Abstract
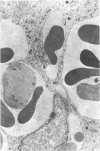
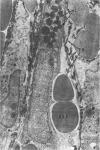
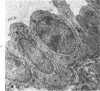
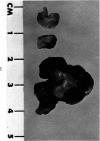
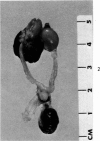
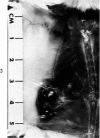
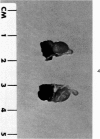
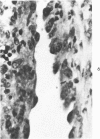
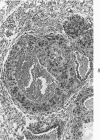
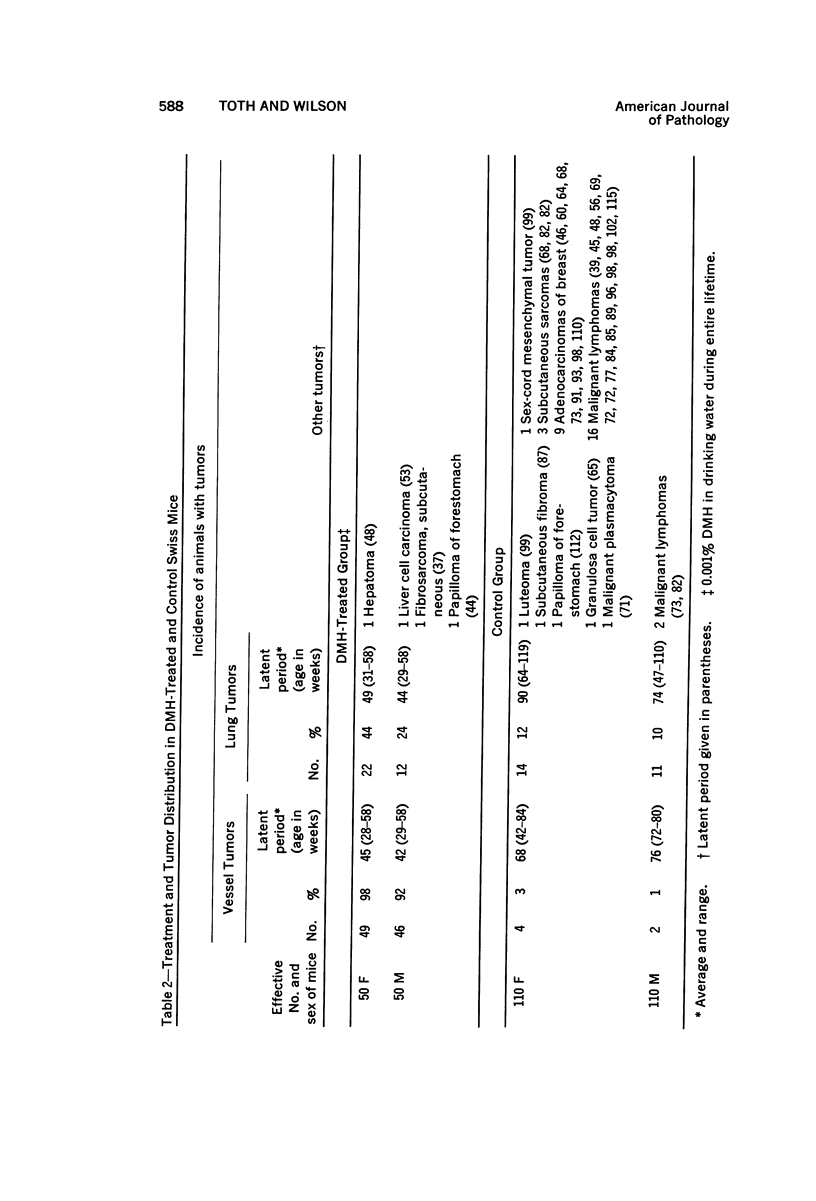
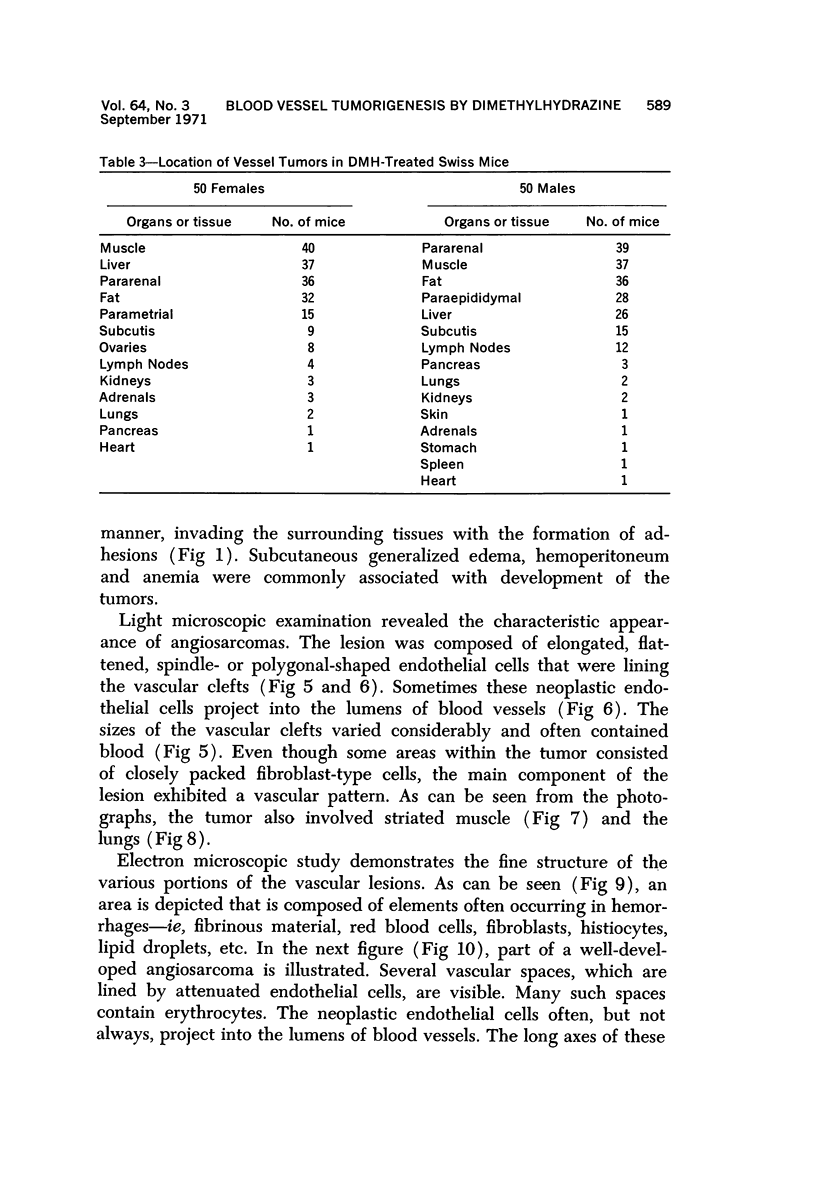
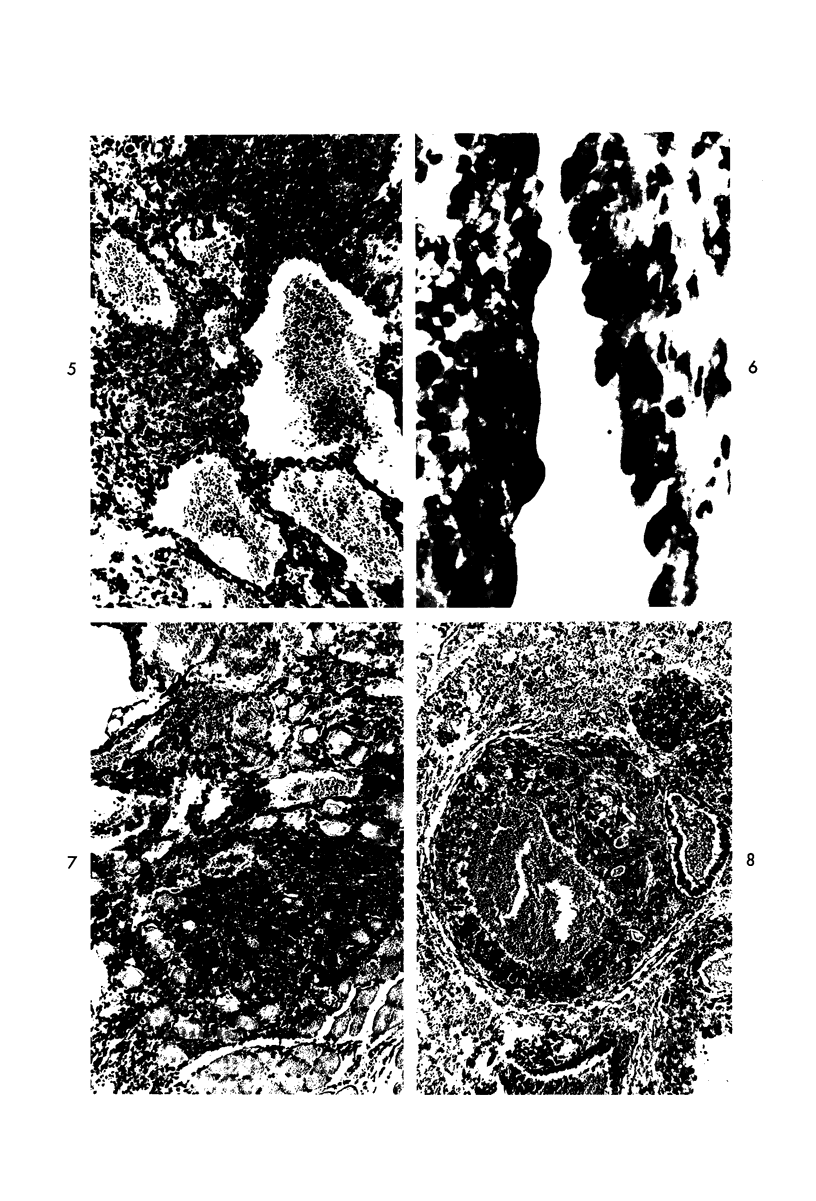
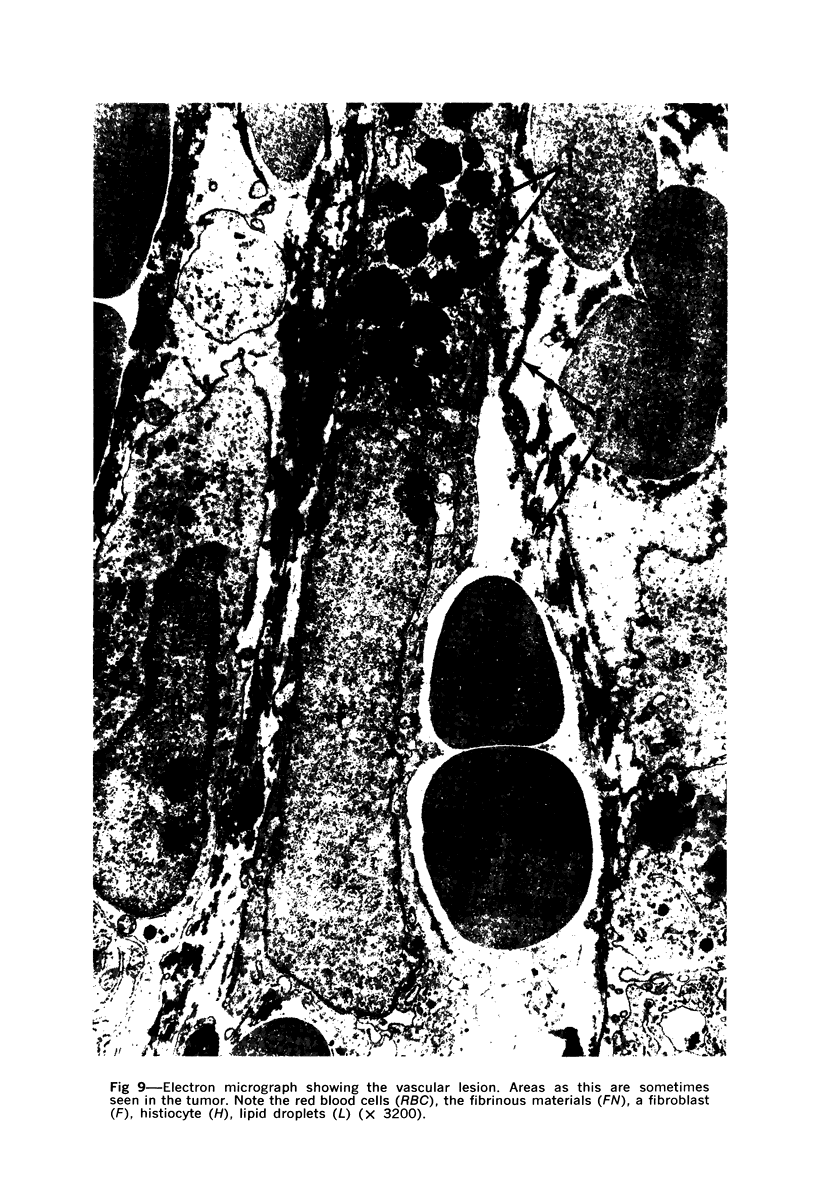
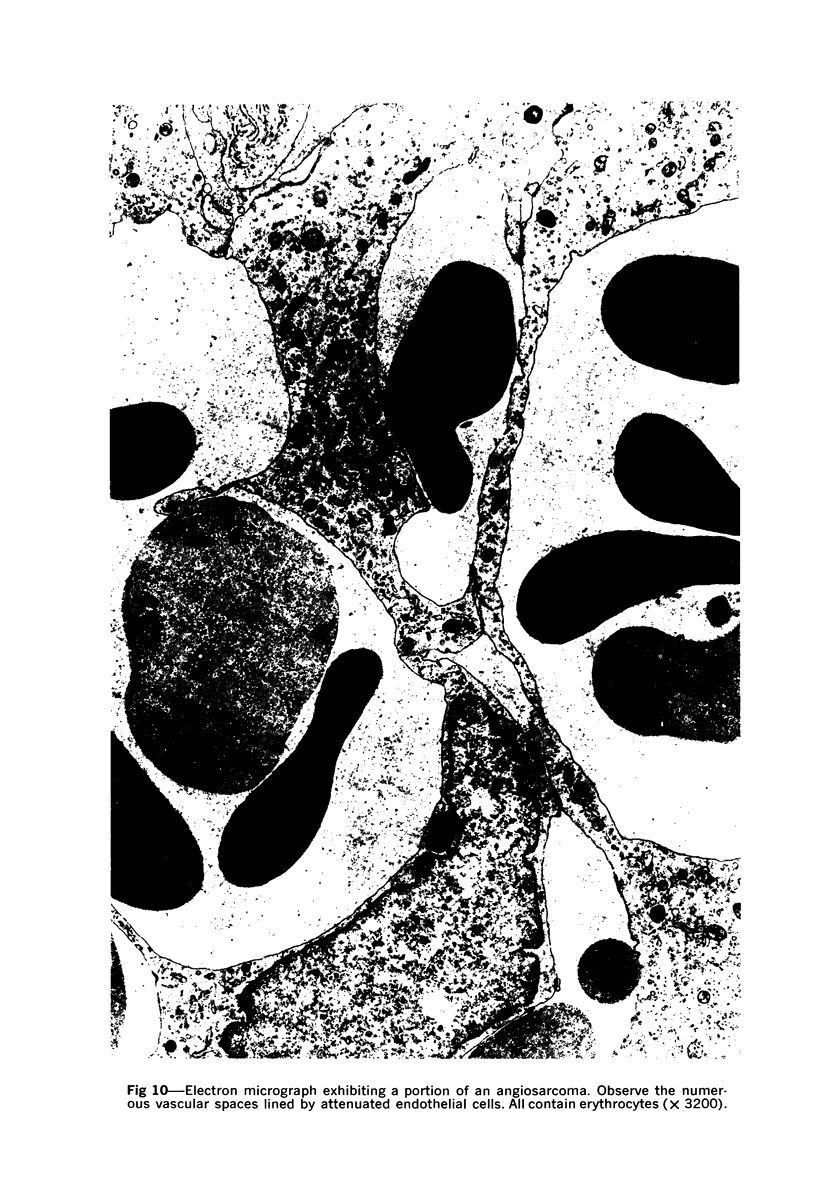
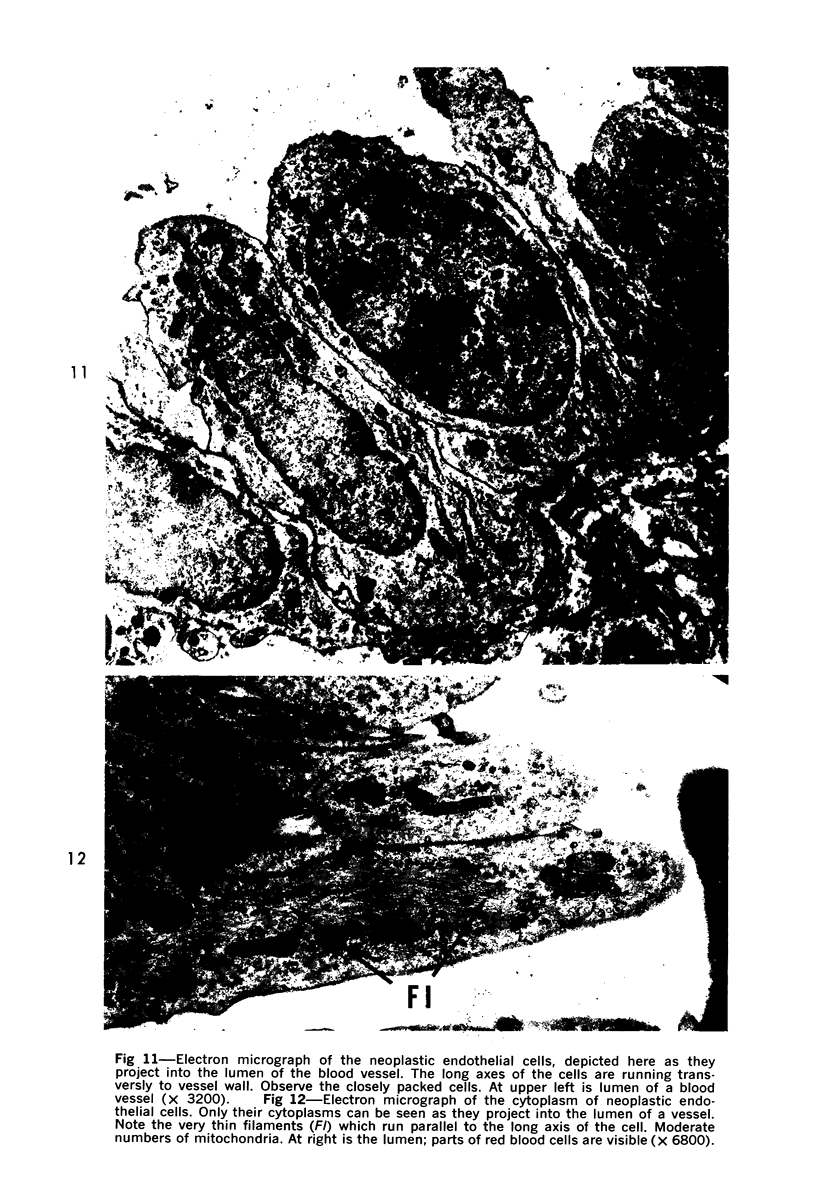
Administration of 0.001% 1,2-dimethylhydrazine dihydrochloride, symmetrical, in the drinking water of 7-week-old randomly bred Swiss mice for the remainder of their lifetime induced blood vessel tumors and enhanced the incidence of lung neoplasms. Ninety-eight percent of the females and 92% of the males developed vascular lesions, whereas among the controls the incidence was 3% in the females and 1% in the males. In addition, the incidence of lung tumors rose from 12 to 44% in the females and from 10 to 24% in the males, as compared with the controls. The occurrence of the vascular tumors in order of decreasing frequency was as follows: muscle, pararenal, fat, liver, parametrial, paraepididymal tissues, etc. Gross, light and electron microscopic examinations of vascular lesions revealed the characteristic appearance of angiosarcomas. The type and extent of macroscopic and histologic involvements of the various tissues by the tumors are presented. The ultrastructural descriptions of hemorrhagic areas, vascular spaces, neoplastic endothelial cells, their cytoplasms and organelles are illustrated in detail.
