Abstract
Recent studies indicate that reactive oxygen species (ROS) play an important role in neuropathic pain, predominantly through spinal mechanisms. Since the data suggest that ROS are involved in central sensitization, the present study examines the levels of activated N-methyl-D-aspartate (NMDA) receptors in the dorsal horn before and after removal of ROS with a ROS scavenger, phenyl-N-t-butyl nitrone (PBN), in animal models of pain. Tight ligation of the L5 spinal nerve was used for the neuropathic pain model, and intradermal injection of capsaicin was used for the inflammatory pain model. Foot withdrawal thresholds to von Frey stimuli to the paw were measured as pain indicators. The number of neurons showing immunoreactivity to phosphorylated NMDA-receptor subunit 1 (pNR1) and the total amount of pNR1 proteins in the spinal cord were determined using immunohistochemical and Western blotting techniques, respectively. Hyperalgesia and increased pNR1 expression were observed in both neuropathic and capsaicin-treated rats. A systemic injection of PBN (100 mg/kg, i.p.) dramatically reduced hyperalgesia and blocked the enhancement of spinal pNR1 in both pain models within 1 hour after PBN treatment. The data suggest that ROS are involved in NMDA-receptor activation, an essential step in central sensitization, and thus contribute to neuropathic and capsaicin-induced pain.
Keywords: free radical, hyperalgesia, NMDA-receptor activation, spinal dorsal horn neuron, PBN, central sensitization
Introduction
Reactive oxygen species (ROS) have been implicated in many degenerative neurological conditions such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, as well as brain dysfunction due to injury or aging (Olanow, 1992; Coyle and Puttfarcken, 1993; Jenner, 1994; Balazs and Leon, 1994; Gerlach et al., 1994; Hensley et al., 1997; Lewen et al., 2000). Recent studies indicate that ROS are also involved in persistent pain, including neuropathic and inflammatory pain (Tal, 1996; Salvemini et al., 1999; Kim et al., 2004; Wang et al., 2004). In neuropathic rats, the allodynia that developed after nerve ligation was temporarily but significantly reversed after treatment with various ROS scavengers (Kim et al., 2004, 2006). The data suggest that ROS are involved in the maintenance of neuropathic pain. Furthermore, the superoxide dismutase (SOD) mimetic, M40403, which converts free radical superoxide to hydrogen peroxide, was very effective in reducing inflammation indicators and hyperalgesia after carrageenan injection into the rat paw (Wang et al., 2004). The transient but dramatic analgesic effect of free radical scavengers in persistent pain models suggests that ROS are critically involved in the maintenance of persistent pain.
Central sensitization is believed to be an important mechanism underlying persistent pain, including neuropathic pain (Woolf and Thompson, 1991; Dubner and Ruda, 1992; Willis, 1994) and inflammatory pain (Coderre and Melzack, 1992; Ma and Woolf, 1996; Willis, 2002). It is also well accepted that NMDA receptors are critically involved in central sensitization (Haley et al., 1990; Woolf and Thompson, 1991; Dubner and Ruda, 1992). Specifically, NMDA-receptor phosphorylation is greatly increased in the spinal dorsal horn neurons in animals showing neuropathic pain or capsaicin induced hyperalgesia (Zou et al., 2000; Gao et al., 2005), suggesting that enhanced NMDA-receptor phosphorylation can be related to central sensitization. On the other hand, various ROS scavengers, including phenyl-N-t-butyl nitrone (PBN), 5,5-dimethylpyroline N-oxide (DMPO), 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol) (Kim et al., 2004; Schwartz et al., 2006; Tal, 1996; Yowtak et al., 2006) and vitamin E (Kim et al., 2006), reduce pain behaviors predominantly through spinal mechanisms, suggesting that ROS may be involved in central sensitization. While behavioral and electrophysiological data indicate that ROS scavengers reverse central sensitization (Kim et al., 2006), the effect of ROS scavengers on NMDA-receptor activation in the spinal cord has not been explored. Thus, the present study examined the changes of the levels of phosphorylated NMDA receptor subunit 1 (pNR1), presumably indicating activated NMDA receptors, in the spinal dorsal horn in spinal-nerve-ligated neuropathic rats before and after treatment with the ROS scavenger, PBN. The number of neurons expressing pNR1 was determined by immunohistochemical staining, and the total amount of pNR1 protein was quantified by Western blotting. In addition, the study was expanded to another well-established pain model, capsaicin-induced hyperalgesia, which involves central sensitization. The data showed that pNR1 expression was enhanced when animals show signs of hyperalgesia, and that both hyperalgesia and enhanced pNR1 expression quickly returned to normal levels after ROS were removed by systemic treatment with PBN in both pain models.
Materials and Methods
Animals
Young adult male CD rats (200–225 g) were purchased from Harlan Sprague-Dawley Co. (Madison, WI) and were housed two per cage with free access to food and water under a reversed 12/12 hour light/dark cycle (light cycle: 8:00 p.m.–8:00 a.m.). Rats were acclimated for at least 5 days before any experimental procedures. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and are in accordance with NIH guidelines.
Neuropathic pain model and drug treatment
Peripheral nerve injury was induced by a tight ligation of the L5 spinal nerve (SNL) while the animal was under halothane anesthesia (3% for induction and 1.5% for maintenance in the flow of oxygen). After the paraspinal muscles and the transverse process of the L6 vertebra were removed, the ventral ramus of the left L5 spinal nerve was exposed and then tightly ligated with 6–0 silk thread. The wounds were closed, and anesthesia was discontinued. Rats were returned to their cages when they recovered from anesthesia. Seven days after nerve ligation, the rats were randomly divided into two groups. Rats in one group were treated with a ROS scavenger, phenyl-N-t-butyl nitrone (PBN, Sigma Chemical Company, St. Louis, MO) (SNL-PBN). Since our previous study has shown that graded doses of PBN produces corresponding graded analgesia in neuropathic rats and that 100 mg/kg of PBN produces the maximum effect (Kim et al., 2004), we have chosen to use a systemic injection of 100 mg/kg PBN in this study. 100 mg of PBN was dissolved in 5 ml of saline, then, 1 ml of this PBN solution was injected intraperitoneally into a 200 g rat. Rats in the other group were treated with the same volume of saline in order to serve as controls (SNL-S). Two other groups of rats, normal naive rats (Normal, or Pre) and normal rats treated with the same dose of PBN were used as controls for behavioral testing and for the Western blot study for pNR1 and NR1 proteins. Mechanical thresholds were measured before and 1 hour after treatment with PBN (or saline) in all groups of rats.
