Abstract
Infection of macrophages by Yersinia species results in YopJ-dependent apoptosis, and naïve macrophages are highly susceptible to this form of cell death. Previous studies have demonstrated that macrophages activated with lipopolysaccharide (LPS) prior to infection are resistant to YopJ-dependent cell death; we found this simultaneously renders macrophages susceptible to killing by YopJ− Yersinia pseudotuberculosis (Yptb). YopJ− Yptb-induced macrophage death was dependent on caspase-1 activation, resulting in rapid permeability to small molecules, followed by membrane breakdown and DNA damage, and accompanied by cleavage and release of proinflammatory interleukin-18. Induction of caspase-1-dependent death, or pyroptosis, required the bacterial type III translocon but none of its known translocated proteins. Wild-type Yptb infection also triggered pyroptosis: YopJ-dependent activation of proapoptotic caspase-3 was significantly delayed in activated macrophages and resulted in caspase-1-dependent pyroptosis. The transition to susceptibility was not limited to LPS activation; it was also seen in macrophages activated with other Toll-like receptor (TLR) ligands and intact nonviable bacteria. Yptb infection triggered macrophage activation and activation of caspase-1 in vivo. Y. pestis infection of activated macrophages also stimulated caspase-1 activation. These results indicate that host signaling triggered by TLR and other activating ligands during the course of Yersinia infection redirects both the mechanism of host cell death and the downstream consequences of death by shifting from noninflammatory apoptosis to inflammatory pyroptosis.
Author Summary
Pathogenic Yersinia are bacteria capable of interacting with host immune cells and inhibiting their function. Macrophages are potent antimicrobial immune cells that eliminate invading microbes, and represent a major target for Yersinia during infection. Yersinia triggers death of resting macrophages by apoptosis, a process thought to be advantageous for Yersinia growth during early stages of infection because important host cells are eliminated without perturbing the surrounding tissue. However, activated macrophages with enhanced antimicrobial activity play a crucial role in controlling Yersinia infection. To elucidate the mechanisms involved in successful defense against infection, the authors investigated the response of activated macrophages to Yersinia, which revealed induction of a proinflammatory cell death pathway termed pyroptosis. Unlike apoptosis, pyroptosis unleashes inflammatory mediators capable of enhancing immune responses and clearing bacteria. Macrophage activation and pyroptosis was observed in infected host tissue. Thus, regulating the mechanism of cell death is important for effective responses to Yersinia infection: activated macrophages resisting apoptosis are redirected to utilize pyroptosis, an inflammatory process facilitating host resistance.
Introduction
The genus Yersinia includes three species pathogenic for humans: Yersinia pestis, the causative agent of plague, and Y. entercolitica and Y. pseudotuberculosis, which cause gastroenteritis and lymphadenitis and occasionally systemic infection. All pathogenic Yersinia species harbor a 70 kb virulence plasmid, which encodes a type III secretion system (T3SS) and the effector proteins translocated by this system [1]. The structural components of the T3SS include the needle complex and the secreted proteins YopB and YopD, which form a conduit through the host cell membrane to allow entry of bacterial effector proteins directly into the host cytosol. Once the effector proteins (Yops E, H, O, M, and J) reach the cytosol, they function primarily to inhibit phagocytosis and suppress the host inflammatory response triggered upon bacterial interaction [2]. In addition, all three pathogenic Yersinia species are able to induce cell death in naïve macrophages, and this requires the translocated effector YopJ [3–5].
Two signals are required for maximal induction of cell death in Yersinia-infected naïve macrophages, YopJ and signaling through host Toll-like receptor 4 (TLR4) [6,7]. Upon contact with a macrophage, Yersinia lipopolysaccharide (LPS) recognized by host TLR4 simultaneously initiates apoptotic signaling through the adapter protein TRIF [8,9] as well as mitogen-activated protein kinase (MAPK)- and nuclear factor kappa B (NF-κB)-dependent up-regulation of inflammatory cytokine production and cell survival genes [10–12]. However, YopJ inhibits the activation of NF-κB and MAPKs [13–15], allowing apoptotic signaling to predominate. TRIF-dependent signaling leads to cleavage of the apoptotic initiator caspase-8 [9] and release of cytochrome c from the mitochondria [16]. This leads to activation of downstream executioner caspases-9, −7, and −3 and apoptosis of Yersinia-infected naïve macrophages [16]. Although inducing cell death via apoptosis could potentially suppress inflammation and eliminate macrophages, a host cell type hypothesized to play an important role in combating Yersinia infection [5,17], the relative importance of these YopJ-dependent processes during Yersinia infection is somewhat controversial. Some groups report no change in virulence of YopJ mutant bacteria [18–20] and others observe varying degrees of attenuation [21,22].
