Abstract
We have found that Pseudomonas putida ATCC 17642 cells grown in a medium containing D-threonine as the sole nitrogen source produce an enzyme that catalyzes epimerization of threonine. Proton nuclear magnetic resonance analysis of the enzyme reaction in deuterium oxide clearly showed epimerization from L- to D-allo-threonine and also from D- to L-allo-threonine. This is the first example of an enzyme that was clearly shown to epimerize threonine. The enzyme has been purified to homogeneity, which was shown by polyacrylamide gel electrophoresis. The enzyme has a molecular weight of about 82,000 and consists of two subunits identical in molecular weight (about 41,000). The enzyme contains 1 mol of pyridoxal 5'-phosphate per mol of subunit as a cofactor, and its absorption spectrum exhibits absorption maxima at 280 and 420 nm. The enzyme catalyzes not only epimerization of threonine by stereoconversion at the alpha position but also racemization of various amino acids, except acidic and aromatic amino acids. The enzyme is similar to amino acid racemase with low substrate specificity (EC 5.1.1.10) in enzymological properties but is distinct from it in the action on threonine.
Full text
PDF

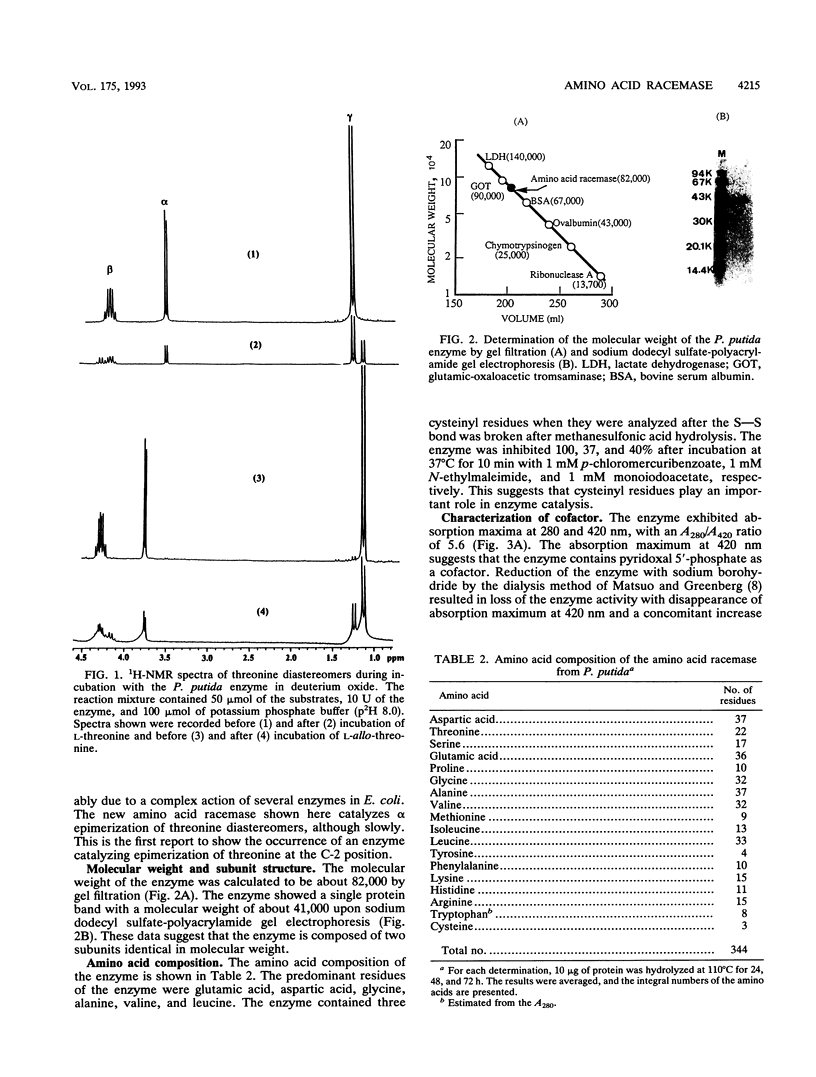


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. Fluorometric determination of pyridoxal phosphate in enzymes. Methods Enzymol. 1979;62:407–410. doi: 10.1016/0076-6879(79)62249-8. [DOI] [PubMed] [Google Scholar]
- Chiou S. H., Wang K. T. Simplified protein hydrolysis with methanesulphonic acid at elevated temperature for the complete amino acid analysis of proteins. J Chromatogr. 1988 Sep 16;448(3):404–410. doi: 10.1016/s0021-9673(01)84603-3. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Tanizawa K., Badet B., Walsh C. T., Tanaka H., Soda K. Thermostable alanine racemase from Bacillus stearothermophilus: molecular cloning of the gene, enzyme purification, and characterization. Biochemistry. 1986 Jun 3;25(11):3268–3274. doi: 10.1021/bi00359a028. [DOI] [PubMed] [Google Scholar]
- Kusakabe H., Kodama K., Kuninaka A., Yoshino H., Misono H., Soda K. A new antitumor enzyme, L-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J Biol Chem. 1980 Feb 10;255(3):976–981. [PubMed] [Google Scholar]
- MATSUO Y., GREENBERG D. M. A crystalline enzyme that cleaves homoserine and cystathionine. III. Coenzyme resolution, activators, and inhibitors. J Biol Chem. 1959 Mar;234(3):507–515. [PubMed] [Google Scholar]
- Walsh C. T. Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem. 1989 Feb 15;264(5):2393–2396. [PubMed] [Google Scholar]
- Yorifuji T., Ogata K. Arginine racemase of Pseudomonas graveolens. I. Purification, crystallization, and properties. J Biol Chem. 1971 Aug 25;246(16):5085–5092. [PubMed] [Google Scholar]