Capsaicin-induced inflammatory pain model and drug treatment
To produce capsaicin-induced hyperalgesia, 20 μl of capsaicin (0.15%, 30 μg capsaicin in 20 μl olive oil) was injected intradermally (i.d.) into the mid-plantar region of the left hind paw of the rat under halothane anesthesia. The same volume of vehicle, olive oil, was injected into the rats in the control group. For drug treatment, 100 mg/kg of PBN was injected intraperitoneally 10 minutes after capsaicin injection (CAP-PBN). The same volume of saline, without PBN, was injected in the control groups (CAP-S). Two other groups of rats, normal naive rats (Normal) and normal rats treated with the same amount of PBN (Normal-PBN) were used as controls for behavioral testing and for the Western blot study for pNR1 and NR1 proteins. Mechanical thresholds were measured before and 1 hour after treatment with capsaicin and saline or capsaicin and PBN.
Behavioral test
The foot withdrawal threshold to mechanical stimuli (mechanical threshold) was used as an indicator of mechanical sensitivity of the affected paw. The mechanical thresholds were measured by using the “up-down” method (Chaplan et al., 1994), following the procedures described in previous studies (Park et al., 2000; Xie et al., 2001). In brief, rats were placed in a transparent plastic box on a metal wire mesh floor. Eight von Frey filaments with bending forces at approximately equal logarithmic increments (0.22) were chosen (von Frey numbers: 3.65, 3.87, 4.10, 4.31, 4.52, 4.74, 4.92, and 5.16; equivalent to 0.45, 0.74, 1.26, 2.04, 3.31, 5.50, 8.32, and 14.45 g, respectively). Starting with filament 4.31, the filaments were applied perpendicular to the ventral surface of the base and proximal part of the third or fourth toe for 2–3 seconds. Whenever a positive response to a stimulus occurred, the next-smaller von Frey filament was applied. Whenever a negative response occurred, the next-higher filament was applied. The test was continued until responses to six stimuli had been obtained after the first change in response. The 50% threshold value was calculated using Dixon’s formula (Dixon, 1980): 50% threshold = 10(X+kd)/104, where X is the value of the final von Frey hair used (in logarithmic units), k is the tabular value for the pattern of positive/negative responses, and d is the mean difference between stimuli in logarithmic units (0.22). In the cases where continuous positive or negative responses were observed all the way to the end of the stimulus spectrum, values of 3.54 (0.34 g) or 5.28 (18.72 g) were assigned, respectively.
Immunostaining
All rats were sacrificed 1 hour after PBN (or saline) treatment, immediately after behavioral testing. At the time of sacrifice, rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and then perfused through the ascending aorta with 100 ml of saline followed by 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The L5 spinal cord segment was removed and post-fixed in the perfusion fixative overnight, and then cryoprotected for 2 days in 30% sucrose in 0.1 M PB. Frozen sections were cut at 16 μm on a cryostat and mounted on gelatinized slides. Immunostaining was performed for phospho-NR1 subunits (pNR1) of NMDA receptors by an indirect fluorescent immunostaining method on the slides. In brief, the sections were incubated in 5% normal goat serum with 10% Fc-receptor blocker (DAKO Protein Block Serum, DAKO Corporation, CA) for 1 hour and then incubated in rabbit immunoaffinity-purified phospho-NR1 antibody (pNR1 antibody, 1:1000 dilution with phosphate-buffered saline [PBS], Upstate Biotech, NY) for 1 hour at room temperature and then overnight at 4°C. The antibodies are specific for NR1 phosphorylated on serine residue 897 and NR1 that is dually phosphorylated on serine 896 and 897 (Tingley et al., 1997). The sections were washed and incubated in the secondary antibody solution containing rhodamine-conjugated goat anti-rabbit IgG (1:200; Chemicon International, CA) for 2 hours at room temperature. Slides were washed and dried. To confirm the specificity of immunostaining, some sections were processed as above, but without primary antibodies. The sections from both normal controls and experimental animals were processed together in each staining process to minimize staining variability.
Tissue sections were examined under a Zeiss epifluorescence microscope with a rhodamine fluorescence filter. Images of each section were photographed using the SpotRT digital camera at a magnification of 100X and stored as image files. To ensure consistency, all photographs were taken at the same exposure setting. Cell counts were obtained in the dorsal horn (laminae I–VI) in 10 randomly selected sections from each animal by an experimenter who did not know the origin of the tissue. Cells were analyzed using the Image Pro-Plus image analysis system. Only the cellular profiles that showed immunostaining density greater than 3X standard deviation from the average density of the unlabeled cells and also enclosed a nucleus in the center were counted as positively labeled cells. The labeled and unlabeled cells were thus clearly separated in almost all cases, so the identification of labeled neurons was unambiguous. The average number of pNR1-immunoreactive (pNR1-ir) neurons per section was calculated and presented as data.
Western blot
All rats were sacrificed 1 hour after PBN (or saline) treatment and immediately after behavioral testing. At the time of sacrifice, rats were anesthetized by sodium pentobarbital (50 mg/kg, i.p.) and then perfused quickly through the ascending aorta with cold saline. The L4/5 spinal cord segments (ipsilateral and contralateral sides separately) were quickly removed, frozen immediately on dry ice, and stored at −70°C until use. At the time of assay, each spinal cord sample was thawed and homogenized in 300 μl of lysis buffer (composed of 20 mM Tris, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1% SDS, 2 μl protease inhibitor cocktail from SIGMA, and 4 μl phosphatase inhibitor cocktail from SIGMA). The homogenate was centrifuged at 14,000 g for 25 minutes at 4°C. The supernatant was decanted from the pellet and used for Western blot analyses. The concentration of protein in the homogenate was measured using the Bio-Rad Protein Assay Kit I (Bio-Rad, Hercules, CA). The homogenates of equal amounts of protein (80 μg) were fractionated by 10% (w/v) SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membrane was then incubated anti-phospho-NR1 antibody (rabbit immunoaffinity purified IgG, 1:500, Upstate Biotechnology) or anti-NR1 antibody (mouse monoclonal IgG, 1:800, Upstate Biotechnology) and monoclonal anti-β-actin antibody (1:50000, Sigma, Saint Louis, MO) for 2 hr at room temperature. The blots were washed three times for 20 minutes each with washing buffer and then incubated with horseradish peroxidase conjugated IgG (1:3000, goat anti-rabbit, Upstate Biotechnology; 1:3000, donkey anti-mouse, Amersham Bioscience), diluted in washing buffer. The membranes were washed with buffer three times for 20 minutes and enhanced with a chemiluminescence reagent (ECL kit, Amersham Pharmacia Biotech, Arlington Heights, IL). The blots were exposed to autoradiographic film (Eastman Kodak Co., Rochester, NY), the films were scanned into a computer, and the intensity of immunoreactive bands of interest was quantified using Meta Image series software. The density was calculated using the formula Density = Log (255/Intensity). Using the β-actin as the internal standard, the ratios of the pNR1 and NR1 density to the β-actin density were calculated and compared among the different groups.
