Abstract
In Alzheimer's disease (AD) the accumulation of pathological forms of the beta-amyloid (Aβ) peptide are believed to be causal factors in the neurodegeneration that results in the loss of cognitive function in patients. Anti-Aβ antibodies have been shown to reduce Aβ levels in transgenic mouse models of AD and in AN-1792 clinical trial on AD patients; however, the clinical trial was halted when some patients developed meningoencephalitis. Theories on the cause of the adverse events include proinflammatory “primed patients,” a Th1-inducing adjuvant, and Aβ autoreactive T cells. New immunotherapy approaches are being developed to eliminate these putative risk factors. Mannan, which is recognized by pattern recognition receptors of the innate immune system, can be utilized as a molecular adjuvant to promote a Th2-mediated immune response to conjugated B cell epitopes. The N-terminus of Aβ was conjugated to mannan, and used to immunize mice with low concentrations of immunoconjugate, without a conventional adjuvant. Mannan induced a significant and highly polarized toward Th2 phenotype anti-Aβ antibody response not only in BALB/c, but also in B6SJL F1 mice. New preclinical trials in AD mouse models may help to develop novel immunogen–adjuvant configurations with the potential to avoid the adverse immune response that occurred in the first clinical trial.
INTRODUCTION
Alzheimer's disease (AD) is a most common form of dementia in the elderly and is characterized by a progressive loss of memory and general cognitive decline. The neuropathological features of the disease include neurofibrillary tangles (NFT), deposition of amyloid-beta (Aβ) in senile plaques, and neuronal loss in affected brain regions (Price and Sisodia, 1994). The amyloid cascade hypothesis that proposed a central role of Aβ deposition in the brain in the onset and progression of AD (Hardy and Higgins, 1992; Hardy and Selkoe, 2002), remains to be a rationale for therapeutic strategies (Golde, 2005). Thus, reduction of the level of Aβ in the brain may diminish learning and memory deficits observed in AD patients. Recently, several groups have demonstrated that active immunization of amyloid precursor protein (APP) transgenic (Tg) mice with fibrillar Aβ, as well as passive transfer of anti-Aβ antibodies, significantly reduced Aβ plaque deposition, neuritic dystrophy, and astrogliosis in the brains of these mice (Schenk et al., 1999; Bard et al., 2000; Morgan et al., 2000; Wilcock et al., 2004a). Improvements in learning and memory were also observed after either active or passive immunization of APP/Tg mice (Janus et al., 2000; Morgan et al., 2000; Dodart et al., 2002; Sigurdsson et al., 2004; Wilcock et al., 2004a; 2004b).
Based on these results, the AN-1792 vaccine clinical trial was initiated with AD patients, but was halted because a subset of participants developed meningoencephalitis. Although the results of the first vaccination of elderly AD patients with Aβ42 peptide raised concerns about the safety of AN-1792 vaccine, follow-up studies suggest that anti-Aβ antibodies were capable of reducing AD pathology and, at least in some patients, diminishing the progressive cognitive decline associated with the disease (Hock et al., 2003; Nicoll et al., 2003; Ferrer et al., 2004; Bayer et al., 2005; Fox et al., 2005; Gilman et al., 2005; Masliah et al., 2005). Second-generation vaccines, which induce a Th2-polarized immune response or utilize nonself T-cell epitopes in the immunogen to amplify the antibody response to the B-cell epitope of Aβ, may provide safer alternatives for active immunization (Cribbs et al., 2003b; Agadjanyan et al., 2005; Cribbs and Agadjanyan, 2005). Previously, it was demonstrated that a Th2-type of humoral immune response in APP/Tg mice was therapeutically effective (Weiner et al., 2000). Thus, an adjuvant that can direct the immune response towards a Th2 phenotype may be critical for the design of a safe and effective immunotherapy for AD patients.
Mannan has been investigated extensively as a molecular adjuvant due to its ability to enhance both B- and T-cell immune responses (Okawa et al., 1992; Apostolopoulos et al., 1995, 2000; Karanikas et al., 1997; Vaughan et al., 2000; Stambas et al., 2002a, 2002b, 2005). The adjuvant function is dependent on the ability to target the immunogen to antigen-presenting cells expressing receptors specific to mannosylated sugars. Mannose-binding receptors (MBRs) are expressed on dendritic cells, some endothelial cells, and macrophages (Engering et al., 1997a; Gröger et al., 2000; Linehan et al., 2000). In addition, Mannose-binding lectin (MBL) (Turner, 1996; Tenner, 1999; Vasta et al., 1999), which also has an opsonic function similar to complement C1q (homologue of MBL), binds to complement receptor type 1 (CD35) (Ghiran et al., 2000), and therefore stimulates phagocytosis of antigen conjugated to mannan. Dendritic cells are able to present very low concentrations of mannosylated antigen 100–1000 fold more efficiently than non-mannosylated antigen (Engering et al., 1997a). Besides promoting phagocytosis and antigen presentation, antigens conjugated to mannan may also provide a stronger signal to antigen-specific B cells by simultaneous triggering of BCR and CD21 and/or BCR and CD35 C' receptors (Molina et al., 1996; Kozono et al., 1998). Enhancement of immune responses against several mannosylated antigens, including peptide antigens, has been demonstrated (Okawa et al., 1992; Apostolopoulos et al., 1995, 2000). Under certain conditions, mannosylated antigens induced strong Th2 type anti-inflammatory responses with high levels of IgG1 antibodies, IL4 and IL10 production (Okawa et al., 1992; Apostolopoulos et al., 1995, 1996; Vaughan et al., 1999; Apostolopoulos and McKenzie, 2001).
In this paper we report the development of a novel AD vaccine consisting of the N-terminus of Aβ (Aβ28) conjugated to mannan. We demonstrated that low doses of Aβ28 conjugated with mannan, in the absence of conventional adjuvant, are capable of eliciting a robust Th2-type anti-Aβ immunity in mice. The antibodies were specific for the N-terminus and were judged functional based on strong binding to Aβ-plaques in brain tissue from an AD case.
