Abstract
The fate of heteroduplex molecules containing 5-, 7-, 9-, 192-, 410-, and 514-base loops after transformation of wild-type and various mutant strains of Escherichia coli has been examined. No evidence for repair was obtained for the wild type or for strains with mutations in the following genes: mutS, recA, recBC sbcBC, recD, recF, recJ, recN, recO, recR, recBC sbcBC recF uvrA, recG ruvC, ruvB, lexA3, lexA51, uvrA, nfo xth nth, polA(Ts), or pcnB. These results rule out the involvement of the SOS system and most known recombination and repair pathways. Repair of heteroduplex molecules containing 410- and 514-base loops was observed when a 1-base deletion-insertion mismatch was present nearby. The repair of both the mismatch and the loops was directed by the state of dam methylation of the DNA chains and was dependent on the product of the mutS gene. A high efficiency of repair (95%) was found even when the mismatch and the loops were 1,448 nucleotides apart. We conclude that multibase loops in DNA can be removed only as a consequence of corepair by dam-directed mismatch repair.
Full text
PDF


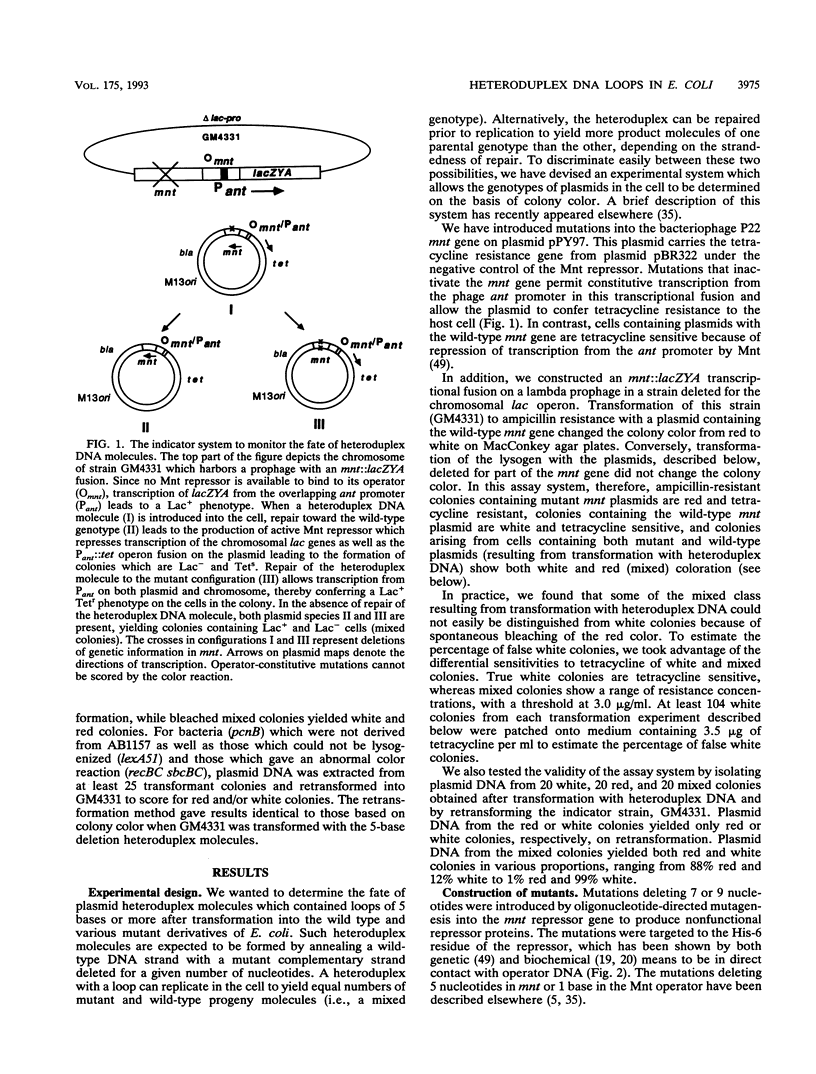




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Bebenek K., Kunkel T. A. The use of native T7 DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 1989 Jul 11;17(13):5408–5408. doi: 10.1093/nar/17.13.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe L., Marinus M. G. Role of plasmid multimers in mutation to tetracycline resistance. Mol Microbiol. 1991 Oct;5(10):2541–2545. doi: 10.1111/j.1365-2958.1991.tb02100.x. [DOI] [PubMed] [Google Scholar]
- Carraway M., Rewinski C., Marinus M. G. Mutations produced by DNA polymerase III holoenzyme of Escherichia coli after in vitro synthesis in the absence of single-strand binding protein. Mol Microbiol. 1990 Oct;4(10):1645–1652. doi: 10.1111/j.1365-2958.1990.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Carraway M., Youderian P., Marinus M. G. Spontaneous mutations occur near dam recognition sites in a dam- Escherichia coli host. Genetics. 1987 Jul;116(3):343–347. doi: 10.1093/genetics/116.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B., Parsons C. A., Benson F. E., Dunderdale H. J., Sharples G. J., Lloyd R. G., West S. C. Resolution of Holliday junctions in vitro requires the Escherichia coli ruvC gene product. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6063–6067. doi: 10.1073/pnas.88.14.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohet C., Dzidić S., Wagner R., Radman M. Large non-homology in heteroduplex DNA is processed differently than single base pair mismatches. Mol Gen Genet. 1987 Jan;206(1):181–184. doi: 10.1007/BF00326556. [DOI] [PubMed] [Google Scholar]
- Dunderdale H. J., Benson F. E., Parsons C. A., Sharples G. J., Lloyd R. G., West S. C. Formation and resolution of recombination intermediates by E. coli RecA and RuvC proteins. Nature. 1991 Dec 19;354(6354):506–510. doi: 10.1038/354506a0. [DOI] [PubMed] [Google Scholar]
