Abstract
Aberrant promoter hypermethylation, also known as epigenetics, is thought to be a promising biomarker approach to diagnose malignancies. Kidney repair after injury is a recapitulation of normal morphogenesis, with similarities to malignant transformation. We hypothesized that changes in urine epigenetics could be a biomarker approach during early kidney transplant injury and repair. We examined urine DNA for aberrant methylation of 2 gene promoters (DAPK CALCA) by quantitative methylation specific PCR from 13 deceased and 10 living donor kidney transplant recipients on postoperative day 2 and 65 healthy controls. Results were compared with clinical outcomes and to results of the kidney biopsy. Transplant recipients were significantly more likely to have aberrant hypermethylation of the CALCA gene promoter in urine than healthy controls (100% vs. 31%, p<0.0001). There was increased CALCA hypermethylation in the urine of deceased vs. living donor transplants (21.60 +/− 12.5 vs. 12.19 +/− 4.7 P=0.04). Furthermore, there was a trend towards increased aberrant hypermethylation of urine CALCA in patients with biopsy-proven acute tubular necrosis vs. acute rejection and slow or prompt graft function (mean: 20.40 +/− 6.9, 13.87 +/− 6.49, 17.17 +/− 13.4, P=0.67). However, there was no difference of CALCA hypermethylation in urine of patients with delayed graft function vs. those with slow or prompt graft function (16.9 +/− 6.2 vs. 18.5 +/− 13.7, respectively; P=0.5). There was no aberrant hypermethylation of DAPK in the urine of transplant patients. Urine epigenetics is a promising biomarker approach for acute ischemic injury in transplantation that merits future study.
Keywords: biomarker, hypermethylation, acute kidney injury
Introduction
There is an increasing incidence of delayed graft function (DGF) (8–50%) after deceased donor kidney transplantation (1–6). This is due, in part, to the need to use kidneys from donors with risk factors for acute tubular necrosis which is driven by the increasing numbers of patients wait listed for transplantation with little increase of the donor pool (7). It has been shown in many, but not all studies, that the presence of DGF with or without rejection is associated with decreased long term graft survival (8). Because of the insults on the allograft from early procurement injury, deceased donor transplants suffer more ischemia and injury than living donor kidneys (7). However, approximately 10% patients with DGF can have other causes of acute kidney injury besides acute tubular necrosis, mainly acute rejection and drug toxicity. At present, the etiology of the DGF can only be reliably diagnosed with a kidney biopsy during the first 7–10 days to exclude acute rejection. Kidney biopsies are associated risks of bleeding and possible loss of the allograft (9, 10). Some clinicians consider the risk of bleeding to be high during the first 7 days following surgery and advocate avoiding this procedure until after one week. However, acute rejections can occur even in the first postoperative week (5). Clinical management of patients with DGF would be improved by the development of non-invasive tests such as urinary biomarkers that can be measured early and safely. The goal of this study was to begin to explore if the novel diagnostic modality of urine epigenetics, that is promising for kidney cancer detection (11, 16–18, 19–21, 27–29), can be used as a novel biomarker approach in the early transplantation setting.
Epigenetics is a new field of molecular medicine and merits introduction for those in transplantation. Epigenetics refers to a heritable change in the pattern of gene expression that is mediated by mechanisms other than the primary nucleotide sequence of gene. Reactions using S-adenosyl-methionine as a methyl donor catalyzed by enzymes called DNA methyltransferases (DNMTs) add a methyl group to the cytosine ring to form methyl cytosine. In humans and other mammals, this modification is imposed only on cytosines that precede a guanosine in the DNA sequence (the CpG dinucleotide). In normal cells, most CpG sites outside of the CpG Island are methylated and CpG Island sites are unmethylated. The methylated state in the normal genome suppresses unwanted transcription whereas the unmethylated state in the CpG Island permits active gene transcription. In virtually all types of cancer, most CpG sites in the bulk of the genome and in the coding regions of genes, which should be methylated, become unmethylated, and a growing list of genes have been identified as having abnormal methylation of promoters containing CpG islands with associated transcriptional silencing (11). The presence of abnormally high methylated DNA concentrations in the serum and urine of patients with various malignancies has been confirmed (11–22) but this concept has yet to be tested in the kidney transplant setting.