In conclusion, whereas hydrazine enhanced the development of lung tumors, when the dimethyl group was attached to it at symmetrical positions, it evoked vascular tumors. Thus, the present study provides evidence for the possible relationship between chemical structure and tumor induction at specific organ sites.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADGER G. M. Chemical constitution and carcinogenic activity. Adv Cancer Res. 1954;2:73–127. doi: 10.1016/s0065-230x(08)60492-3. [DOI] [PubMed] [Google Scholar]
- BUU-HOI N. P., DAUDEL R., LACASSAGNE A., ZAJDELA F. The relation between carcinogenic activity and the physical and chemical properties of angular benzacridines. Adv Cancer Res. 1956;4:315–369. doi: 10.1016/s0065-230x(08)60727-7. [DOI] [PubMed] [Google Scholar]
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLAPORTA G., CAPITANO J., MONTIPO W., PARMI L. STUDIO SULL'AZIONE CANCEROGENA DELL'URETANO NEL TOPO. Tumori. 1963 Nov-Dec;49:413–427. [PubMed] [Google Scholar]
- Deringer M. K. Response of strain DBA-2eBDe mice to treatment with urethan. J Natl Cancer Inst. 1965 Jun;34(6):841–847. [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten. Z Krebsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Matzkies F., Ivankovic S. Selektive Erzeugung von Darmkrebs bei Ratten durch 1,2-Dimethyl-hydrazin. Naturwissenschaften. 1967 Jun;54(11):285–286. doi: 10.1007/BF00620890. [DOI] [PubMed] [Google Scholar]
- Florey The endothelial cell. Br Med J. 1966 Aug 27;2(5512):487–490. doi: 10.1136/bmj.2.5512.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESTON W. E., VLAHAKIS G., DERINGER M. K. High incidence of spontaneous hepatomas and the increase of this incidence with urethan in C3H, C3Hf, and C3He male mice. J Natl Cancer Inst. 1960 Feb;24:425–435. [PubMed] [Google Scholar]
- KAWAMOTO S., KIRSCHBAUM A., IBANEZ M. L., TRENTIN J. J., TAYLOR H. G. Influence of urethan on the development of spontaneous leukemia and on the induction of hemagiomas in the AKR and C58 strains of mice. Cancer Res. 1961 Jan;21:71–74. [PubMed] [Google Scholar]
- Kelly M. G., O'Gara R. W., Yancey S. T., Gadekar K., Botkin C., Oliverio V. T. Comparative carcinogenicity of N-isopropyl-alpha-(2-methylhydraziono)-p-toluamide. HCI (procarbazine hydrochloride), its degradation products, other hydrazines, and isonicotinic acid hydrazide. J Natl Cancer Inst. 1969 Feb;42(2):337–344. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. N., Barnes J. M. Carcinogenic nitroso compounds. Adv Cancer Res. 1967;10:163–246. doi: 10.1016/s0065-230x(08)60079-2. [DOI] [PubMed] [Google Scholar]
- Osswald H., Krüger F. W. Die cancerogene Wirkung von 1,2-Dimethylhydrazin beim Goldhamster. Arzneimittelforschung. 1969 Nov;19(11):1891–1892. [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe F. J., Grant G. A., Millican D. M. Carcinogenicity of hydrazine and 1,1-dimethylhydrazine for mouse lung. Nature. 1967 Oct 28;216(5113):375–376. doi: 10.1038/216375a0. [DOI] [PubMed] [Google Scholar]
- Schauer A., Völlnagel T., Wildanger F. Cancerisierung des Rattendarmes durch 1,2-Dimethylhydrazin. Z Gesamte Exp Med. 1969;150(1):87–93. [PubMed] [Google Scholar]
- TOTH B., MAGEE P. N., SHUBIK P. CARCINOGENESIS STUDY WITH DIMETHYLNITROSAMINE ADMINISTERED ORALLY TO ADULT AND SUBCUTANEOUSLY TO NEWBORN BALB-C MICE. Cancer Res. 1964 Nov;24:1712–1721. [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Terracini B., Palestro G., Gigliardi M. R., Montesano G., Montesano R. Carcinogenicity of dimethylnitrosamine in Swiss mice. Br J Cancer. 1966 Dec;20(4):871–876. doi: 10.1038/bjc.1966.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B. Lung tumor induction and inhibition of breast adenocarcinomas by hydrazine sulfate in mice. J Natl Cancer Inst. 1969 Mar;42(3):469–475. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebecke B., Löhrs U., Gimmy J., Eder M. Erzeugung von Darmtumoren bei Mäusen durch 1,2-Dimethylhydrazin. Z Gesamte Exp Med. 1969;149(3):277–278. [PubMed] [Google Scholar]