- Feinstein S. I., Low K. B. Hyper-recombining recipient strains in bacterial conjugation. Genetics. 1986 May;113(1):13–33. doi: 10.1093/genetics/113.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R., Kolodner R. Gene conversion in Escherichia coli: the recF pathway for resolution of heteroduplex DNA. J Bacteriol. 1989 Jun;171(6):3046–3052. doi: 10.1128/jb.171.6.3046-3052.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Initiation of replication of plasmid ColE1 DNA by RNA polymerase, ribonuclease H, and DNA polymerase I. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):409–417. doi: 10.1101/sqb.1979.043.01.047. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Takahagi M., Shiba T., Nakata A., Shinagawa H. Escherichia coli RuvC protein is an endonuclease that resolves the Holliday structure. EMBO J. 1991 Dec;10(13):4381–4389. doi: 10.1002/j.1460-2075.1991.tb05016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. A., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982 Sep 25;160(3):411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Sauer R. T. DNA binding specificity of the Arc and Mnt repressors is determined by a short region of N-terminal residues. Proc Natl Acad Sci U S A. 1989 Feb;86(3):797–801. doi: 10.1073/pnas.86.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Sauer R. T. Identification of functionally important residues in the DNA binding region of the mnt repressor. J Biol Chem. 1989 Aug 15;264(23):13706–13710. [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn B. A., Grafstrom R. H. Methyl-directed repair of frameshift heteroduplexes in cell extracts from Escherichia coli. J Bacteriol. 1989 Dec;171(12):6473–6481. doi: 10.1128/jb.171.12.6473-6481.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Buckman C., Benson F. E. Genetic analysis of conjugational recombination in Escherichia coli K12 strains deficient in RecBCD enzyme. J Gen Microbiol. 1987 Sep;133(9):2531–2538. doi: 10.1099/00221287-133-9-2531. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G. Conjugational recombination in resolvase-deficient ruvC mutants of Escherichia coli K-12 depends on recG. J Bacteriol. 1991 Sep;173(17):5414–5418. doi: 10.1128/jb.173.17.5414-5418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J., Bortner S., Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986 Nov;205(2):285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- Lu A. L., Clark S., Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4639–4643. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi A. A., Lloyd R. G. Identification of the recR locus of Escherichia coli K-12 and analysis of its role in recombination and DNA repair. Mol Gen Genet. 1989 Apr;216(2-3):503–510. doi: 10.1007/BF00334397. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Konrad E. B. Hyper-recombination in dam mutants of Escherichia coli K-12. Mol Gen Genet. 1976 Dec 22;149(3):273–277. doi: 10.1007/BF00268528. [DOI] [PubMed] [Google Scholar]