Since injury and repair are a recapitulation of morphogenesis and methylation of cytosine nucleotides in human cancer cells have been shown to participate in the inactivation of several apoptotic, cell cycle, and DNA repair pathways, we hypothesized that methylation based urinary biomarkers could be useful for the early detection of ischemic reperfusion injury. We evaluated the diagnostic potential of DNA methylation-based markers in the urine of both deceased and living donor kidney transplants. We compared these results to those from previously collected age matched non kidney transplant patients. Using quantitative methylation specific PCR (QMSP), we analyzed the promoter hypermethylation pattern in 2 cancer related genes, calcitonin gene (CALCA) and death associated protein Kinase (DAPK) with matched urine DNA. CALCA gene was chosen for its important role in T and B cell regulation in certain malignancies; whereas DAPK was tested for hypermethylation as this gene participates in regulation of various apoptotic systems of tumor cells that undergo initiation, progression, and metastasis which has similarities to the injury and repair process that occurs in ischemic reperfusion injury (IRI). The results of urine epigenetic data were correlated to clinical outcomes as well as the kidney biopsy.
Methods
Study Cohorts
After obtaining approval from the Institutional Review Board of the Johns Hopkins University, approximately 50 cc of urine was collected from 13 deceased and 10 living donor transplant recipients on postoperative day 2 following kidney transplantation at Johns Hopkins Hospital. The mean age in this cohort was 49.6 (+/− 13.9) years. There were 13 (56.5%) deceased donor and 10 (43.5%) living donor renal transplant recipients. Deceased donors were defined mostly as donors who had clinical evidence of brain death. The cohort was 43.5% female, 56.5% Caucasian, 39.1% African American and 4.3 % Asian. Nearly 48% had delayed graft function (DGF) defined as the need for dialysis within the first week post transplantation (1, 6). Patients who did not have sufficient graft dysfunction to be classified as having DGF were defined to have slow graft function (23). The mean cold ischemia time in deceased donor recipient transplants was 28.63 +/− 13.6 hours vs. 1–2 hours in living donor transplants. In addition, 65 urines collected from healthy adults served as controls in this study. The control samples were collected as part of a previous pilot study, which analyzed similar genes. The controls had a median age of 61 (range 28–64), were 17% female, 34% Caucasian, 12% African American, 1.5% Asian and 1.5% Hispanic, with 51% race not reported.
The urine was collected via an aseptic technique from a Foley catheter. Clinical data including the patients' age, sex, cause of end stage renal disease, serum creatinine on day of collection, presence of DGF, and immunosuppression regimen was collected on the day of the sample. Hypermethylation levels were correlated with the findings of a subsequent kidney biopsy, which was performed if deemed necessary by the patient’s primary physicians for the evaluation of DGF on postoperative day 7 through 10. Formalin–fixed, paraffin embedded renal biopsy specimens were stained with hematoxylin and eosin, periodic acid-Schiff and Mason’s trichrome stains and were scored according to the Banff 97 classification (24). Immunosuppression mainly consisted of a calcineurin inhibitor based regimen (tacrolimus) with the concurrent use of glucocorticoids, mycophenolate mofetil, and an induction agent: either Il-2 receptor antagonist, antithymocyte globulin, or rituximab. However, many patients with DGF did not receive a calcineurin inhibitor when the urine was obtained. The study population were adults age 18 and over with a history of advanced kidney failure that were status post kidney transplantation at Johns Hopkins Hospital. There were no major exclusion criteria.
Fifty milliliters of voided urine was collected from all controls and cases. Urine samples were spun at 3000xg for 10 min and washed twice with phosphate-buffered saline. All samples were stored at −80°C. Frozen urine cell pellets were digested with 1% SDS and 50μg/ml proteinase K (Boehringer Mannheim, Germany) at 48ºC overnight, followed by phenol/chloroform extraction and ethanol precipitation of DNA as previously described (56).
Bisulfite treatment
DNA from urine sediment was subjected to bisulfite treatment, as described previously (26). Briefly, 2 μg of genomic DNA was denatured in 0.2 M NaOH for 20 min at 50°C. The denatured DNA was diluted in 500 μl of freshly prepared solution of 10 mM hydroquinone and 3 M sodium bisulfite, and incubated for 3 hours at 70° C. After incubation, the DNA sample was desalted through a column (Wizard DNA Clean-Up System, Promega), treated with 0.3 M NaOH for 10 min at room temperature, and precipitated with ethanol. The bisulfite-modified genomic DNA was resuspended in 120 μl of LoTE (2.5 mM EDTA, 10 mM Tris-HCL) and stored at −80°C.
