed] [Google Scholar]
- 27.Lee MH, Osaki T, Lee JY, Baek MJ, Zhang R, Park JW, Kawabata S, Söderhäll K, Lee BL. J. Biol. Chem. 2004;279:3218–3227. doi: 10.1074/jbc.M309821200. [DOI] [PubMed] [Google Scholar]
- 28.Kurata S. Dev. Comp. Immunol. 2004;28:89–95. doi: 10.1016/s0145-305x(03)00121-6. [DOI] [PubMed] [Google Scholar]
- 29.Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O‘Donnell E, Salvesen GS, Travis J, Whisstock JC. J. Biol. Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 31.Gettins PGW. Chem. Rev. 2002;102:4751–4803. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Wang Z, Canagarajah B, Jiang H, Kanost MR, Goldsmith EJ. Structure (Lond.) 1999;7:103–109. doi: 10.1016/s0969-2126(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 33.Elliott PR, Pei XY, Dafforn TR, Lomas DA. Protein Sci. 2000;9:1274–1281. doi: 10.1110/ps.9.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potempa J, Korzus E, Travis J. J. Biol. Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 35.Irving JA, Pike RN, Lesk AM, Whisstock JC. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- 36.Kanost MR, Prasad SV, Wells MA. J. Biol. Chem. 1989;264:965–972. [PubMed] [Google Scholar]
- 37.Jiang H, Wang Y, Kanost MR. J. Biol. Chem. 1994;269:55–58. [PubMed] [Google Scholar]
- 38.Jiang H, Wang Y, Huang Y, Mulnix AB, Kadel J, Cole K, Kanost MR. J. Biol. Chem. 1996;271:28017–28023. doi: 10.1074/jbc.271.45.28017. [DOI] [PubMed] [Google Scholar]
- 39.Gan H, Wang Y, Jiang H, Mita K, Kanost MR. Insect Biochem. Mol. Biol. 2001;31:887–898. doi: 10.1016/s0965-1748(01)00034-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Wang Y, Gorman MJ, Jiang H, Kanost MR. J. Biol. Chem. 2003;278:46556–46564. doi: 10.1074/jbc.M309682200. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Jiang H. Insect Biochem. Mol. Biol. 2004;34:387–395. doi: 10.1016/j.ibmb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Kanost MR. J. Biol. Chem. 1997;272:1082–1087. doi: 10.1074/jbc.272.2.1082. [DOI] [PubMed] [Google Scholar]
- 43.Tong Y, Kanost MR. J. Biol. Chem. 2005;280:14923–14931. doi: 10.1074/jbc.M500531200. [DOI] [PubMed] [Google Scholar]
- 44.Dunn PE, Drake D. J. Invertebr. Pathol. 1983;41:77–85. doi: 10.1016/0022-2011(83)90219-7. [DOI] [PubMed] [Google Scholar]
- 45.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 46.Jiang H, Wang Y, Kanost MR. Insect Mol. Biol. 1999;8:39–53. doi: 10.1046/j.1365-2583.1999.810039.x. [DOI] [PubMed] [Google Scholar]
- 47.Kawabata S, Muta T, Iwanaga S. In: New Directions in Invertebrate Immunology. Söderhäll K, Iwanaga S, Vasta GR, editors. Fair Haven, NJ: SOS Publications; 1996. pp. 255–284. [Google Scholar]
- 48.Ji C, Wang Y, Guo X, Hartson S, Jiang H. J. Biol. Chem. 2004;279:34101–34106. doi: 10.1074/jbc.M404584200. [DOI] [PubMed] [Google Scholar]
- 49.Jiang H, Wang Y, Ma C, Kanost MR. Insect Biochem. Mol. Biol. 1997;27:835–850. doi: 10.1016/s0965-1748(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Jiang H. Insect Biochem. Mol. Biol. 2004;34:731–742. doi: 10.1016/j.ibmb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Kanost MR, Jiang H, Yu XQ. Immunol. Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- 52.Arlaud GJ, Gaboriaud C, Thielens NM, Rossi V, Bersch B, Hernandez JF, Fontecilla-Camps JC. Immunol. Rev. 2001;180:136–145. doi: 10.1034/j.1600-065x.2001.1800112.x. [DOI] [PubMed] [Google Scholar]
- 53.Thiel S, Petersen SV, Vorup-Jensen T, Matsushita M, Fujita T, Stover CM, Schwaeble WJ, Jensenius JC. J. Immunol. 2000;165:878–887. doi: 10.4049/jimmunol.165.2.878. [DOI] [PubMed] [Google Scholar]
- 54.Matsushita M, Fujita T. Immunol. Rev. 2001;180:78–85. doi: 10.1034/j.1600-065x.2001.1800107.x. [DOI] [PubMed] [Google Scholar]
- 55.Ross J, Jiang H, Kanost MR, Wang Y. Gene (Amst.) 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- 56.Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]


