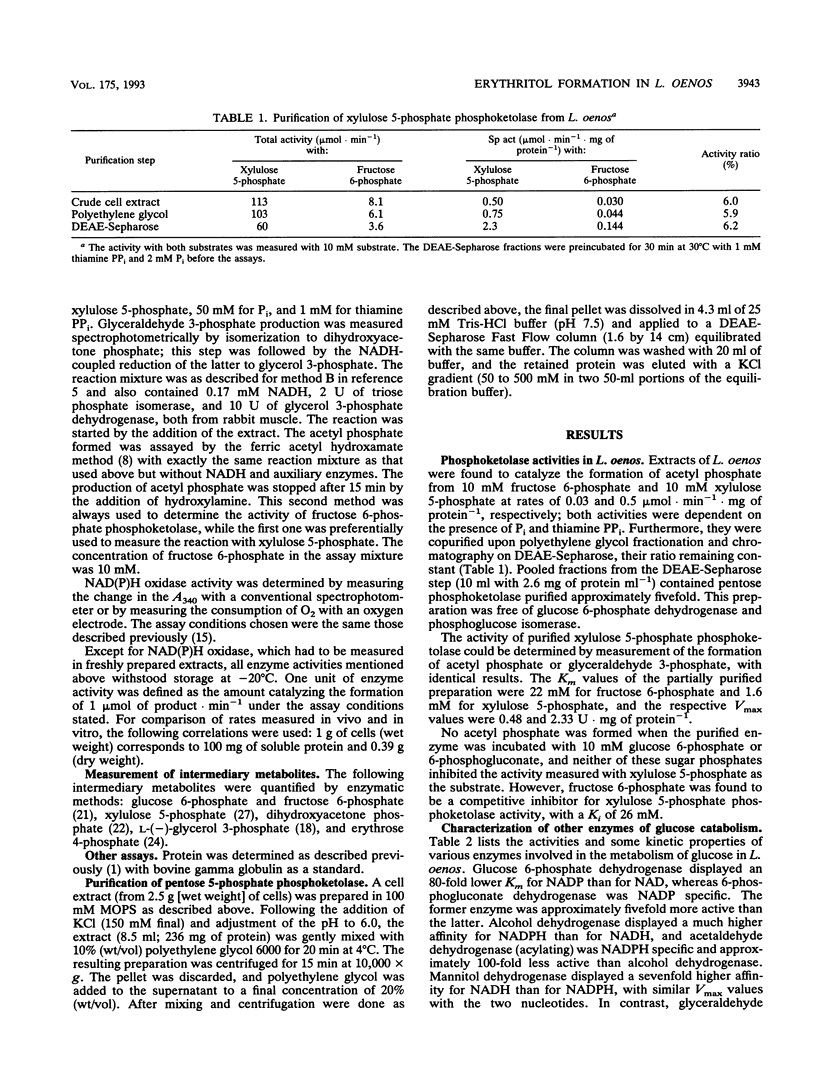
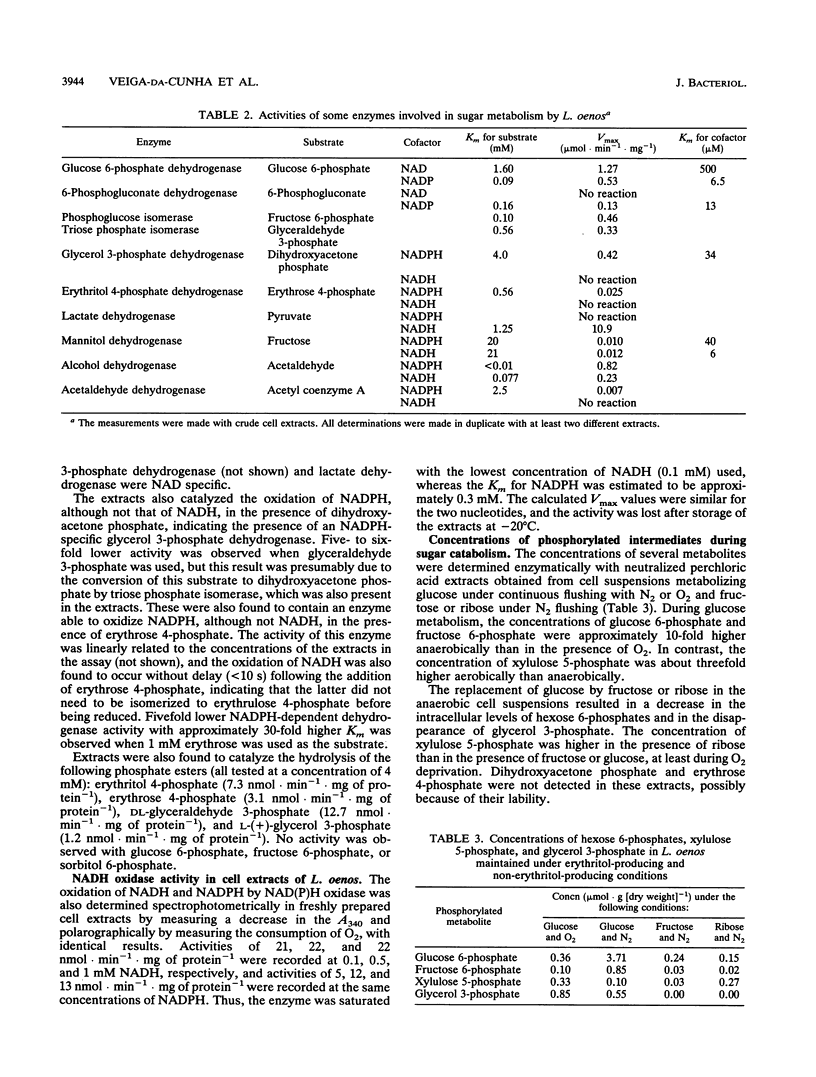
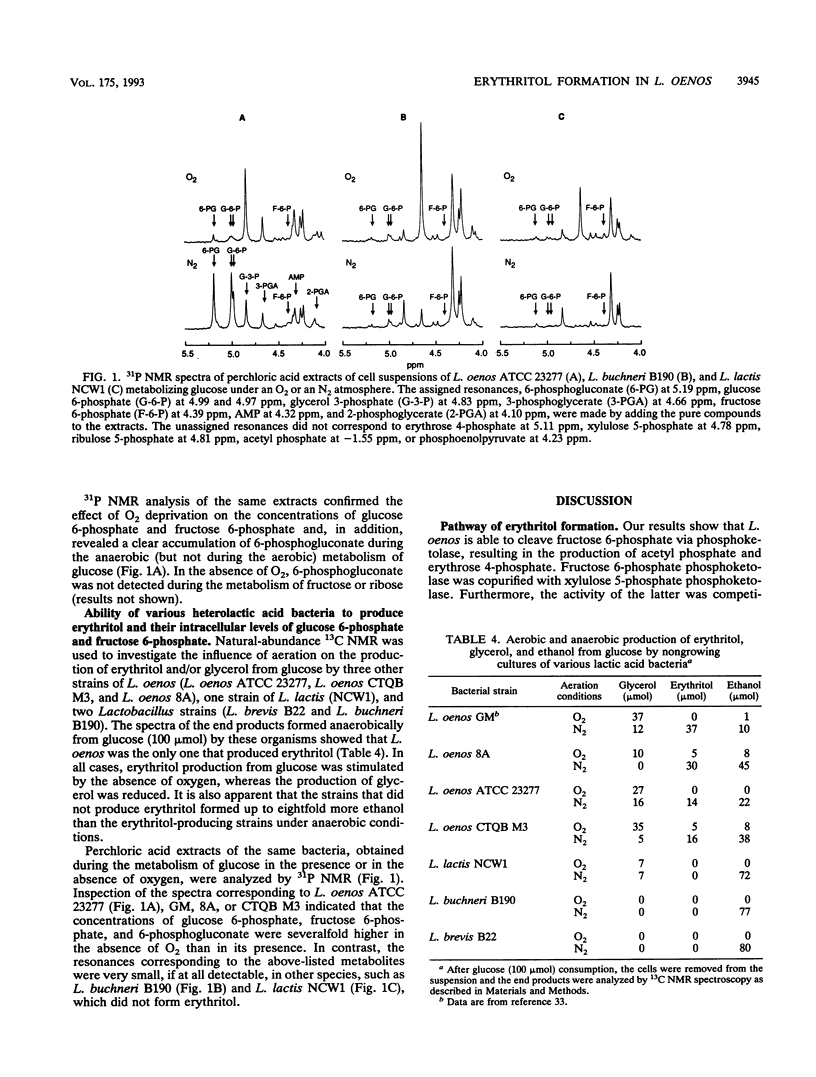
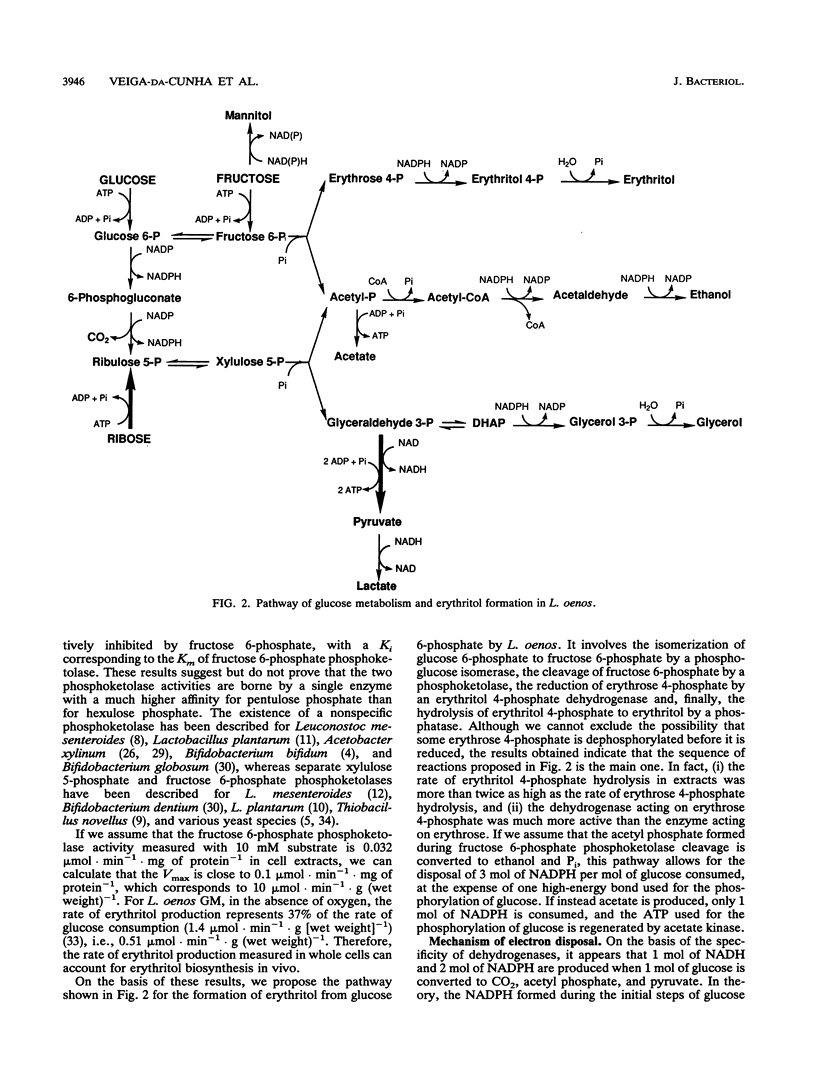
Abstract
It was recently observed that Leuconostoc oenos GM, a wine lactic acid bacterium, produced erythritol anaerobically from glucose but not from fructose or ribose and that this production was almost absent in the presence of O2. In this study, the pathway of formation of erythritol from glucose in L. oenos was shown to involve the isomerization of glucose 6-phosphate to fructose 6-phosphate by a phosphoglucose isomerase, the cleavage of fructose 6-phosphate by a phosphoketolase, the reduction of erythrose 4-phosphate by an erythritol 4-phosphate dehydrogenase and, finally, the hydrolysis of erythritol 4-phosphate to erythritol by a phosphatase. Fructose 6-phosphate phosphoketolase was copurified with xylulose 5-phosphate phosphoketolase, and the activity of the latter was competitively inhibited by fructose 6-phosphate, with a Ki of 26 mM, corresponding to the Km of fructose 6-phosphate phosphoketolase (22 mM). These results suggest that the two phosphoketolase