Methylation analysis
Templates were amplified by a fluorescence based-real-time PCR as previously described (28). In brief, primers and probes were designed to specifically amplify the bisulfite-converted promoter of the gene of interest. The ratios between the values of the gene of interest and the internal reference gene, β-actin, obtained by Taqman analysis, were used as a measure for representing the relative level of methylation in the particular sample (Target gene/β–actin X 1000). Fluorogenic PCRs were carried out in triplicate in a reaction volume of 20 μl consisting of 600 nM of each primer, 200 nM of probe, 5 units of Taq Polymerase, 200 μM each of dATP, dCTP, and dGTP; 400 of M dTTP; and 5.5 mM MgCl2. Three microliters of treated DNA solution were used in each real-time MSP reaction. Amplifications were carried out in 384-well plates in a 7900 Sequence detector (Perkin-Elmer Applied Biosystems). Each plate consisted of patient samples and multiple water blanks, as well as positive and negative controls. Leukocytes from a healthy individual were methylated in vitro with excess SssI methyltransferase (New England Biolabs Inc., Beverly, MA) to generate completely methylated DNA and serial dilutions of this DNA were used for constructing the calibration curves on each plate.
Statistical Analysis
Descriptive statistics were done to compare demographic and other characteristics between groups of interest. Student t-test was performed to compare mean CALCA values between two groups while analysis of variance (ANOVA) was performed to compare mean values among more than two groups for normally distributed data. For markedly skewed data, Wilcoxon rank-sum (Mann-Whitney) test was done to compare between two groups, and Kruskal Wallis test was performed to compare among more than two groups. Statistical analyses were performed using Stata Statistical Software version 9 (Stata Corporation, College Station, TX).
RESULTS
We examined two genes of diverse function (DAPK and CALCA) in the urine of 23 patients status post either deceased donor (13), living donor related (6), or living donor unrelated kidney transplantation (4) (Table 1).
Table 1.
Characteristics of the transplant patients
| No | Type transplant | DGF | Age | Sex | Race | Cause of ESRD | Creatinine on day prior to transplant mg/dl | Creatinine on day of collection mg/dl | Biopsy results | CALCA/AC TBX | IMMUNO SUPPRESSION |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Deceased | yes | 58 | male | White | HTN, FSGS | 4.4 | 4.0 | ATN | 23.58 | Thymoglobulin MMF, FK-506 prednisone |
| 2 | Deceased | yes | 56 | female | Black | Lithium toxicity | 4.9 | 4.1 | Antibody mediated rejection | 21.37 | Rituximab, Thymoglobulin, FK-506 MMF, prednisone |
| 3 | Deceased | yes | 54 | female | White | DM | 4.8 | 4.8 | Antibody mediated rejection | 10.19 | FK-506, MMF prednisone |
| 4 | Deceased | yes | 52 | male | Black | HTN, DM | 9.1 | 8.0 | Focal, moderate ATN, focal isometric vacuolization | 29.96 | MMF, FK-506 thymoglobulin, prednisone |
| 5 | Deceased | yes | 38 | male | Black | DM | 5.8 | 7.0 | Focal ATN, isometric vacuolization | 17.84 | MMF, FK-506 thymoglobulin, prednisone |
| 6 | Deceased | yes | 41 | male | Asian | IgA nephropathy | 6.0 | 5.5 | N/A | 9.73 | FK-506, MMF prednisone |
| 7 | Deceased | no | 70 | male | White | FSGS | 7.7 | 6.5 | N/A | 15.39 | MMF, FK-506 thymoglobulin, prednisone |
| 8 | Deceased | no | 47 | female | Black | HTN | 8.3 | 4.5 | N/A | 5.54 | MMF, FK-506 thymoglobulin, prednisone |