MATERIALS AND METHODS
Preparation of peptides and conjugation with mannan
Aβ peptides spanning aa 1–42 (Aβ42), 1–28 (Aβ28), 1–15 (Aβ1–15), 6–20 (Aβ6–20), 11–25 (Aβ11–25), and 16–30 (Aβ16–30) of Aβ42 were synthesized at the UCI core facility (Cribbs et al., 2003b; Petrushina et al., 2003). The Aβ28 peptide with an N-terminal linker (n-CAGA) sequence was synthesized, and mannan–Aβ28 conjugate was prepared as previously described (Inman, 1993; Lees et al., 1996). More specifically, mannan from Saccharomyces cerevisiae (cat #M-3640; Sigma, St. Louis, MO) was further purified by passage over a Q Sepharose FF column. Purified mannan (10 mg/ml) was activated by addition of the organic cyanylating reagent 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) (25 μl/ml, 100 mg/ml in acetonitrile). After 30 sec 25 μl of aqueous 0.2 M triethylamine (TEA) was added. After another 2 min an equal volume of 0.5 M hexanediamine, pH 9, was added and the reaction allowed to continue overnight. The solution was then dialyzed against water, and the mannan concentration and the free amine content were determined as previously described (Lees et al., 1996). The amino-mannan was bromoacetylated using NHS bromoacetate and desalted by dialysis. The n-CAGA–Aβ28 peptide (5.0 mg/ml) was dissolved in 0.15 M HEPES, 2 mM EDTA, pH 7.3, and the free thiol content determined using Ellman's reagent. The peptide was combined with the bromoacetylated mannan at a molar ratio of 30 thiols/100 kDa of carbohydrate under a stream of nitrogen. After an overnight reaction, the solution was quenched by the addition of mercaptoethanol, concentrated with an Ultra 4 (10-kDa cutoff) device (Millipore, Bedford, MA), and free peptide removed by gel filtration on a Superose 12 column equilibrated with 0.1 M HEPES, pH 8. The void volume peak was pooled. The peptide content was determined from the UV spectrum and its calculated extinction coefficient. The final product contained 50 μM peptide and five molecules of peptides per 100 kDa of mannan formulated in PBS.
Immunization of mice
Six- to 8-week old mice of different immune haplotypes BALB/c) and APP/Tg 2576 animals were immunized four times (biweekly) subcutaneously (s.c.) with 100 μg of fibrillar Aβ28 in alum as previously described (Cribbs et al., 2003b). To evaluate the efficacy of mannan as an adjuvant we immunized BALB/c mice s.c. four times (biweekly) with 2.5, 5, or 10 μg of mannan–Aβ28 peptide or with 10 μg of fibrillar Aβ28 peptide without alum. Two months later, the BALB/c mice were boosted one more time with the same dose of the appropriate immunogen.
Detection of anti-Aβ42 antibodies
Eight to 9 days after each immunization, sera from mice were collected, and anti-Aβ42 antibodies, as well as their isotypes, were determined by ELISA (Cribbs et al., 2003b; Ghochikyan et al., 2003; Petrushina et al., 2003; Agadjanyan et al., 2005). To identify the B-cell antigenic determinant/s within Aβ28, we used four overlapping 15-mer peptides (Aβ1–15, Aβ6–20, Aβ11–25, and Aβ16–30) for epitope mapping. Since adsorption of a peptide to an ELISA plate may mask some of the peptide's epitopes, we detected B-cell antigenic determinants using a competition ELISA as previously described (Cribbs et al., 2003b).
Detection of anti-Aβ T-cell proliferation and production of cytokines by immune splenocytes
Nine days after the last boost, BALB/c mice were sacrificed, and anti-Aβ T-cell responses were analyzed using splenocyte cultures from individual mice. We used HL-1 serum-free synthetic medium (Cambrex, Baltimore, MD) for our T-cell stimulation assays, because it significantly decreases nonspecific activation of splenocytes, allowing measurement of T-cell activation (proliferation, cytokine production, and Th1 and Th2 subsets) more accurately. To detect proliferation of splenocytes, we restimulated individual culture of cells with Aβ40 peptide and measured 3[H]thymidine uptake, as described previously (Cribbs et al., 2003b). Data are presented as the Stimulation Index (SI), and were calculated for each mouse.
We used the ELISPOT technique to detect production of IFNγ-Th1) or IL4 (Th2) lymphokines, as well as TNFα (proinflammatory) cytokine in restimulated splenocytes from experimental mice. Experiments were conducted as recommended by the manufacturer (PharMingen, San Diego, CA) and as we described previously (Cribbs et al., 2003b). The colored spots were counted, and the results were examined for differences between stimulated and nonstimulated conditions for each experiment using one-way ANOVA and Tukey's posttest, Graph Pad Prism 3.03.
Detection of Aβ plaques in human brain tissues
Sera from immunized mice were screened for the ability to bind to Aβ plaques on tissue sections from an AD case as we described previously (Ghochikyan et al., 2003; Agadjanyan et al., 2005). Briefly, pooled sera (dilution 1:500) were added to the serial 50-μm brain sections of formalin-fixed frontal cortical tissue from patients with neuropathological and behavioral patterns typical of severe AD. Sections were pretreated with 90% formic acid, and exogenous peroxidase was quenched. As a negative control, we used the same dilutions of preimmune sera. As a positive control, monoclonal antihuman Aβ antibody 6E10 (Signet Laboratories, Dedham, MA) was used. Binding of antibodies to the brain sections was detected by Vectastain Elite ABC Mouse anti-IgG/biotin–avidin/HRP system (Vector Labs, Burlingame, CA) with DAB, according to the manufacturer's recommendations. A digital camera (Olympus, Japan) was used to collect images of the plaques at 20× image magnification.
RESULTS
Immunogenicity of Aβ28 peptide
Prior to testing the Aβ28–mannan conjugates, we first evaluated the immunogenicity of the peptide alone in different strains of mice. Previously, we showed that BALB/c mice recognized the B- and T-cell antigenic determinants of Aβ42 within the first 28 aa of this peptide (Cribbs et al., 2003b). In fact, BALB/c mice of H-2d immune haplotype generated high titers of anti-Aβ antibodies (Petrushina et al., 2003; Gevorkian et al., 2004), and the highest level of anti-Aβ T cell responses after immunizations with fibrillar Aβ42 peptides (Cribbs et al., 2003b). We directly analyzed the immunogenicity of fibrillar Aβ28 peptide in this strain of mice and compared it with that in mice of H-2b (C57BL6), H-2s (SJL), and H-2b×s(B6SJL F1), immune haplotypes, as well as with antibody responses generated in APP/Tg 2576 animals, which have H-2b×s background.