Statistics
All data are expressed as mean plus and minus standard error of the mean (SEM). The mechanical thresholds of paw withdrawal were plotted on a logarithmic scale (indicated on the left side with the corresponding gram values indicated on the right side of the graph), because the von Frey filaments chosen for the test had equal logarithmic intervals. Changes in mechanical thresholds from the controls to the experimental manipulations were compared using a non-parametric paired test (the Wilcoxon Signed Rank Test) since some data sets failed the normality test, making it inappropriate to conduct parametric tests. The changes in the number of pNR1-ir neurons among various groups were tested with one way ANOVA, followed by all pairwise multiple comparison procedures (Bonferroni test). The data from the Western blot analysis were expressed as the ratios of pNR1 and NR1 density to β-actin density. The changes of pNR1 and NR1 density ratios among different groups were also tested by one way ANOVA, followed by Dunnett’s multiple comparison tests, using normal values as controls. P values less than 0.05 were considered significant.
Results
Systemic PBN reduced the number of pNR1-immunoreactive neurons in the dorsal horn and alleviated hyperalgesia
When the L5 spinal cord of the normal naive rat was immunostained for phosphorylated NR1 subunits (pNR1) of NMDA receptors, many neurons in the dorsal horn showed immunostaining, as shown in Figure 1 (panels A and E). The pNR1-immunoreactive (pNR1-ir) neurons were found throughout the entire dorsal horn, and a significantly higher number of pNR1-ir neurons was found in the deep layer (laminae III–VI) compared to that in the superficial layer (laminae I–II). The total number of pNR1-ir neurons in each dorsal horn section (laminae I–VI) ranged from 14 to 26, and the average ± SEM was 20.5 ± 1.9 (n = 6).
Fig. 1.
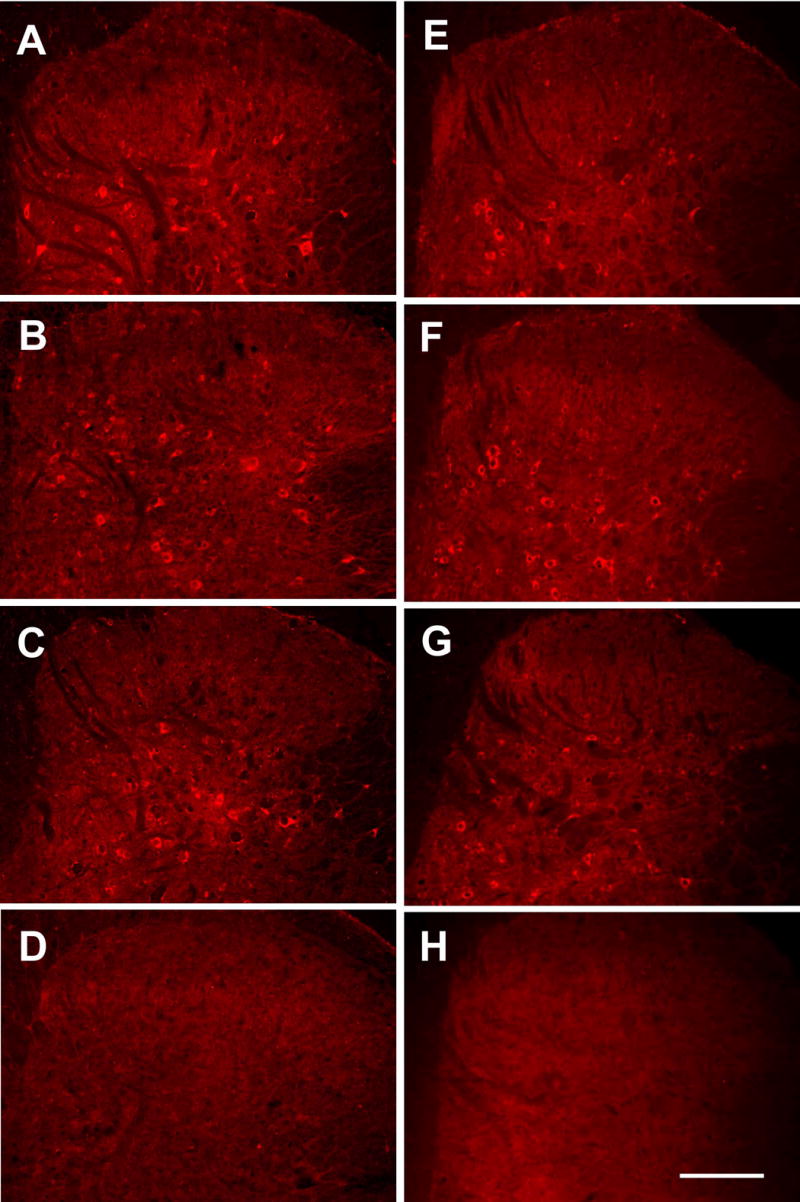
Photomicrographs of pNR1-immunostained ipsilateral L5 spinal dorsal horn sections of neuropathic (A-D) and capsaicin-treated (E-H) rats. A & E: normal control rats, B: 7 days after left L5 spinal nerve ligation with saline treatment, C: 1 hour after PBN treatment (100 mg/kg, i.p.) in 7-day postoperative neuropathic rats, D & H: negative control tissues that were immunostained without a primary antibody, F: 1 hour after capsaicin and saline treatment, and G: 1 hour after capsaicin and PBN (100 mg/kg, i.p.) treatment. There was a significant increase in the number of pNR1-ir neurons in the dorsal horns of neuropathic and capsaicin-treated rats, and this increase returned to normal levels with PBN treatments. Bar = 200 μm.