| 9 | Deceased | no | 65 | male | White | Chronic GN | 9.2 | 2.8 | N/A | 16.55 | FK-505, MMF prednisone |
| 10 | Deceased | no | 38 | female | White | ADPKD | 12.1 | 2.4 | N/A | 16.33 | MMF, FK-506, thymoglobulin, prednisone |
| 11 | Deceased | no | 50 | female | Black | HTN | 12.2 | 2.0 | N/A | 38.07 | MMF, FK-506, thymoglobulin, prednisone |
| 12 | Deceased | no | 55 | male | White | Secondary FSGS | 10.5 | 8.0 | N/A | 50.30 | MMF, FK-506, thymoglobulin, prednisone |
| 13 | Deceased | no | 31 | female | Black | DM | 6.9 | 2.2 | Focal ATN, isometric vacuolization | 25.95 | MMF, FK-506, thymoglobulin, and prednisone |
| 14 | Living Related | no | 38 | male | Black | HTN | 5.6 | 1.4 | Mild focal ATN, no rejection | 21.72 | Certican,, MMF, prednisone FK-506 |
| 15 | Living Related | no | 61 | female | White | DM | 3.5 | 0.9 | N/A | 8.32 | FK-506, MMF prednisone |
| 16 | Living Related | no | 34 | male | White | DM | 2.4 | 1.2 | N/A | 8.71 | Simulect, FK-506, MMF |
| 17 | Living Related | no | 81 | female | Black | HTN, DM | 6.5 | 0.8 | N/A | 13.59 | FK-506, MMF prednisone |
| 18 | Living Related | no | 39 | male | White | SLE | 8.7 | 2.3 | N/A | 6.71 | zenapax, MMF, prednisone FK-506 |
| 19 | Living Related | yes | 36 | male | White | Unilateral stenosis | 2.7 | 2.8 | N/A | 16.93 | FK-506, prednisone MMF |
| 20 | Living Unrelated | yes | 54 | male | White | ADPKD | 17.4 | 3.5 | borderline inflammation, no rejection | 16.89 | Daclizumab MMF FK-506 prednisone |
| 21 | Living Unrelated | yes | 62 | female | White | Postinfectious GN | 6.0 | 4.7 | N/A | 10.57 | Thymoglobulin, FK-506, prednisone, MMF |
| 22 | Living Unrelated | yes | 33 | female | Black | Primary FSGS | 6.6 | 1.2 | focal inflammation with eosinophils mild ATN | 13.24 | FK-506, MMF and prednisone |
| 23 | Living Unrelated | yes | 36 | male | Black | Reflux nephropathy | 8.0 | 3.6 | Severe acute rejection, intimal arteritis | 10.05 | Rituximab, Thymoglobulin, FK-506, MMF and prednisone |
Frequency of aberrant hypermethylation in transplant patients versus controls
Aberrant promoter hypermethylation of one of gene promoters, CALCA, was more likely to be detected among kidney transplant recipients as compared to healthy controls (100% vs. 31%, respectively; p<0.0001). Aberrant methylation of the CALCA gene promoter had no correlation with patient demographic data, including age, gender or thymoglobulin induction status.
Aberrant hypermethylation of CALCA gene promoter in deceased versus living donor transplants
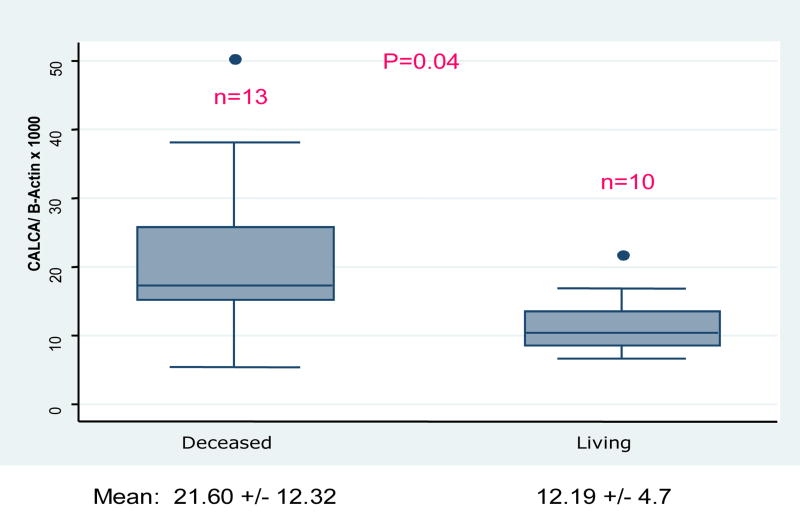
There were no differences in baseline characteristics between deceased and living donor transplant recipients (Table 2). Aberrant hypermethylation of urine CALCA was significantly higher among deceased-donor recipients compared to living-donor recipients, both in unadjusted analysis (21.6 +/− 12.3 vs. 12.7 +/− 4.7, p=0.04) and after controlling for DGF status (p=0.048) (Figure 1).
Table 2.
Characteristics of patients by type of transplant
| Living Donor (n=10) | Deceased Donor (n=13) | p-value | |
|---|---|---|---|
| Age (mean) | 47.4 +/− 16.3 | 51.3 +/− 12.2 | 0.52 |
| Female | 4 (40%) | 6(46%) | 0.55 |
| Black race | 3 (30%) | 6 (46%) | 0.67 |
| Primary Disease | 0.95 | ||
| Diabetes Mellitus | 3 (30%) | 5 (39%) | |
| Hypertension | 1 (10%) | 2 (15%) | |
| ADPKD | 1 (10%) | 1 (8%) | |
| IgA Nephropathy | 0 | 1 (8%) | |
| SLE | 1 (10%) | 0 | |
| FSGS | 1 (10%) | 2 (15%) | |
| Other | 3 (30%) | 2 (15%) | |
| Delayed Graft Function | 5(50%) | 6 (46%) | 1.00 |
| Cold ischemia Time | 1–2 hours | 28.63 +/− 13.6 hours | 0.005 |
Figure 1.