activities are borne by a single enzyme. Extracts of L. oenos were also found to contain NAD(P)H oxidase, which must be largely responsible for the reoxidation of NADPH and NADH in cells incubated in the presence of O2. In cells incubated with glucose, the concentrations of glucose 6-phosphate and of fructose 6-phosphate were higher in the absence of O2 than in its presence, explaining the stimulation by anaerobiosis of erythritol production. The increase in the hexose 6-phosphate concentration is presumably the result of a functional inhibition of glucose 6-phosphate dehydrogenase because of a reduction in the availability of NADP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cavin J. F., Prevost H., Lin J., Schmitt P., Divies C. Medium for Screening Leuconostoc oenos Strains Defective in Malolactic Fermentation. Appl Environ Microbiol. 1989 Mar;55(3):751–753. doi: 10.1128/aem.55.3.751-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG M. L., RACKER E. Formation and isolation of a glycoladehyde-phosphoketolase intermediate. J Biol Chem. 1962 Dec;237:3841–3842. [PubMed] [Google Scholar]
- HEATH E. C., HURWITZ J., HORECKER B. L., GINSBURG A. Pentose fermentation by Lactobacillus plantarum. I. The cleavage of xylulose 5-phosphate by phosphoketolase. J Biol Chem. 1958 Apr;231(2):1009–1029. [PubMed] [Google Scholar]
- HURWITZ J. Pentose phosphate cleavage by Leuconostoc mesenteroides. Biochim Biophys Acta. 1958 Jun;28(3):599–602. doi: 10.1016/0006-3002(58)90526-2. [DOI] [PubMed] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- Kanodia S., Roberts M. F. Methanophosphagen: Unique cyclic pyrophosphate isolated from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5217–5221. doi: 10.1073/pnas.80.17.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Kobayashi T., Ito S., Saitoh M. Purification and characterization of NADH oxidase from a strain of Leuconostoc mesenteroides. J Biochem. 1985 May;97(5):1279–1288. doi: 10.1093/oxfordjournals.jbchem.a135179. [DOI] [PubMed] [Google Scholar]
- PEYNAUD E., GUIMBERTEAU G. SUR LES POLYOLS FORM'ES DANS LA FERMENTATION LACTIQUE DES GLUCIDES. C R Hebd Seances Acad Sci. 1964 May 4;258:4626–4628. [PubMed] [Google Scholar]