In neuropathic rats, at 7 days after the L5 spinal nerve ligation with saline treatment, the number of pNR1-ir neurons was significantly increased to 31.1 ± 0.8 in the ipsilateral spinal dorsal horn (Fig. 1B and Exp. Side in SNL-S in Fig. 2A, n = 6). This represented an approximately 52% increase in the total number of pNR1-ir neurons after spinal nerve ligation. In addition, many pNR1-ir neurons were immunostained more intensely than those in the normal cord. One hour after PBN treatment in 7-day postoperative neuropathic rats (SNL-PBN), the number of pNR1-ir neurons in the dorsal horn of the experimental side was reduced to 23.0 ± 1.6; this number is significantly different from saline treatment (SNL-S Exp. side), but not significantly different from the levels of normal rats (Fig. 1C and Fig. 2A). On the contrary, there was no significant change in the number of pNR1-ir neurons in the contralateral dorsal horn (Cont. Side) of all different groups: 20.5 ± 1.9, 23.1 ± 1.6, and 19.8 ± 1.3 for normal, saline-treated SNL, and PBN-treated SNL rats, respectively.
Fig. 2.
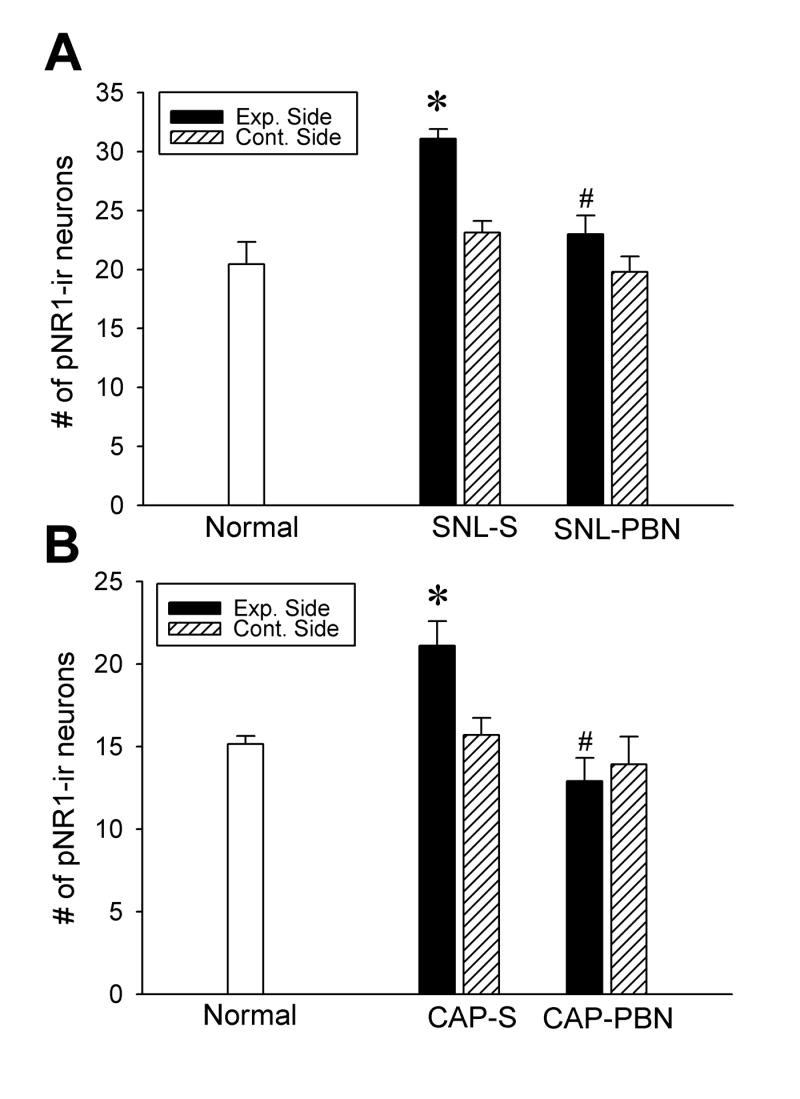
The number of pNR1-ir neurons in the dorsal horn of three groups of neuropathic (A) and capsaicin-treated (B) rats. The data are presented as average ± SEM. The spinal cord of ipsilateral to either nerve ligation or capsaicin treatment is designated as the Exp. Side and that of the contralateral side is labeled as Cont. Side. Normal: normal naive rats; SNL-S: 1 hour after saline (1 ml, i.p.) treatment in 7-day postoperative L5 spinal-nerve-ligated neuropathic rats; SNL-PBN: 1 hour after PBN treatment (100 mg/kg, i.p.) in 7-day postoperative L5 spinal-nerve-ligated rats; CAP-S: 1 hour after capsaicin and saline treatment; and CAP-PBN: 1 hour after capsaicin and PBN treatment (100 mg/kg, i.p.). Each value is the average number of pNR1-ir neurons per section (16 μm thickness) obtained from 6 animals from each group. The number of pNR1-ir neurons was significantly increased only in the ipsilateral dorsal horn in SNL-S and CAP-S rats compared to the normal controls. After PBN treatment (SNL-PBN and CAP-PBN), the number of pNR1-ir neurons reduced significantly (indicated as #) compared to saline treated groups in both neuropathic and capsaicin-treated animals. The contralateral side (Cont. Side) did not show any change from the normal in all groups. Asterisks indicate values significantly different from normal rats and #s indicate values significantly different from saline treated groups (Exp. Side of SNL-S and CAP-S) at P < 0.05.
In rats with inflammatory pain induced by intradermal capsaicin (CAP), there was an approximately 40% increase in the number of pNR1-ir neurons in the ipsilateral dorsal horn with a systemic saline treatment (Fig. 1F and Exp. side in the CAP-S group in Fig. 2B). A systemic PBN treatment (CAP-PBN), however, reversed the capsaicin-induced enhancement of pNR1-ir neurons to normal levels (Fig. 1G and Fig. 2B). The number of pNR1 neurons did not change significantly in the contralateral dorsal horns (Cont. Side, Fig. 2B) in any of the experimental groups compared to normal rats.
Mechanical thresholds of the paw for foot withdrawals were measured before (Pre) and after nerve ligation (SNL) in 12 rats. Nerve ligation produced a dramatic reduction in thresholds when measured 1 week later (Fig. 3A), confirming successful production of an SNL model. These 12 rats were randomly divided into 2 groups of 6 each, one group received intraperitoneal injection of PBN (100 mg/kg in saline, SNL-PBN) and the other group received an equal volume of saline injection (SNL-S). Mechanical thresholds, measured 1 hour after either PBN or saline injection, showed that PBN produced significant reversal of the threshold whereas saline did not. PBN treatment in normal rats did not show any effect on mechanical thresholds (data not shown).
Fig. 3.