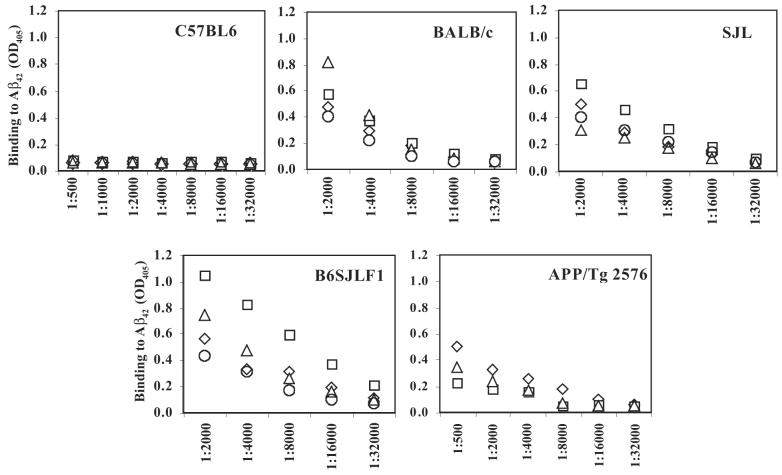
As shown in Figure 1, three biweekly injections with 100 μg of fibrillar human Aβ28 peptide formulated in a Th2-type adjuvant (Alum) induced anti-Aβ antibodies in all immune haplotypes except H-2b. C57BL6 had not responded to immunizations with fibrillar Aβ28 at all, indicating that this immune haplotype did not recognize a T-cell epitope within this immunogen. B6SJL F1 animals induced the highest titer of anti-Aβ antibodies, whereas the levels of anti-Aβ antibody in SJL and BALB/c mice were moderate. However, a difference in the level of anti-Aβ antibody response between these groups is not significant (p > 0.05). Immunization of APP/Tg 2576 mice of H-2b×s background induced the lowest level of anti-Aβ42 antibody response. These results were consistent with our previous findings that C57BL6 do not respond to Aβ28, and that for APP/Tg 2576 mice human Aβ28 represents a self-antigen. However, collectively these data confirmed that wildtype mice of H-2s, H-2b×s, H-2d immune haplotypes, as well as APP/Tg 2576 mice recognized Aβ28 immunogen and produced anti-Aβ antibodies. Next, we tested the potency of mannan as an adjuvant in BALB/c wild-type mice.
FIG. 1.
The Aβ28 sequence of the human Aβ42 peptide is immunogenic in B6SJL F1, SJL, and BALB/c, but not in the C57BL/6 strain of mice (for each immune haplotype n = 4). Total Ig specific to Aβ42-coated wells was detected in serum from individual mice after immunization and two biweekly boosts with fibrillar Aβ28 formulated in alum, a Th2-type adjuvant.
Conjugation with mannan significantly enhances immunogenicity of the Aβ28 peptide
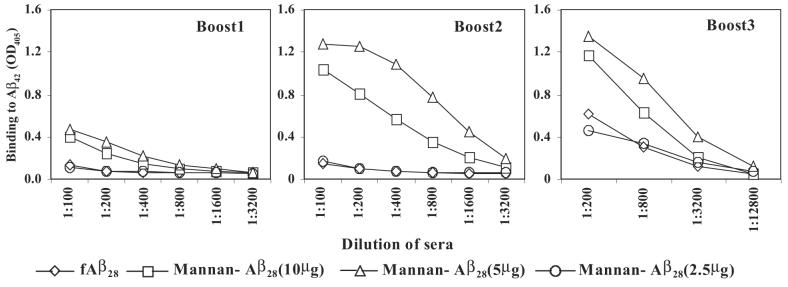
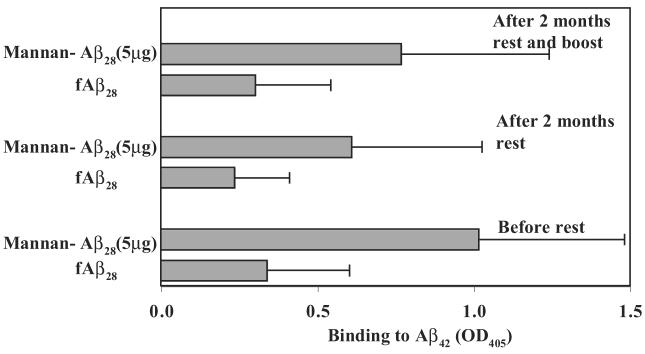
To determine the ability of mannan to enhance the immunogenicity of Aβ28 peptide, we vaccinated BALB/c mice with mannan–Aβ28 conjugate or fibrillar Aβ28. The groups of experimental mice were injected with 2.5, 5, or 10 μg of Aβ28 conjugated with mannan, whereas control mice were immunized with 10 μg Aβ28. Of note, we did not use any additional adjuvant, and prepared both immunogens in PBS. The sera collected from experimental and control mice were analyzed for the presence of anti-Aβ42 antibodies after one, two, and three biweekly boosts (Fig. 2). Three immunizations of mice with 10 μg of fibrillar Aβ28 were not sufficient to generate a detectable anti-Aβ42 antibody response. However, after a third boost this group of mice generated a low level of antibody response that was equal to that induced in animals immunized four times with 2.5 μg of mannan–Aβ28 conjugate. In contrast, mice immunized with 5 and 10 μg of mannan–Aβ28 induced low titers of anti-Aβ antibodies after the first boost. The second and third boosts with these doses of mannan–Aβ28 significantly enhanced anti-Aβ antibody production (≥4–8 times). Of note, although 5 μg peptide induced higher response than 10 μg, the difference was not statistically significant. These mice were boosted one more time after a 2-month rest period. Anti-Aβ42 antibody responses were analyzed before and 9 days after the fourth boost (Fig. 3). After 2 months of rest, the level of anti-Aβ42 antibodies decreased slightly (32.5 ± 6% on average). As expected, a single boost enhanced a production of anti-Aβ42 antibodies with the largest increase detected in groups of mice immunized with 5 and 10 μg (data not shown) mannan–Aβ28. We concluded that mannan was an effective molecular adjuvant that induced long-lasting anti-Aβ antibody responses in mice immunized with 5 and 10 μg of mannosylated Aβ28 peptide, although variability of humoral immune responses in individual mice was significant.
FIG. 2.
Mannan enhanced immunogenicity of Aβ28 peptide. BALB/c mice were immunized and boosted one, two, or three times with the indicated dose of mannan–Aβ28 or fibrillar Aβ28. Pooled sera were used for detection of binding to Aβ42-coated wells. Representative ELISA data from three experiments are presented.