- SCHRAMM M., KLYBAS V., RACKER E. Phosphorolytic cleavage of fructose-6-phosphate by fructose-6-phosphate phosphoketolase from Acetobacter xylinum. J Biol Chem. 1958 Dec;233(6):1283–1288. [PubMed] [Google Scholar]
- Sgorbati B., Lenaz G., Casalicchio F. Purification and properties of two fructose-6-phosphate phosphoketolases in Bifidobacterium. Antonie Van Leeuwenhoek. 1976;42(1-2):49–57. doi: 10.1007/BF00399448. [DOI] [PubMed] [Google Scholar]
- Tseng C. P., Montville T. J. Enzyme Activities Affecting End Product Distribution by Lactobacillus plantarum in Response to Changes in pH and O(2). Appl Environ Microbiol. 1990 Sep;56(9):2761–2763. doi: 10.1128/aem.56.9.2761-2763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Da-Cunha M., Firme P., Romão M. V., Santos H. Application of C Nuclear Magnetic Resonance To Elucidate the Unexpected Biosynthesis of Erythritol by Leuconostoc oenos. Appl Environ Microbiol. 1992 Jul;58(7):2271–2279. doi: 10.1128/aem.58.7.2271-2279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Gerbrandy S. J., Stouthamer A. H. Carbohydrate metabolism in Bifidobacterium bifidum. Biochim Biophys Acta. 1967 Apr 25;136(3):415–425. doi: 10.1016/0304-4165(67)90001-3. [DOI] [PubMed] [Google Scholar]