Distribution of CALCA/ -Actin ratio by type of kidney transplant
Aberrant promoter hypermethylation in urine of patients with delayed graft function vs. those with prompt or intermediate function
There was no difference in mean CALCA/β Actin ratio between patients with DGF (n=11) and patients slow or prompt function (n=12) (16.9 +/− 6.2 vs. 18.5 +/− 13.7, respectively; P=0.5) (Figure 2). Controlling for donor type did not alter these findings (Figure 3).
Figure 2.
Distribution of CALCA/ -Actin ratio by delayed graft function
Figure 3.
Distribution of CALCA/ -Actin ratio by type of transplant and delayed graft function
Among patients who were not dialysis dependent, there was no significant association between CALCA and creatinine on the day of collection (p=0.09). Also, sensitivity analysis excluding 1 outlier of CALCA/B-actin levels > 50 did not alter the finding.
Aberrant Hypermethylation of CALCA and type of allograft injury
Of the 23 renal transplant recipients, 10 subsequently required allograft biopsy to investigate the cause of persistent allograft failure. Biopsies were performed 3–5 days after drawing the CALCA levels. Of these, 7 were found to have acute tubular necrosis and 3 were found to have acute allograft rejection. There was a trend towards elevated mean CALCA hypermethylation levels among patients with biopsy proven acute tubular necrosis, vs. acute rejection or a functioning allograft (20.4 +/− 6.9, 13.9 +/− 6.5, 17.2 +/− 12.9; p=0.67) (Figure 4). This finding was not statistically significant likely secondary to small sample size within the individual groups.
Fig 4.
Distribution of CALCA/ -Actin ratio by acute tubular necrosis and acute rejection
Discussion
Recently, there was been a great deal of interest in the search for novel noninvasive urine and serum biomarkers to diagnose and define etiologies of renal dysfunction (57). Certain blood and urine markers of kidney injury such as serum creatinine, urea nitrogen as well as urine markers such as casts, fractional excretion of Na are insensitive and nonspecific for the diagnosis of acute kidney injury (32). Recently, urinary molecules have shown promise in both urine and animal studies including, neutrophil gelatinase associated lipocalcin (NGAL) (33, 34), Kidney Injury Molecule-1 (Kim 1) (35, 36), sodium/hydrogen exchanger isoform 3 (NHE3) (37), cytokines (38, 39) cysteine rich protein 61(40), actin (38), glutathione-S-transferases (41, 42). These biomarkers have a great deal of promise in the ability to detect early kidney injury and aid with its subsequent management but need to be validated and confirmed in larger studies.
Molecular signatures of cancers of all types have been used to improve cancer detection and assess disease risk. Promotor- hypermethylation events provide some of the most promising markers for such purposes (43). DNA –based markers have advantages because of the inherent stability of DNA as compared to RNA and proteins. Hypermethylation of CpG Islands in gene promoters can and do appear very early in the progression of lung tumors (44) and colon cancer (45, 46) and have been to be helpful in detecting premalignant lesions. There have been no publications to date in determining the utility of this diagnostic modality in the kidney transplantation setting for the purposes of early detection of kidney dysfunction.
In this pilot study, we tested aberrant methylation to two gene promoters, CALCA and DAPK. It has been well established that the calcitonin gene, which lies on chromosome 11p15.2 is a “hot spot” for methylation in leukemia (50). It also has been described in lymphomas (47) and well as myelodysplastic syndrome (48). The CpG rich 5’ region of the calcitonin gene was found to be “methylated” in 75–95% of acute leukemias by Southern analysis (47) or genomic sequencing (49). It has also been shown that CALCA gene hypermethylation is probably a crucial event in acute lymphocytic leukemia (ALL) that appears in a significant proportion (43%) of patients with adult and childhood ALL. Aberrant CALCA gene methylation arises universally and de novo during leukemic transformation in hematopoietic progenitors developing in the lymphoid pathway (B or T cell lineage) (51). Since it has been shown that CD4+ T cells may play a pathogenic role in ischemic acute renal failure (52, 53) as well as in acute rejection, we believed that aberrant hypermethylation of the CALCA gene which seems to be involved in the lymphoid pathway may be a biomarker of the two main causes of acute kidney injury in transplantation; mainly acute rejection and acute tubular necrosis.
In our subset of transplant patients compared to the age matched controls, we found that CALCA was found in a greater proportion of patients’ status post kidney transplantation than in controls. This suggests a potential importance of this marker as a sign of injury and repair in the setting of transplantation either from acute kidney injury vs. surgical manipulation. This is a nonspecific finding as 30% of our normal healthy controls had aberrant hypermethylation of CALCA in the urine. However, it is important to note that age related methylation in normal controls has not been found in the CALCA gene (54).