FIG. 3.
Long-lasting anti-Aβ antibody responses in mice immunized with 5 μg of Aβ28 conjugated with mannan (the data with similar profile were obtained with 2.5 and 10 μg mannan-Aβ28). After vaccination and three booster injections mice were rested for 2 months, and the immune response was recalled with the appropriate antigen. Individual sera from animals were diluted 1:250 and tested for detection of anti-Aβ42 antibodies in ELISA. The experiment was repeated with similar results.
Mannosylated Aβ28 induced a Th2 polarized immune response
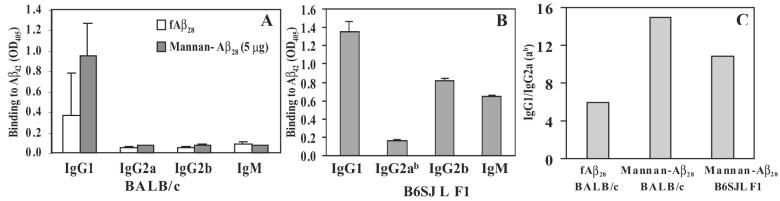
Antibody isotyping has been used as an indirect measure of the contribution of Th1 (IgG2a) and Th2 (IgG1) cytokines to the humoral response (Finkelman et al., 1990). In addition, it was demonstrated that the subclass of anti-Aβ42 antibodies might correlate with their therapeutic potential (Solomon et al., 1996; Bard et al., 2000; Dodart et al., 2002; McLaurin et al., 2002). Thus, we measured production of IgG1, IgG2a, IgG2b, and IgM anti-Aβ antibodies in the sera of immunized BALB/c mice. Both fibrillar Aβ28 and mannan–Aβ28 induced primarily IgG1 antibodies after immunization and three boosts (Fig. 4A). The ratio of IgG1 to IgG2a antibody in mice immunized with Aβ28 was 6. However, sera from mice immunized with mannan conjugated to Aβ28 significantly enhanced the highly polarized Th2 type immune response. The IgG1/IgG2a ratios in the sera of these animals increased to 15. Since Balb/c mice are Th2-prone, in order to demonstrate the real contribution of mannan in Th2 polarization of immune response we immunized B6SJL F1 mice with mannan–Aβ28 and measured production of IgG1 and IgG2ab antibodies (Fig. 4B). The ratio of IgG1 to IgG2ab antibody in immunized B6SJL F1 mice was 11 (Fig. 4C). Thus, mannan conjugation enhanced Th2-polarized anti-Aβ antibody responses, as has been observed after conjugation of mannan with other peptide immunogens (Okawa et al., 1992; Apostolopoulos et al., 1995, 1996; Vaughan et al., 1999; Apostolopoulos and McKenzie, 2001).
FIG. 4.
(A) BALB/c mice immunized with mannan–Aβ28 (5 μg) or fibrillar Aβ28 formulated in PBS induced Th2-polarized anti-Aβ42 antibodies of IgG1 isotype (similar results were obtained with 2.5 and 10 μg mannan–Aβ28). Sera for this assay were collected before the rest period (after the third boost) and diluted 1:250 prior to the detection of isotypes. These results were generated with individual mice. (B) Immunization of B6SJL F1 mice with mannan–Aβ28 also induced anti-Aβ42 antibodies of IgG1 isotype. (C) IgG1/IgG2a (ab) ratio for BALB/c mice immunized with mannan–Aβ28 or fibrillar Aβ28 and B6SJL F1 mice immunized with mannan–Aβ28.
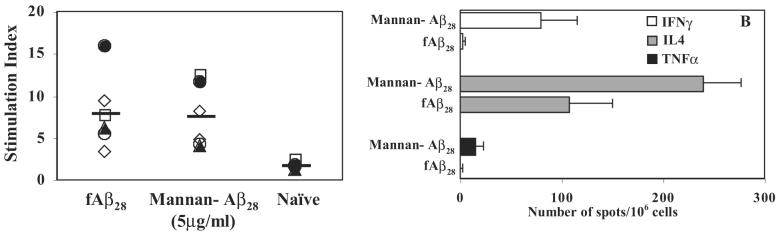
To directly demonstrate a role of the mannan conjugation in Th1– and Th2-type immune responses, we analyzed the cellular immune responses in individual vaccinated mice. The splenocytes from immune mice were restimulated with Aβ40, and the T-cell proliferation was analyzed (Fig. 5A). Both fAβ28 and mannan–Aβ28 induced robust T-cell proliferation with stimulation index of 8.2 and 7.7, respectively (Fig. 5A), although antibody responses in mice immunized with fAβ28 were significantly weaker than in mice administered with mannan–Aβ28 (Figs. 2 and 3). Next, we analyzed a production of Th1 (IFNγ) and Th2 (IL4) lymphokines by immune splenocytes isolated from mice immunized and boosted four times with 5 μg of mannan–Aβ28 and compared it with data obtained after vaccination with fibrillar Aβ28. In addition, we detected a production of the pro-inflammatory cytokine TNFα that is expressed by activated macrophages, monocytes, neutrophils, lymphocytes, and natural killer cells and has been suggested to play a pivotal role in regulation of the synthesis of other pro-inflammatory cytokines (Arend and Dayer, 1995). Our data demonstrated that only a small number of splenocytes from mice immunized with the mannan-conjugated immunogen, but not fibrillar Aβ28, produced this pro-inflammatory cytokine after in vitro restimulation with Aβ40 (Fig. 5B). On the contrary, the same immune splenocytes generated the highest number of cells producing IL4, and immunization of mice with fibrillar Aβ28 was less effective than vaccination of mice with Aβ28 conjugated with mannan.
FIG. 5.
Mannan–Aβ28 and fibrillar Aβ28-induced Th2-type cellular immune responses in BALB/c mice. (A) Immune splenocytes isolated from individual mice immunized with mannan–Aβ28 or fibrillar Aβ28 and in vitro restimulated by Aβ40 peptide-induced robust T-cell proliferation. Data are presented as Stimulation Index (SI). (B) Production of Th1 (IFNγ) and Th2 (IL-4)-type cytokines, as well as pro-inflammatory TNFα by immune splenocytes isolated from mice immunized with 5 μg of mannan–Aβ28 or fibrillar Aβ28. The ELISPOT technique was used as described in Materials and Methods, and data are presented as a delta between number of spots in activated with Aβ40 and nonactivated splenocyte cultures.