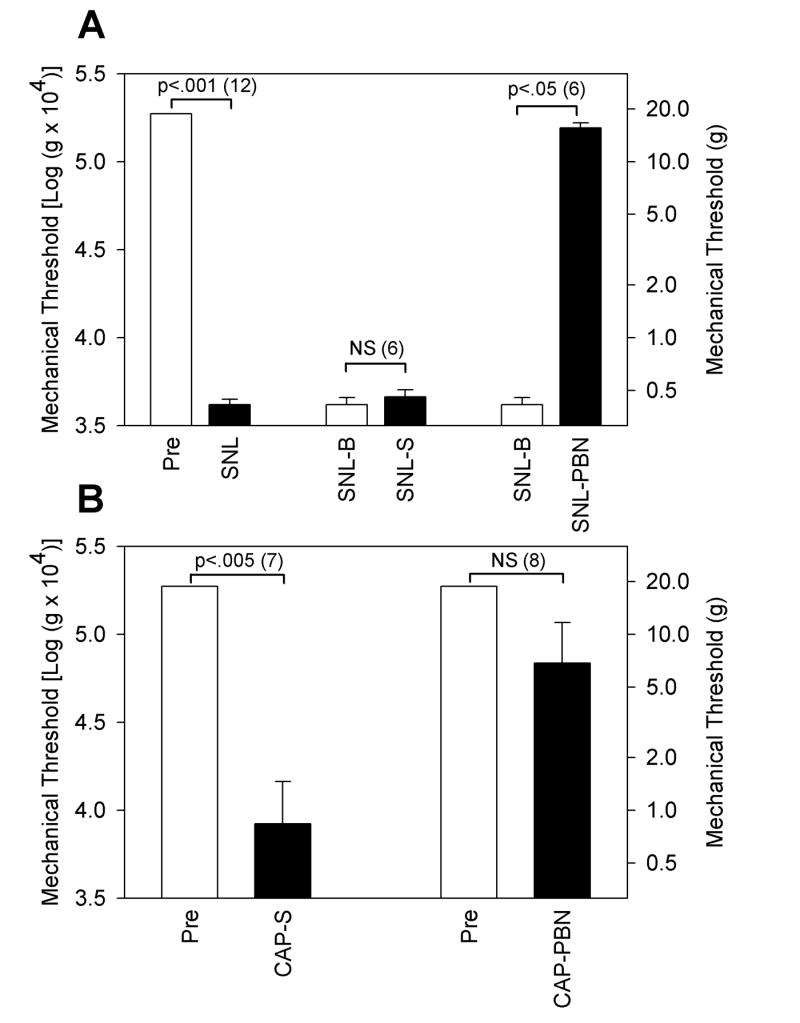
The mechanical thresholds of the ipsilateral paw for foot withdrawals in various groups of rats. The mechanical thresholds are expressed as calculated VF values on the left side and the corresponding gram values on the right side. A: Data from experiments on the SNL model of neuropathic pain are shown. The threshold for pre-operated (Pre) value (which was the upper cut-off value) in 12 rats was lowered to less than 1 g force at 7 days after SNL operation (SNL). Six of these SNL rats received intraperitoneal injection of saline (5 ml/kg) and the threshold did not change much 1 hour after the saline injection (SNL-S) as compared with pre-injection baseline value (SNL-B). On the other hand, the threshold of the other group of 6 SNL rats which received intraperitoneal injection of PBN (100 mg/5 ml saline/kg) increased significantly one hour after the injection (SNL-PBN) as compared to pre-injection baseline (SNL-B). B: Data from experiments on the capsaicin injection are shown. One group of 7 rats received intraperitoneal injection of saline (5 ml/kg) followed by intradermal injection of capsaicin (0.15%, 30 μg capsaicin in 20 μl olive oil). One hour later, the threshold reduced significantly lower levels (CAP-S) as compared to pre-injection value (Pre). The other group of 8 rats received intraperitoneal injection of PBN (100 mg/kg in saline) following intradermal injection of capsaicin. One hour later, the threshold (CAP-PBN) failed to reduce compared to the pre-injection value (Pre). All paired data were compared using a non-parametric test, the Wilcoxon Signed Rank Test, since some pairs failed normality test (all Pre values are the upper cut-off value with no variability). When values are significantly different each other within the pair, the levels of P values are indicated on top of the bracket with the n values in the parenthesis. NS: non-significant
Mechanical thresholds of the paw for foot withdrawals were also measured before (Pre) and after intradermal injection of capsaicin (CAP). Fifteen rats were divided into two groups of 7 and 8 rats. The first group of 7 rats received intradermal injection of capsaicin (0.15%, 30 μg capsaicin in 20 μl olive oil) followed by intraperitoneal injection of saline (5 ml/kg, CAP-S) 10 minutes later. The threshold measurement done at one hour after capsaicin injection showed significant reduction in the thresholds (Fig. 3B), confirming the development of capsaicin-induced hyperalgesia. The other group of 8 rats received intraperitoneal PBN injection (100 mg/kg in saline) 10 minutes after the capsaicin injection (CAP-PBN). The thresholds of this group reduced much less than those in the saline injection group, suggesting that the development of capsaicin-induced hyperalgesia is severely interfered with PBN injection.
Systemic PBN reduced the total amount of pNR1 protein in the spinal cord
To determine the changes in the level of activated NMDA receptors, the total amount of pNR1 and NR1 subunits were measured separately using a Western blotting method in various conditions. The results are shown in Figs. 4 and 5. In both figures, the top panel is an example of Western blot gels showing the typical pattern of pNR1 and NR1 protein bands at various conditions. The bar graph at the bottom shows the summary of the Western blot data.
Fig. 4.