In order to distinguish if hypermethylation of CALCA in urine of transplant patients truly was a marker of ischemia reperfusion injury, we tested aberrant hypermethylation of CALCA gene between deceased and living donor patients. In this analysis, we found a statistically significant difference in urine CALCA between deceased vs. living donor transplants. This suggests that this difference of increased urine CALCA hypermethylation is likely a marker of ischemia since deceased donor transplants suffer procurement injury and undergo cold ischemic time that predisposes this type of kidney injury to delayed graft function from acute tubular necrosis. In a subgroup analysis comparing the few subjects who underwent kidney biopsy and their respective urine CALCA levels, we found a trend of increased promoter hypermethylation of CALCA in patients who had biopsy proven ATN vs. those with rejection or prompt function. This result was an important finding but was not statistically significant as very small proportion of patients in this analysis had undergone a kidney biopsy and there were small number of patients to compare in each group. Interestingly enough, we found no significant correlation of aberrant hypermethylation of urine CALCA overall in patients with DGF vs. those without DGF. We suspect this negative finding was likely secondary to small sample size as well.
The second gene tested in this study was apoptosis related gene, death associated protein (DAP) –kinase (DAPK). DAPK is member of novel subfamily of proapoptotic serine/threonine kinases, and was recently identified as a new tumor suppressor gene with multiple functions in programmed cell death (55). DAPK is known to participate in both the elimination of premalignant cells or cytoprotection in cellular homeostasis. DAPK is frequently inactivated by aberrant promoter hypermethylation in many cancer types and its expression has been useful in cancer prognosis. In our cohort of transplant patients, there was no significant hypermethylation of DAPK in the urine suggesting that there is no role for this gene promoter in the diagnosis of acute kidney injury in the transplant setting.
There were some limitations to this preliminary study. One of most important limitations has been the small sample size of the study which limits many of our findings to be statistically significant. We need to test this novel approach with other kidney specific gene promoters in a larger cohort of transplant patients. Although we did not find any association with elevated urine CALCA levels and creatinine, it would be interesting to test and association with other traditional biomarkers such as B2-microglobulin, fractional excretion of sodium as well as with other novel biomarkers such as KIM-1, NGAL, cystatin C etc. Lastly, it would be important to search for methylation markers in the serum. In conclusion, urine epigenetics is a promising diagnostic modality to evaluate kidney injury in the setting of kidney transplantation. Further studies will be needed with larger cohort of patients to validate and extend the current findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ojo AO, Wolfe RA, Held P, Port FK, Schomder RL. Delayed Graft Function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–74. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 2.The Organ Procurement and Transplantation Network.
- 3.Jacobs SC, Cho E, Foster C, Liao P, Bartlett ST. Laparoscopic donor nephrectomy: the University of Maryland 6-year experience. J Urol. 2004;171(1):47–51. doi: 10.1097/01.ju.0000100221.20410.4a. [DOI] [PubMed] [Google Scholar]
- 4.The Canadian Multicentre Transplant Study Group. A randomized clinical trial of cyclosporine in cadaveric renal transplantation: Analysis at three years. N Engl J Med. 1986;314:1219–25. doi: 10.1056/NEJM198605083141904. [DOI] [PubMed] [Google Scholar]
- 5.Gjertson DW. Impact of delayed graft function and graft function. In: Cecka JM, Terasaki PI, editors. Clinical Transplants. Los Angeles, CA: The Regents of the University of California; 2000. pp. 467–79. [Google Scholar]
- 6.Koning OJ, Ploeg R, Van Beckel JH, et al. Risk Factors for delayed graft function in cadaveric kidney transplantation. Transplantation. 1997;63(11):1620–8. doi: 10.1097/00007890-199706150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Senel FM, Karakayali H, Moray G, Haberal M. Delayed graft function: predictive factors and impact on outcome in living related kidney transplantations. Renal Fail. 1998;20(4):589–95. doi: 10.3109/08860229809045151. [DOI] [PubMed] [Google Scholar]