B-cell epitope specificity of anti-Aβ28 antibodies
To demonstrate the specificity of antibodies and to identify the B-cell antigenic determinant/s within the Aβ28 immunogen, we screened the antisera with a series of short overlapping peptides encompassing entire Aβ28 peptide using a competition ELISA assay (Cribbs et al., 2003b). Preincubation of antisera with 2.5 μM of the full-length Aβ42 peptide resulted in strong inhibition of antibody binding to Aβ42 on the plate (Fig. 6). At 2.5 μM, the Aβ1–15 peptide was equally effective at blocking the binding of antibodies from mice immunized with fibrillar Aβ28 or the mannan–Aβ28 immunogen. Notably, Aβ6–20, Aβ11–25 or Aβ16–30 peptides were ineffective (Fig. 6). Thus, vaccination with both fibrillar Aβ28 and mannosylated Aβ28 activated B cells specific to the B-cell epitope in the Aβ1–15 peptide.
FIG. 6.
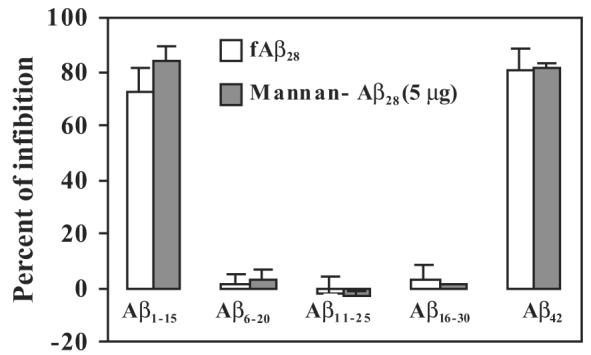
Mannan–Aβ28, as well as fibrillar Aβ28 induced predominantly antibodies specific to the N-terminal region of Aβ42. Mapping of B-cell epitopes was conducted by competition ELISA as we previously described (Cribbs et al., 2003b). Individual sera from immune mice (final dilution 1:250) were collected and preincubated with Aβ1–15 Aβ6–20, Aβ11–25, Aβ16–30, or Aβ42 peptides with the final concentration of each peptide being 2.5 μM before binding to Aβ42-coated wells. Representative ELISA data from two experiments.
To further analyze the potential therapeutic efficacy of anti-Aβ antibodies generated in response to the Aβ28–mannan conjugate vaccine, we also determined binding to amyloid plaques in human brain tissue. We used pooled sera from mice immunized with mannan–Aβ28 and observed that this antiserum bound to amyloid plaques on the brain sections of cortical tissues from a severe AD case (Fig. 7). Preimmune sera from these mice and irrelevant immune sera did not bind to the amyloid plaques (data not shown). These data suggest that anti-Aβ antibodies raised after immunizations with Aβ28–mannan conjugate were potentially functional, as it was demonstrated previously with antibodies specific to fibrillar Aβ42 (Schenk et al., 1999; Bard et al., 2000; Janus et al., 2000; Morgan et al., 2000) or Aβ1–15 peptide fused with foreign T-cell epitope (Agadjanyan et al., 2005).
FIG. 7.
Mannan–Aβ28 induced potentially therapeutic anti-Aβ1–15 antibodies, which are capable of binding to amyloid plaques in human brain tissue (A). Coincubation with Aβ28 peptide blocked this binding (B). 6E10 antibodies were used as a positive control (C). Picture represents binding of 1:500 diluted pooled sera to a 50-μm brain section of formalin-fixed cortical tissue from an elderly individual with neuropathological and behavioral patterns typical to severe AD. Original magnification, ×20.
DISCUSSION
Anti-Aβ-immunotherapy is a novel strategy to induce antigen-specific humoral immune responses therapeutic for AD. Although the failure of the first anti-Aβ-immunotherapy clinical trial was disappointing, the follow-up studies indicate that Th2-prone immune response may be more beneficial and safer than a Th1 response. Mannan has previously been shown to be a potent molecular adjuvant enhancing both B- and T-cell immune responses, as well as antigen uptake and presentation (Okawa et al., 1992; Apostolopoulos et al., 1995, 2000; Engering et al., 1997a, 1997b; Karanikas et al., 1997; Gröger et al., 2000; Linehan et al., 2000; Vaughan et al., 2000; Stambas et al., 2002a, 2002b, 2005). This molecular adjuvant not only enhanced antibody responses specific to appropriate peptide attached to it (Okawa et al., 1992; Apostolopoulos et al., 1995, 2000), but under certain conditions also induced Th2-polarized immunity (Okawa et al., 1992; Apostolopoulos et al., 1995, 1996; Vaughan et al., 1999; Apostolopoulos and McKenzie, 2001). Taking advantage of this property of mannan, we designed a novel AD vaccine that will induce Th2-prone immune responses directed to the Aβ peptide. The data presented here further support the previous observations and demonstrate that mannan conjugates can enhance Th2-polarized immune responses to the Aβ28 peptide immunogen.
Previously, we have demonstrated that Aβ28 peptide possessed both B- and T-cell antigenic determinants of Aβ42 in BALB/c mice (Cribbs et al., 2003a). In this study, we confirm this observation by demonstrating production of anti-Aβ42 antibodies in wild-type mice of H-2d, H-2s, and H-2b×s immune haplotypes immunized with 100μg of Aβ28 peptide formulated in alum, which is a Th2-type adjuvant (Fig. 1). Of note, previously several groups, including us (Das et al., 2003; Petrushina et al., 2003; Seabrook et al., 2004; Kutzler et al., 2005), demonstrated that C57BL6 mice respond to fibrillar Aβ42 poorly. The results generated here indicate that H-2b immune haplotype does not respond to Aβ28 peptide immunization, suggesting that these mice may not recognize a T-cell epitope within this peptide. While further investigation of these data is required in order to demonstrate an exact mechanism for the lack of response in H-2b mice, it does emphasize the significant hurdle facing development of a small epitope vaccine in humans, which have multiple MHC haplotypes. We chose the Aβ28 peptide as a prototype immunogen to test the effectiveness of mannan as a molecular adjuvant to break tolerance against a self-peptide. We hypothesized that mannan should not only enhance antigen uptake and presentation, but may also induce better crosslinking of B-cell receptors, which amplifies the signal to Aβ specific B cells (Reth and Wienands, 1997; Wagle et al., 2000). The data presented here showed that very low doses of mannan–Aβ28 induced activation of B cells and generation of anti-Aβ42 antibodies after only one boost (Fig. 2). Even 2.5 μg of mannan–Aβ28 was active after two additional boosts with mannan–Aβ28. In fact, the level of the humoral immune response in this group was similar to that generated in BALB/c mice immunized with 10μg of fibrillar Aβ28 (Fig. 2). To check the longevity of the immune responses, mice from all groups were allowed to rest for 2 months. Importantly, mice boosted with mannan–Aβ28 responded to a single recall injection with the same antigen, suggesting that significant immunological memory was present in these mice (Fig. 3). The specificity of these antibodies was confirmed by a competition ELISA in which antisera from immune mice were preadsorbed by small overlapping linear peptides Aβ1–15, Aβ6–20, Aβ11–25, or Aβ16–30. Consistent with previous studies with fibrillar Aβ42 vaccine (Cribbs et al., 2003b), antisera raised in mice immunized with mannan–Aβ28 was specific only to Aβ1–15 (Fig. 6) and bound to amyloid plaques in cortical tissue from an AD patient (Fig. 7).