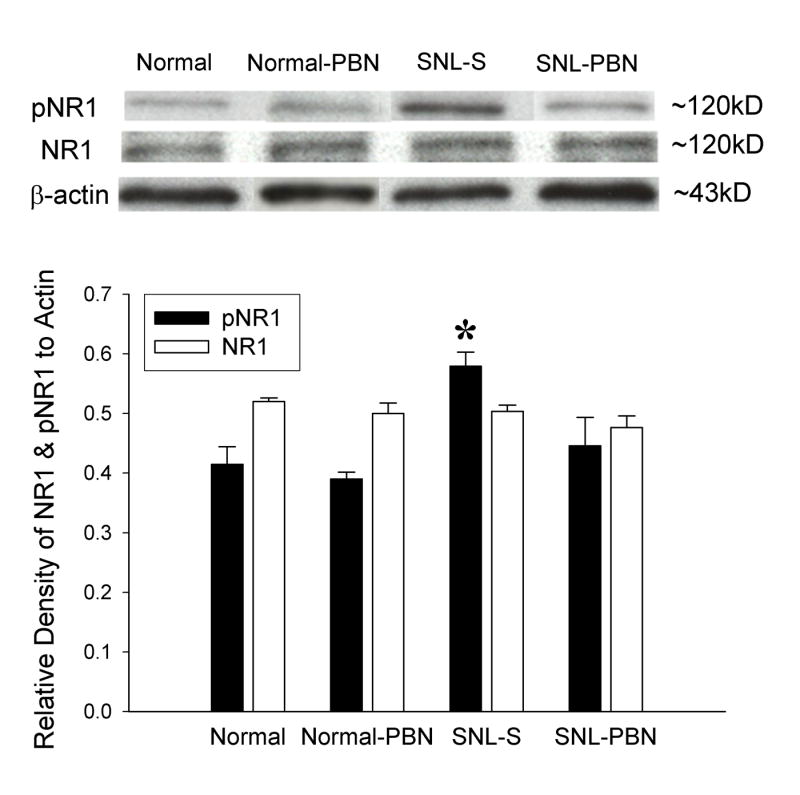
An example of a Western blot gel and the average gel density ratio of pNR1 and NR1 in various groups of neuropathic rats. Normal: normal naive rats (n = 5); Normal-PBN: 1 hour after PBN injection in normal rats (n = 3); SNL-S: 1 hour after saline treatment in 7-day postoperative neuropathic rats (n = 6); SNL-PBN: 1 hour after PBN treatment in 7-day postoperative neuropathic rats (n = 6). Each value in the graph represents the average (± S.E.M.) gel density ratio for each group. Beta-actin was used as an internal control. The density of pNR1 protein in the ipsilateral L4/5 spinal cord increased significantly in the SNL-S group compare to the normal. PBN treatment (100 mg/kg, i.p., SNL-PBN) significantly decreased the total amount of pNR1 protein compared to saline treatment in SNL rats. Injection of PBN in normal rats did not affect pNR1 levels. The density of NR1 protein was unchanged in all groups of animals.
Fig. 5.
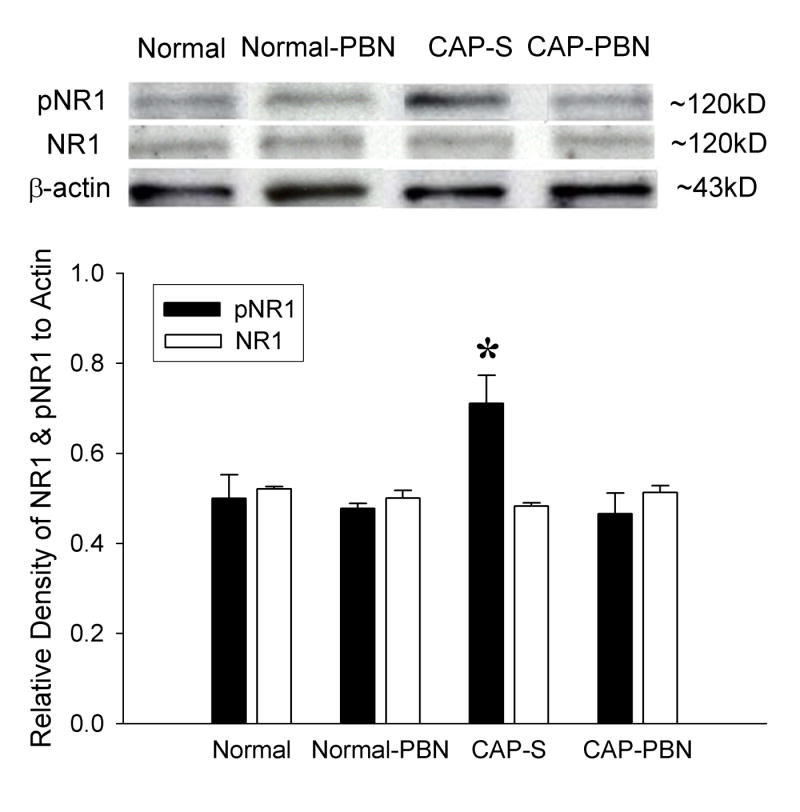
An example of a Western blot gel and the average value of pNR1 and NR1 gel density ratio of various groups of capsaicin-treated rats. Normal: normal naive rats (n = 5); Normal-PBN: 1 hour after PBN injection in normal rats (n = 3); CAP-S: 1 hour after capsaicin and saline treatment (n = 6); CAP-PBN: 1 hour after capsaicin and PBN treatment (n = 6). Saline and PBN were injected intraperitonially 10 minutes after capsaicin treatment. Each value in the graph represents the average (± S.E.M.) gel density ratio for each group. Beta-actin was used as an internal control. The density of pNR1 protein in the ipsilateral L4/5 spinal cord increased significantly in the CAP-S group compared to normal rats. PBN treatment (100 mg/kg, i.p., CAP-PBN) significantly decreased the total amount of pNR1 protein compared to the saline treatment (CAP-S). PBN treatment in normal rats did not affect pNR1 levels. The density of NR1 protein was unchanged in all groups of rats.
In saline treated SNL rats (SNL-S), the relative levels of pNR1 protein was increased approximately 41% compared to that of normal rats. The data confirmed that the amount of NMDA-receptor phosphorylation is significantly increased in SNL neuropathic rats. These elevated pNR1 levels, however, quickly returned back to normal levels within one hour after PBN treatment (Fig. 4, SNL-PBN group). PBN injection in normal rats (Normal-PBN) had no effect on pNR1 protein expression. The levels of NR1 proteins remained the same levels in all different conditions, control and experimental.
In capsaicin and saline treated rats (CAP-S), the levels of phosphorylated NMDA-receptors, measured by the total amount of pNR1 proteins in the dorsal horn, were increased approximately 42% compared to those of normal rats. The enhanced NMDA-receptor phosporylation was quickly decreased back to normal levels one hour after PBN treatment (CAP-PBN, Fig. 5). PBN injection in normal rats had no effect on pNR1 protein expression. The levels of NR1 proteins, however, remained the same in all different experimental conditions.
In both pain models, the total amount of pNR1 protein was significantly increased when animals showed hyperalgesia after nerve ligation or capsaicin injection, and then returned to normal levels when hyperalgesia was reversed by PBN treatment.
Discussion
This study shows that a free radical scavenger, PBN, reverses the enhancement of NMDA-receptor phosphorylation in the spinal cord and alleviates hyperalgesia in two different animal models of pain. The data suggest that elevated levels of free radicals play an important role in the phosphorylation of NMDA receptors in the spinal cord, leading to central sensitization and thus pain in a rapid and reversible manner.