- 8.Shoskes DA, Cecka JM. Effect of delayed graft function on short and long term kidney graft survival. Clinical Transplantation. 1997:297–303. [PubMed] [Google Scholar]
- 9.Huraib S, Goldberg H, Katz A, et al. Percutaneous needle biopsy of the transplanted kidney: technique and complications. Am J Kidney Dis. 1989;14(1):13–7. doi: 10.1016/s0272-6386(89)80087-3. [DOI] [PubMed] [Google Scholar]
- 10.Beckingham IJ, Nicholson ML, Bell PR. Analysis of factors associated with complications following renal transplant needle core biopsy. Br J Urol. 1994;73(1):13–5. doi: 10.1111/j.1464-410x.1994.tb07449.x. [DOI] [PubMed] [Google Scholar]
- 11.Herman JG, Baylin SB. Mechanisms of Disease: Gene Silencing in Cancer in Association with Promoter Hypermethylation. New England Journal of Medicine. 2003;349(21):2042–2051. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 12.Geddes C, Woo M, Jardine A. The Impact of delayed graft function on the long-term outcome of renal transplantation. J Nephrology. 2002;15(1):17–21. [PubMed] [Google Scholar]
- 13.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000;16(4):168–74. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 14.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 15.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 16.Jeronimo C, Henrique R, Hoque MO, et al. A quantitative promoter methylation profile of prostate cancer. Clinical Cancer Res. 2004;10(24):8472–8. doi: 10.1158/1078-0432.CCR-04-0894. [DOI] [PubMed] [Google Scholar]
- 17.Hoque MO, Begum S, Topaloglu O, et al. Quantitative detection of promoter hypermethylation of multiple genes in the tumor, urine, and serum DNA of patients with renal cancer. Cancer Res. 2004;64(15):5511–7. doi: 10.1158/0008-5472.CAN-04-0799. [DOI] [PubMed] [Google Scholar]
- 18.Jeronimo C, Henrique R, Hoque MO, et al. Quantitative RARbeta2 hypermethylation: A promising prostate cancer marker. Clinical Cancer Res. 2004;10(12 Pt 1):4010–4. doi: 10.1158/1078-0432.CCR-03-0643. [DOI] [PubMed] [Google Scholar]
- 19.Xing M, Cohen Y, Mambo E, et al. Early occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesis. Cancer Research. 2004;64(5):1664–8. doi: 10.1158/0008-5472.can-03-3242. [DOI] [PubMed] [Google Scholar]
- 20.Umetani N, Mori T, Koyanagi K, et al. Aberrant hypermethylation of ID4 gene promoter region increases risk of lymph node metastasis in T1 breast cancer. Oncogene. 2005;24(29):4721–7. doi: 10.1038/sj.onc.1208538. [DOI] [PubMed] [Google Scholar]
- 21.Shinozaki M, Hoon DS, Giuliano AE, et al. Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clinical Cancer Res. 2005;11(6):2156–62. doi: 10.1158/1078-0432.CCR-04-1810. [DOI] [PubMed] [Google Scholar]
- 22.Costello JF, Fruhwald MC, Smiraglia DJ, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24(2):132–8. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 23.Humar A, Ramchandran T, Kandaswamy R, Gillingham K, Payne WD, Matas A. Risk factors for slow graft function after kidney transplants: a multivariate analysis. Clinical Transplant. 2002;16(6):425–429. doi: 10.1034/j.1399-0012.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 93(18):9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraga MF, Esteller M. DNA Methylation: a profile of methods and applications. Biotechniques. 2002;33(3):632, 634, 636–49. doi: 10.2144/02333rv01. [DOI] [PubMed] [Google Scholar]
- 27.Topaloglu O, Hoque MO, Tokumaru Y, et al. Detection of promoter hypermethylation of multiple genes in the tumor and bronchoalveloar lavage of patients with lung cancer. Clinical Cancer Res. 2004;10(7):2284–8. doi: 10.1158/1078-0432.ccr-1111-3. [DOI] [PubMed] [Google Scholar]
- 28.Harden SV, Tokumaru Y, Westra WH, et al. Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clinical Cancer Res. 2003;9(4):1370–5. [PubMed] [Google Scholar]
- 29.Eads CA, Lord RV, Wickramasinghe K, et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;15 61(8):3410–8. [PubMed] [Google Scholar]
- 30.Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28(8):E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999;59(10):2302–6. [PubMed] [Google Scholar]
- 32.Star RA. Treatment of acute renal failure. Kidney International. 1998;54(6):1817–31. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 33.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrology. 2003;14(10):2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 34.Mishra J, Mori K, Ma Q, et al. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrology. 2004;24(3):307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 35.Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 36.Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. American J Physiology Renal Physiology. 2004;286(3):F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 37.du Cheyron D, Daubin C, Poggioli J, et al. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. American J Kidney Kidney Dis. 2003;42(3):497–506. doi: 10.1016/s0272-6386(03)00744-3. [DOI] [PubMed] [Google Scholar]