- Marinus M. G. Location of DNA methylation genes on the Escherichia coli K-12 genetic map. Mol Gen Genet. 1973 Dec 14;127(1):47–55. doi: 10.1007/BF00267782. [DOI] [PubMed] [Google Scholar]
- Murphy K. C. Lambda Gam protein inhibits the helicase and chi-stimulated recombination activities of Escherichia coli RecBCD enzyme. J Bacteriol. 1991 Sep;173(18):5808–5821. doi: 10.1128/jb.173.18.5808-5821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. O., Marinus M. G. Repair of DNA heteroduplexes containing small heterologous sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1730–1734. doi: 10.1073/pnas.89.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B., Marinus M. G. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene. 1988 Dec 20;73(2):531–535. doi: 10.1016/0378-1119(88)90517-3. [DOI] [PubMed] [Google Scholar]
- Pasta F., Lefèvre J. C., Guillot E., Sicard A. M. Conversion of large heterologies in Streptococcus pneumoniae. Biochimie. 1991 Apr;73(4):353–355. doi: 10.1016/0300-9084(91)90100-f. [DOI] [PubMed] [Google Scholar]
- Pukkila P. J., Peterson J., Herman G., Modrich P., Meselson M. Effects of high levels of DNA adenine methylation on methyl-directed mismatch repair in Escherichia coli. Genetics. 1983 Aug;104(4):571–582. doi: 10.1093/genetics/104.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa S., Fox M. S. Some features of base pair mismatch and heterology repair in Escherichia coli. Genetics. 1987 Nov;117(3):381–390. doi: 10.1093/genetics/117.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayssiguier C., Thaler D. S., Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989 Nov 23;342(6248):396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- Rewinski C., Marinus M. G. Mutation spectrum in Escherichia coli DNA mismatch repair deficient (mutH) strain. Nucleic Acids Res. 1987 Oct 26;15(20):8205–8215. doi: 10.1093/nar/15.20.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. REPLICAtion of small plasmids in extracts of Escherichia coli: requirement for both DNA polymerases I and II. Mol Gen Genet. 1976 Dec 8;149(2):151–158. doi: 10.1007/BF00332883. [DOI] [PubMed] [Google Scholar]
- Wagner R., Jr, Meselson M. Repair tracts in mismatched DNA heteroduplexes. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4135–4139. doi: 10.1073/pnas.73.11.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg J., Meselson M. Mismatch repair in heteroduplex DNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2202–2206. doi: 10.1073/pnas.72.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. H., Clarke C. H., Marinus M. G. Specificity of Escherichia coli mutD and mutL mutator strains. Gene. 1990 Mar 1;87(1):1–5. doi: 10.1016/0378-1119(90)90488-d. [DOI] [PubMed] [Google Scholar]
- Youderian P., Vershon A., Bouvier S., Sauer R. T., Susskind M. M. Changing the DNA-binding specificity of a repressor. Cell. 1983 Dec;35(3 Pt 2):777–783. doi: 10.1016/0092-8674(83)90110-1. [DOI] [PubMed] [Google Scholar]