We interpreted that the analgesic effect and the reduction of NMDA-receptor phosphorylation are due to ROS scavenging action of PBN in the spinal cord. PBN is a well utilized free radical scavenger with few side effects, but it also is known to inhibit gene induction of inducible NOS (iNOS) and activation of NFkB (Kotake, 1999). Thus it is possible that PBN’s analgesic effect is due to something other than its ROS scavenging action. Despite that possibility, we propose PBN’s analgesic effect is due to it’s free radical scavenging action based on the followings. The time course of PBN induced analgesia is comparable to the effects of other ROS scavengers such as 5,5-dimethyl-pyrroline-N-oxide (DMPO), 4-hydorxy-2,2,6,6-tetramethyl piperidine-1-oxyl (TEMPOL), nitrosobenzene (NBZ), and vitamin E (Kim et al., 2004; 2006; Schwartz et al., 06; Yowtak et al., 06). Electrophysiological recordings show that both PBN and vitamin E reduce responsiveness of dorsal horn neurons in response to peripheral stimuli (Kim et al., 2006; unpublished data), suggesting their effect on the reduction of central sensitization. Furthermore, analgesia and dephosphorylation of NMDA-receptors produced by PBN have a rapid onset with a peak effect within 1-2 hours after injection, which speaks against the analgesia being due to gene induction. Thus we propose that the analgesic effect of PBN is mainly through ROS scavenging actions in the spinal cord.
The elevated level of pNR1 is interpreted as an indicator of central sensitization in this study. Physiologically, central sensitization in the spinal cord is defined by increased responsiveness of dorsal horn neurons to nociceptive peripheral stimulation (Woolf, 1983). Excitatory amino acids are critically involved in central sensitization through NMDA-receptor activation (Woolf and Thompson, 1991; Coderre and Melzack, 1992; Ren et al., 1992; Ma and Woolf, 1995) and NR1 subunits are essential components of functional NMDA receptors (Masu et al., 1993; Mori and Mishina, 1995). Furthermore, phosphorylation enhances the responses of NMDA receptors to NMDA in various neurons in the central nervous system (Cerne et al., 1992; Li and Zhuo, 1998; Christie et al., 1999), thus indicating that NMDA receptors are activated by phosphorylation. In line with this evidence, an intradermal capsaicin injection increases the amount of pNR1 proteins and the number of pNR1-ir neurons in the dorsal horn (Zou et al., 2000; Zou et al., 2002). In spinal-nerve-ligated neuropathic rats, the enhancement of pNR1 expression in the ipsilateral L4/5 dorsal horn is synchronized with the development of pain behaviors (Gao et al., 2005). In this study, we thus confirm the increased spinal expression of pNR1 is associated with hyperalgesia in two different animal models. Our most important finding, however, is that removal of ROS by PBN not only alleviates hyperalgesia but also reduces dorsal horn pNR1 expression to normal levels in neuropathic and capsaicin-treated rats. Data suggest that oxidative stress in the spinal cord plays a critical role in maintaining a high level of phosphorylated NMDA receptors in dorsal horn neurons, thus contributing to central sensitization. Since the levels of spinal pNR1 and mechanical thresholds do not change after PBN treatment in normal naive rats, PBN may not influence the baseline level of NMDA-receptor activation and thus normal physiological sensations. While the changes of pNR1 levels correlate with pain behaviors, the levels of NR1 proteins in the dorsal horn do not change from normal in all tested groups, in accord with findings in other studies (Zou et al., 2000, Zou et al., 2002).
Although the study shows that removal of ROS reverses enhanced pNR1 expression and hyperalgesia, the mechanism of ROS involvement in central sensitization is unclear. In cultured hippocampal pyramidal neurons, application of a glutamate agonist induces superoxide production (Bindokas et al., 1996), and an NMDA-receptor antagonist blocks superoxide generation (Li et al., 2001). Furthermore, NMDA-receptor activation and subsequent production of superoxide are required for the induction of long-term potentiation (LTP) in hippocampal CA1 neurons (Klann et al., 1998; Klann, 1998). The data indicate that NMDA-receptor activation induces superoxide production, which in turn leads to sensitization of pyramidal neurons. A similar mechanism may be involved in dorsal horn neuron sensitization in persistent pain. Therefore, elevated spinal ROS levels may be caused by persistent nociceptive afferent inputs from pathological peripheral tissue and through NMDA-receptor activation. The excessive level of ROS, in turn, either activates many second messengers of glutamate receptors in neurons (Ali and Salter, 2001; Zhang et al., 2003) or activates spinal glial cells (Raghavendra et al., 2003) and leads to a hyperexcitable state of dorsal horn neurons. In support of this possibility, a number of intracellular signaling pathways, including protein phosphatases, protein kinases, and transcription factors, can be modified by ROS (Maher & Schubert, 2000).
For detection of NMDA-receptor phosphorylation, the antibody used in this study recognizes the NR1 subunit that is phosphorylated either by the Ser-897 alone or both the Ser-897 and Ser-896 together, but not Ser-896 alone. Since Ser-897 is phosphorylated by PKA while Ser-890 and Ser-896 are phosphorylated by PKC (Tingley et al., 1997), our method detects NR1 subunits phosphorylated mainly by PKA and partially by PKC. Thus our data indicate that PKA and PKC activation is involved for enhancement of NR1 phosphorylation, suggesting enhanced activation of protein kinases by ROS in neuropathic pain and its reversal by the ROS scavenger PBN. ROS involvement in activation of protein kinases has been shown in many different tissues. For example, ROS activates adenylyl cyclase thus production of cAMP, and then cAMP activates protein kinase A (PKA) during sperm capacitation (O’Flaherty et al., 2004; 2006). In the hippocampal CA1 region, superoxides increase the activity of protein kinase C and protein tyrosine kinase and decrease the activity of protein phosphatases 2A and 2B, and a protein tyrosine phosphatase (Klann & Thiels, 1999) during LTP. In rat skeletal muscle, ROS activate mitogen-activated protein kinases (Ji et al., 2006). In the spinal dorsal horn neurons, peripheral noxious stimulation increases NR1 phosphorylation through a PKA and PKC dependent manner (Zou et al., 2002; 2004; Brenner et al., 2004). Based on NMDA-receptor dephosporylation and alleviation of pain behaviors by PBN, we now propose that ROS are involved in NR1 phosphorylation through PKA and PKC activation. The current study in our laboratory confirms the enhanced activation of PKA and PKC in the spinal cord of neuropathic rats and that its reversal by PBN (data not published). Although indirect, our data suggest that elevated levels of spinal ROS increase the levels of activated protein kinases, which in turn increase levels of activated NMDA receptors and thus maintain central sensitization.