Next, we tested the Th1 and Th2 phenotype of humoral immune responses by analyzing isotypes of anti-Aβ antibodies generated in vaccinated mice. Antibody isotyping has been used as an indirect measure of the contribution of Th1 (IgG2a) and Th2 (IgG1) cytokines to the humoral response (Finkelman et al., 1990); thus, we measured anti-Aβ42 IgG2a and IgG1 antibodies in the sera of immune mice. Data indicated that both mannan–Aβ28 and fibrillar Aβ28 in PBS induced highly polarized Th2-type humoral immune responses (IgG1/IgG2a ratios were equal to 15 and 6, respectively) (Fig. 4). To confirm that mannan–Aβ28 induces Th2-type immune responses not only in typically Th2-prone Balb/c mice, we measured the production of anti-Aβ42 IgG2ab and IgG1 antibodies in B6SJL F1 mice immunized with mannan–Aβ28 and showed that IgG1/IgG2ab ratio is 11. Analysis of T-cell responses supported these results. More specifically, immune splenocytes from BALB/c mice immunized with mannan–Aβ28 after restimulation with Aβ40 peptide induced robust T-cell proliferation and generated substantial amounts of CD4+Th2 cells, as well as a higher percent of cells producing IL4 (Th2) than IFNγ (Th1) cytokines (Fig. 5). Thus, mannan–Aβ28 induced predominantly anti-inflammatory Th2 type immune responses in BALB/c mice immunized with mannan–Aβ28 peptide. Th1 cytokines (IL12, IL18, and IFNβ) have been implicated in many autoimmune disorders, whereas Th2 type responses (IL-4, IL-10, and TGFβ) in some cases have been shown to attenuate cell-mediated immunity and inhibit autoimmune disease (Smeltz and Swanborg, 1998; Aharoni et al., 2000; O'Shea et al., 2001; Swanborg, 2001; Weiner and Selkoe, 2002). Therefore, the bias of the anti-Aβ immune responses towards a Th2 phenotype may be potentially beneficial for AD patients. As stated above, we are currently investigating neuropathological changes in APP/Tg 2576 mice vaccinated with mannan–Aβ28, and preliminary data suggest that these animals generated robust anti-Aβ antibody production that can clear/inhibit AD-like pathology in the brains of these animals (Petrushina et al., 2006). The development of second-generation vaccine candidates, which promote a Th2-mediated immune response and the removal of the self T-cell epitope of the Aβ from the immunogen, may help to develop novel immunogen–adjuvant configurations which reduce the risk of adverse events that occurred during the first clinical trial in AD patients.
ACKNOWLEDGMENTS
This work was supported by NIH R01 grants NIA AG20241 and NINDS NS50895 to D. H. Cribbs, and by the Alzheimer's Association IIRG grant IIRG-03-6279 to M.G. Agadjanyan.
REFERENCES
- AGADJANYAN MG, GHOCHIKYAN A, PETRUSHINA I, VASILEVKO V, MOVSESYAN N, MKRTICHYAN M, SAING T, CRIBBS DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J. Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- AHARONI R, TEITELBAUM D, LEITNER O, MESHORER A, SELA M, ARNON R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc. Natl. Acad. Sci. USA. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APOSTOLOPOULOS V, MCKENZIE IF. Role of the mannose receptor in the immune response. Curr. Mol. Med. 2001;1:469–474. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- APOSTOLOPOULOS V, PIETERSZ GA, LOVELAND BE, SANDRIN MS, MCKENZIE IF. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc. Natl. Acad. Sci. USA. 1995;92:10128–10132. doi: 10.1073/pnas.92.22.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APOSTOLOPOULOS V, PIETERSZ GA, MCKENZIE IF. Cell-mediated immune responses to MUC1 fusion protein coupled to mannan. Vaccine. 1996;14:930–938. doi: 10.1016/0264-410x(95)00258-3. [DOI] [PubMed] [Google Scholar]
- APOSTOLOPOULOS V, BARNES N, PIETERSZ GA, MCKENZIE IFC. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine. 2000;18:3174–3184. doi: 10.1016/s0264-410x(00)00090-6. [DOI] [PubMed] [Google Scholar]
- AREND WP, DAYER J-M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor a in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- BARD F, CANNON C, BARBOUR R, BURKE RL, GAMES D, GRAJEDA H, GUIDO T, HU K, HUANG J, JOHNSON-WOOD K, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- BAYER AJ, BULLOCK R, JONES RW, WILKINSON D, PATERSON KR, JENKINS L, MILLAIS SB, DONOGHUE S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- CRIBBS DH, AGADJANYAN MG. Immunotherapy for Alzheimer's disease: Potential problems and possible solutions. Curr. Immunol. Rev. 2005;1:139–155. [Google Scholar]
- CRIBBS DH, LEES A, GHOCHIKYAN A, VASILEVKO V, PETRUSHINA I, BABIKYAN D, MOVSESYAN N, TRAN MAD, AGADJANYAN MG. Mannan as a molecular adjuvant for Aβ-immunotherapy. In: Hanin I, Fisher A, Cacabelos R, editors. Collection of Selected Free Papers of the 6th International Conference on Progress in Alzheimer's and Parkinson's Disease AP/DP—New Trends in Alzheimer and Parkinson Related Disorders. Monduzzi Editore; Seville, Spain: 2003a. pp. 81–85. [Google Scholar]
- CRIBBS DH, GHOCHIKYAN A, TRAN M, VASILEVKO V, PETRUSHINA I, SADZIKAVA N, KESSLAK P, KIEBER-EMMONS T, COTMAN CW, AGADJANYAN MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 2003b;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAS P, CHAPOVAL S, HOWARD V, DAVID CS, GOLDE TE. Immune responses against Abeta1-42 in HLA class II transgenic mice: implications for Abeta1–42 immune-mediated therapies. Neurobiol. Aging. 2003;24:969–976. doi: 10.1016/s0197-4580(03)00036-8. [DOI] [PubMed] [Google Scholar]