- 38.Kwon O, Molitoris BA, Pescovitz M, et al. Urinary actin, interleukin-6, and interleukin-8 may predict sustained ARF after ischemic injury in renal allografts. Am J Kidney Dis. 2003;41(5):1074–87. doi: 10.1016/s0272-6386(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 39.Parikh CR, Jani A, Melnikov VY, et al. Urinary interleukin-18 is a marker of human acute tubular necrosis. American J Kidney Disease. 2004;43(3):405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Muramatsu Y, Tsujie M, Kohda Y, et al. Early detection of cysteine rich protein 61 (CYR61, CCN1) in urine following renal ischemic reperfusion injury. Kidney International. 2002;62(5):1601–10. doi: 10.1046/j.1523-1755.2002.00633.x. [DOI] [PubMed] [Google Scholar]
- 41.Usuda K, Kono K, Dote T, et al. Urinary biomarkers monitoring for experimental fluoride nephrotoxicity. Arch Toxicology. 1998;72(2):104–9. doi: 10.1007/s002040050475. [DOI] [PubMed] [Google Scholar]
- 42.Branten AJ, Mulder TP, Peters WH, et al. Urinary excretion of glutathione S transferases alpha and pi in patients with proteinuria: reflection of the site of tubular injury. Nephron. 2000;85(2):120–6. doi: 10.1159/000045644. [DOI] [PubMed] [Google Scholar]
- 43.Laird Peter. The Power and the Promise of DNA Methylation Markers. Nat Rev Cancer. 2003;3(4):253–66. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 44.Virmani A, Tsou J, Siegmund K, Shen L, et al. Hierarchical Clustering of Lung Cancer Cell Lines Using DNA Methylation Markers. Cancer Epidemiologic Biomarkers Prev. 2002;11(3):291–7. [PubMed] [Google Scholar]
- 45.Lee S, Hwang KS, Lee HJ, Kim JS, Kang GH. Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Lab Investigation. 2004;84(7):884–93. doi: 10.1038/labinvest.3700108. [DOI] [PubMed] [Google Scholar]
- 46.Cote S, Sinnett D, Momparler RL. Demethylation by 5-aza-2'-deoxycytidine of specific 5-methylcytosine sites in the promoter region of the retinoic acid receptor ß gene in human colon carcinoma cells. Anticancer Drugs. 1998;9(9):743–50. doi: 10.1097/00001813-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Baylin SB, Hoppener JW, de Bustros A, Steenbergh HP, Lips CJ, Nelkin BD. DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res. 1986;46(6):2917–22. [PubMed] [Google Scholar]
- 48.Ihalainen J, Pakkala S, Savolainen ER, Jansson SE, Palotie A. Hypermethylation of the calcitonin gene in the myelodysplastic syndromes. Leukemia (Baltimore) 1993;7:263–267. [PubMed] [Google Scholar]
- 49.Leegwater PA, Lambooy LH, De Abreu RA, Bokkerink JP, van den Heuvel LP. DNA methylation patterns in the calcitonin gene region at first diagnosis and at relapse of acute lymphoblastic leukemia (ALL) Leukemia. 1997;11(7):971–8. doi: 10.1038/sj.leu.2400688. [DOI] [PubMed] [Google Scholar]
- 50.Melki JR, Vincent PC, Clark SJ. Concurrent DNA hypermethylation of multiple genes in acute myeloid leukemia. Cancer Res. 1999;59(15):3730–40. [PubMed] [Google Scholar]
- 51.Roman Jose, Castillejo JA, Antonio Jimenez, et al. Hypermethylation of the calcitonin gene in acute lymphoblastic leukemia is associated with unfavourable clinical outcome. Br J Haematol. 2001;113(2):329–38. doi: 10.1046/j.1365-2141.2001.02764.x. [DOI] [PubMed] [Google Scholar]
- 52.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clinical Investigation. 2001;108(9):1283–90. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokota N, Daniels F, Crosson J, Rabb H. Protective effect of T cell depletion in murine renal ischemia- reperfusion injury. Transplantation. 2002;74(6):759–63. doi: 10.1097/00007890-200209270-00005. [DOI] [PubMed] [Google Scholar]
- 54.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58(23):5489–94. [PubMed] [Google Scholar]
- 55.Schneider-Stock R, Roessner A, Ullrich O. DAP-kinase--protector or enemy in apoptotic cell death. Int J Biochem Cell Bio. 2005;37(9) doi: 10.1016/j.biocel.2005.02.019. Epub 1763–7. [DOI] [PubMed] [Google Scholar]
- 56.Sidransky D, Von Eschenbach A, Tsai YC, et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991;252(5006):706–9. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- 57.Han WK, Bonventre JV. Biologic Markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10(6):476–82. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]