Our data show that the average number of pNR1-ir neurons in the dorsal horn (laminae I–VI) of normal rats is between 14 and 26 neurons per section (16 μm in thickness); the majority (>90%) of them are found in the deep layer (laminae III–VI). In addition, an enhancement of pNR1-ir neurons was found in both superficial and deep layers after SNL or capsaicin treatment. Our data suggest that neurons that acquire increased NMDA-receptor activation are spread throughout all layers of the dorsal horn, although nociceptive C-afferent fibers terminate primarily in the superficial layers. This finding agrees with previous studies that found an increase of pNR1-ir neurons in all layers of the dorsal horn after capsaicin treatment (Zou et al., 2000) and with findings that C-fiber stimulation induces responses not only from superficial but also from many deep dorsal horn neurons (Simone et al., 1991). Many deep dorsal horn neurons are also known to express neurokinin-1 receptors, and their dendrites project to the superficial dorsal horn (Naim et al., 1997). We speculate that many of these activated superficial and deep dorsal horn neurons receive C-afferent inputs directly and/or indirectly and that some may be projection neurons that convey nociceptive information to higher levels of the central nervous system.
One minor concern about our data is that the total number of pNR1-ir neurons in the normal group was different in our two pain models. One possible reason for this discrepancy is that the sensitivity of the primary antibodies used for these two studies was not identical. Although the antibodies had the same product descriptions, they were purchased 1 year apart and had different batch numbers. In our immunostaining process, however, both control and experimental tissues were always processed together, and data were compared only among the tissues processed together. We believe that this precaution eliminated the variability caused by staining variations in the different immunostaining processes.
In conclusion, this study reveals that ROS play an important role in NMDA-receptor phosphorylation, which leads to central sensitization and in turn contributes to persistent pain in spinal-nerve-ligated neuropathic rats and acute inflammatory pain in capsaicin-treated rats.
Acknowledgments
This work was supported by National Institutes of Health Grants NS11255, NS31680, and AT001474.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–42. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Balazs L, Leon M. Evidence of an oxidative challenge in the Alzheimer’s brain. Neurochem Res. 1994;19:1131–37. doi: 10.1007/BF00965146. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–36. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner GJ, Ji R-R, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Cerne R, Jiang M, Randic M. Cyclic adenosine 3’5’-monophosphate potentiates excitatory amino acid and synaptic responses of rat spinal dorsal horn neurons. Brain Res. 1992;596:111–23. doi: 10.1016/0006-8993(92)91538-p. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christie JM, Wenthold RJ, Monaghan DT. Insuline causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J Neurochem. 1999;72:1523–8. doi: 10.1046/j.1471-4159.1999.721523.x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–70. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Ben Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–26. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Hensley K, Pye QN, Tabatabaie T, Stewart CA, Floyd RA. Reactive oxygen involvement in neurodegenerative pathways. In: Wood PL, editor. Neuroinflammation: Mechanisms and Management. Totowa, NJ: Humana Press; 1997. pp. 265–81. [Google Scholar]
- Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994;344:796–8. doi: 10.1016/s0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–35. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–24. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–7. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- Klann E, Roberson ED, Knapp LT, Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273:4516–22. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Progr Neuropsychiatr Biol Psychiatr. 1999;23:359–76. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitone. Antioxiants & Redox Signaling. 1999;1(4):481–499. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–90. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Li L, Shou Y, Borowitz JL, Isom GE. Reactive oxygen species mediate pyridostigmine-induced neuronal apoptosis: involvement of muscarinic and NMDA receptors. Toxicol Appl Pharmacol. 2001;177:17–25. doi: 10.1006/taap.2001.9283. [DOI] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–8. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. Pain. 1995;61:383–90. doi: 10.1016/0304-3959(94)00195-K. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57:1287–305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Nakajima Y, Moriyoshi K, Ishii T, Akazawa C, Nakanashi S. Molecular characterization of NMDA and metabotropic glutamate receptors. Ann N Y Acad Sci. 1993;707:153–64. doi: 10.1111/j.1749-6632.1993.tb38050.x. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–37. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Naim M, Spike RC, Watt C, Shehab SAS, Todd AJ. Cells in laminae III and IV of the rat spinal cord that possess the neurokinin-1 receptor and have dorsally directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci. 1997;17:5536–48. doi: 10.1523/JNEUROSCI.17-14-05536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the arginine-X-X-(serine/threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod. 2004;10:355–63. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radical Biology and Medicine. 2006;41:528–40. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Olanow CW. An introduction to the free radical hypothesis in Parkinson’s disease. Ann Neurol. 1992;32:S2–8. doi: 10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- Park SK, Chung K, Chung JM. Effects of purinergic and adrenergic antagonists in a rat model of painful peripheral neuropathy. Pain. 2000;87:171–9. doi: 10.1016/S0304-3959(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–64. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–44. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, et al. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–6. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- Schwartz E, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord plays an important role in capsaicin-induced hyperalgesia. Neurosci Abstr. 2006;551.21 [Google Scholar]
- Simone DA, Sorkin LS, OH U, Chung JM, Owens C, LaMotte RH, et al. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic track neurons. J Neurophysiol. 1991;66:228–46. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. NeuroReport. 1996;7:1382–4. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyana K, Doherty C, Ptak JB, Riley CT, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–66. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–78. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- Willis WD. Central plastic responses to pain. In: Gebhart GF, et al., editors. Proceedings of the 7th World Congress on Pain, Progress in Pain Research and Management. Seattle: IASP Press; 1994. pp. 301–21. [Google Scholar]
- Willis WD. Long-term potentiation in spinothalamic neurons. Brain Res Rev. 2002;40:202–14. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–8. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implication for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Xie J, Park SK, Chung K, Chung JM. The effect of lumbar sympathectomy in the spinal nerve ligation model of neuropathic pain. J Pain. 2001;2:270–8. doi: 10.1054/jpai.2001.24559. [DOI] [PubMed] [Google Scholar]
- Yowtak J, Kim HK, Chung K, Chung JM. Reactive oxygen species in a mouse model of neuropathic pain. Neurosci Abstr. 2006;644.11 [Google Scholar]
- Zhang X, Wu J, Willis WD. The effects of protein phosphatase inhibitions on nociceptive behavioral responses of rats following intradermal injection of capsaicin. Pain. 2003;106:443–51. doi: 10.1016/j.pain.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–97. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neurosci. 2002;115:775–86. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]