- DODART JC, BALES KR, GANNON KS, GREENE SJ, DeMATTOS RB, MATHIS C, DeLONG CA, WU S, WU X, HOLTZMAN DM, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- ENGERING AJ, CELLA M, FLUITSMA DM, HOEFSMIT EC, LANZAVECCHIA A, PIETERS J. Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv. Exp. Med. Biol. 1997a;417:183–187. doi: 10.1007/978-1-4757-9966-8_31. [DOI] [PubMed] [Google Scholar]
- ENGERING AJ, CELLA M, FLUITSMA D, BROCKHAUS M, HOEFSMIT EC, LANZAVECCHIA A, PIETERS J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur. J. Immunol. 1997b;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- FERRER I, ROVIRA MB, GUERRA MLS, REY MJ, COSTA-JUSSA F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELMAN FD, HOLMES J, KATONA IM, URBAN JF, BECKMANN MP, PARK LS, SCHOOLEY KA, COFFMAN RL, MOSSMANN TR, PAUL WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- FOX NC, BLACK RS, GILMAN S, ROSSOR MN, GRIFFITH SG, JENKINS L, KOLLER M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- GEVORKIAN G, PETRUSHINA I, MANOUCHARIAN K, GHOCHIKYAN A, ACERO G, VASILEVKO V, CRIBBS DH, AGADJANYAN MG. Mimotopes of conformational epitopes in fibrillar beta-amyloid. J. Neuroimmunol. 2004;156:10–20. doi: 10.1016/j.jneuroim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- GHIRAN I, BARBASHOV SF, KLICKSTEIN LB, TAS SW, JENSENIUS JC, NICHOLSON-WELLER A. Complement receptor 1/CD35 is a receptor for mannan-binding lectin. J. Exp. Med. 2000;192:1797–1808. doi: 10.1084/jem.192.12.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOCHIKYAN A, VASILEVKO V, PETRUSHINA I, TRAN M, SADZIKAVA N, BABIKYAN D, MOVSESYAN N, TIAN W, ROSS TM, CRIBBS DH, et al. Generation and chracterization of the humoral immune response to DNA immunization with a chimeric β-amyloid-interleukin-4 minigene. Eur. J. Immunol. 2003;33:3232–3241. doi: 10.1002/eji.200324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILMAN S, KOLLER M, BLACK RS, JENKINS L, GRIFFITH SG, FOX NC, EISNER L, KIRBY L, ROVIRA MB, FORETTE F, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- GOLDE TE. The Abeta hypothesis: leading us to rationally-designed therapeutic strategies for the treatment or prevention of Alzheimer disease. Brain Pathol. 2005;15:84–87. doi: 10.1111/j.1750-3639.2005.tb00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRÖGER M, HOLNTHONER W, MAURER D, LECHLEITNER S, WOLFF K, MAYR BB, LUBITZ W, PETZELBAUER P. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J. Immunol. 2000;165:5428–5434. doi: 10.4049/jimmunol.165.10.5428. [DOI] [PubMed] [Google Scholar]
- HARDY J, SELKOE DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- HARDY JA, HIGGINS GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- HOCK C, KONIETZKO U, STREFFER JR, TRACY J, SIGNORELL A, MULLER-TILLMANNS B, LEMKE U, HENKE K, MORITZ E, GARCIA E, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- INMAN JK. Syntheses of macromolecular immunomodulators and conjugates employing haloacetyl reagents. Ann. N Y Acad. Sci. 1993;685:347–350. doi: 10.1111/j.1749-6632.1993.tb35887.x. [DOI] [PubMed] [Google Scholar]
- JANUS C, PEARSON J, McLAURIN J, MATHEWS PM, JIANG Y, SCHMIDT SD, CHISHTI MA, HORNE P, HESLIN D, FRENCH J, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- KARANIKAS V, HWANG LA, PEARSON J, ONG CS, APOSTOLOPOULOS V, VAUGHAN H, XING PX, JAMIESON G, PIETERSZ G, TAIT B, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J. Clin. Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZONO Y, ABE R, KOZONO H, KELLY RG, AZUMA T, HOLERS VM. Cross-linking CD21/CD35 or CD19 increases both B7-1 and B7-2 expression on murine splenic B cells. J. Immunol. 1998;160:1565–1572. [PubMed] [Google Scholar]
- KUTZLER MA, CAO C, BAI Y, DONG H, CHOE PY, SAULINO V, McLAUGHLIN L, WHELAN A, CHOO AY, WEINER DB, et al. Mapping of immune responses following wild-type and mutant ABeta42 plasmid or peptide vaccination in different mouse haplotypes and HLA Class II transgenic mice. Vaccine. 2006;24:4630–4639. doi: 10.1016/j.vaccine.2005.08.036. [DOI] [PubMed] [Google Scholar]
- LEES A, NELSON BL, MOND JJ. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996;14:190–198. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- LINEHAN SA, MARTINEZ-POMARES L, GORDON S. Mannose receptor and scavenger receptor: Two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv. Exp. Med. Biol. 2000;479:1–14. doi: 10.1007/0-306-46831-X_1. [DOI] [PubMed] [Google Scholar]
- MASLIAH E, HANSEN L, ADAME A, CREWS L, BARD F, LEE C, SEUBERT P, GAMES D, KIRBY L, SCHENK D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- MeLAURIN J, CECAL R, KIERSTEAD ME, TIAN X, PHINNEY AL, MANEA M, FRENCH JE, LAMBERMON MH, DARABIE AA, BROWN ME, et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat. Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- MOLINA H, HOLERS VM, LI B, FUNG Y, MARIATHASAN S, GOELLNER J, STRAUSS-SCHOENBERGER J, KARR RW, CHAPLIN DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN D, DIAMOND DM, GOTTSCHALL PE, UGEN KE, DICKEY C, HARDY J, DUFF K, JANTZEN P, DiCARLO G, WILCOCK D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- NICOLL JA, WILKINSON D, HOLMES C, STEART P, MARKHAM H, WELLER RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- O'SHEA JJ, MA A, LIPSKY P. Cytokines and autoimmunity. Nat. Rev. Immun. 2001;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- OKAWA Y, HOWARD CR, STEWARD MW. Production of anti-peptide specific antibody in mice following immunization with peptides conjugated to mannan. J. Immunol. Methods. 1992;149:127–131. doi: 10.1016/s0022-1759(12)80057-3. [DOI] [PubMed] [Google Scholar]
- PETRUSHINA I, TRAN M, SADZIKAVA N, GHOCHIKYAN A, VASILEVKO V, AGADJANYAN MG, CRIBBS DH. Importance of IgG2c isotype in the immune response to bamyloid in APP/Tg mice. Neurosci. Lett. 2003;338:5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- PETRUSHINA I, MAMIKONYAN G, GHOCHIKYAN A, MKRTICHYAN M, MOVSESYAN N, KARAPETYAN A, LEES A, AGADJANYAN MG, CRIBBS DH. Active immunization of APP/Tg2576 mice with mannan-Ab28 antigen induced clearance of Ab plaques and microhemorrhages in the brains of vaccinated animals. Am. J. Pathol. 2006 submitted. [Google Scholar]
- PRICE DL, SISODIA SS. Cellular and molecular biology of Alzheimer's disease and animal models. Annu. Rev. Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- RETH M, WIENANDS J. Initiation and processing of signals from B cell antigen receptor. Annu. Rev. Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- SCHENK D, BARBOUR R, DUNN W, GORDON G, GRAJEDA H, GUIDO T, HU K, HUANG J, JOHNSON-WOOD K, KHAN K, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- SEABROOK TJ, IGLESIAS M, BLOOM JK, SPOONER ET, LEMERE CA. Differences in the immune response to long term Abeta vaccination in C57BL/6 and B6D2F1 mice. Vaccine. 2004;22:4075–4083. doi: 10.1016/j.vaccine.2004.03.061. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON EM, KNUDSEN E, ASUNI A, FITZER-ATTAS C, SAGE D, QUARTERMAIN D, GONI F, FRANGIONE B, WISNIEWSKI T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-beta derivatives. J. Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMELTZ RB, SWANBORG RH. Concordance and contradiction concerning cytokines and chemokines in experimental demyelinating disease. J. Neurosci. Res. 1998;51:147–153. doi: 10.1002/(SICI)1097-4547(19980115)51:2<147::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- SOLOMON B, KOPPEL R, HANAN E, KATZAV T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc. Natl. Acad. Sci. USA. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMBAS J, PIETERSZ G, McKENZIE I, CHEERS C. Oxidised mannan as a novel adjuvant inducing mucosal IgA production. Vaccine. 2002a;20:1068–1078. doi: 10.1016/s0264-410x(01)00456-x. [DOI] [PubMed] [Google Scholar]
- STAMBAS J, PIETERSZ G, McKENZIE I, NAGABHUSHANAM V, CHEERS C. Oxidised mannan-listeriolysin O conjugates induce Th1/Th2 cytokine responses after intranasal immunisation. Vaccine. 2002b;20:1877–1886. doi: 10.1016/s0264-410x(02)00039-7. [DOI] [PubMed] [Google Scholar]
- STAMBAS J, BROWN SA, GUTIERREZ A, SEALY R, YUE W, JONES B, LOCKEY TD, ZIRKEL A, FREIDEN P, BROWN B, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005;23:2454–2464. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- SWANBORG RH. Experimental autoimmune encephalomyelitis in the rat: Lessons in T-cell immunology and autoreactivity. Immunol. Rev. 2001;184:129–135. doi: 10.1034/j.1600-065x.2001.1840112.x. [DOI] [PubMed] [Google Scholar]
- TENNER AJ. Membrane receptors for soluble defense collagens. Curr. Opin. Immunol. 1999;11:34–41. doi: 10.1016/s0952-7915(99)80007-7. [DOI] [PubMed] [Google Scholar]
- TURNER MW. Mannose-binding lectin: The pluripotent molecule of the innate immune system. Immunol. Today. 1996;17:532–540. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- VASTA GR, QUESENBERRY M, AHMED H, O'LEARY N. C-type lectins and galectins mediate innate and adaptive immune functions: Their roles in the complement activation pathway. Dev. Comp. Immunol. 1999;23:401–420. doi: 10.1016/s0145-305x(99)00020-8. [DOI] [PubMed] [Google Scholar]
- VAUGHAN HA, HO DW, KARANIKAS VA, ONG CS, HWANG LA, PEARSON JM, McKENZIE IF, PIETERSZ GA. Induction of humoral and cellular responses in cynomolgus monkeys immunised with mannan-human MUC1 conjugates. Vaccine. 1999;17:2740–2752. doi: 10.1016/s0264-410x(98)00493-9. [DOI] [PubMed] [Google Scholar]
- VAUGHAN HA, HO DW, KARANIKAS V, SANDRIN MS, MCKENZIE IF, PIETERSZ GA. The immune response of mice and cynomolgus monkeys to macaque mucin 1-mannan. Vaccine. 2000;18:3297–3309. doi: 10.1016/s0264-410x(00)00143-2. [DOI] [PubMed] [Google Scholar]
- WAGLE NM, CHENG P, KIM J, SPROUL TW, KAUSCH KD, PIERCE SK. B lymphocyte signaling receptors and the control of class II antigen processing. Curr. Topics Microbiol. 2000;245:106–126. doi: 10.1007/978-3-642-59641-4_5. [DOI] [PubMed] [Google Scholar]
- WEINER HL, SELKOE DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420:879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- WEINER HL, LEMERE CA, MARON R, SPOONER ET, GRENFELL TJ, MORI C, ISSAZADEH S, HANCOCK WW, SELKOE DJ. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann. Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- WILCOCK DM, ROJIANI A, ROSENTHAL A, SUBBARAO S, FREEMAN MJ, GORDON MN, MORGAN D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J. Neuroinflamm. 2004a;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOCK DM, ROJIANI A, ROSENTHAL A, LEVKOWITZ G, SUBBARAO S, ALAMED J, WILSON D, WILSON N, FREEMAN MJ, GORDON MN, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J. Neurosci. 2004b;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]