In vivo, Yersinia delays inflammation in a T3SS-dependent manner, allowing the bacteria to proliferate [23]. However, the pathology resulting from infection is strikingly biphasic: as infection progresses, Yersinia no longer controls the inflammatory host response resulting in the influx of neutrophils and macrophages, increased inflammatory cytokine production, and tissue necrosis [24–28]. The presence of both host inflammatory mediators and/or bacterial products during infection could result in macrophage activation [29], which is thought to play a critical role in the resolution of Yersinia infection [5,17]. Pretreatment of mice with the macrophage-activating cytokines tumor necrosis factor alpha and interferon gamma confers protection to lethal challenge [23], and dysregulation of the normal LPS modifications made by Yersinia increases their stimulation of macrophages and allows the host to control infection [30]. This suggests that a greater understanding of the differing responses of naïve and activated macrophages to Yersinia infection will provide insight into the immunopathogenesis involved in establishing an ongoing infection, as well as generating protective host immune responses. Activation of macrophages alters their adhesion, migration, and cytokine production, and increases antigen endocytosis, antigen presentation, and activation of effector functions [29]. Importantly, macrophage activation alters the cellular response to death inducing stimuli [10,12,31,32]. Previous studies indicate the toxic effects of YopJ are altered in activated macrophages: LPS pretreated macrophages activate NF-κB, a process inhibited by YopJ in naïve macrophages, and YopJ-dependent apoptosis is correspondingly reduced [33].
In this study we examined the response of activated macrophages to Y. pseudotuberculosis (Yptb) infection in vitro and in vivo. TLR-mediated suppression of apoptosis leads to Yptb-induced macrophage cell death being redirected to a caspase-1-dependent inflammatory pathway called pyroptosis [34], and this process depends upon an intact Yptb T3SS. We also observed the characteristics of this mechanistic shift in host cell execution, macrophage activation and activation of caspase-1, occurring during Yptb infection in vivo. Together our observations suggest modulation of host cell death pathways is an important response to infection.
Results
Activation of Macrophages Increases Susceptibility to Cell Death Induced by YopJ− Yptb
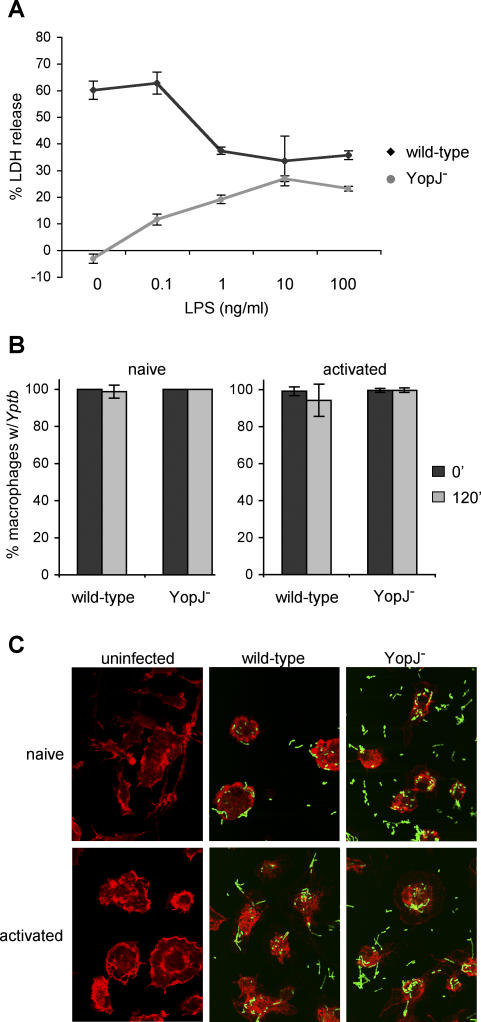
Infection of macrophages with Yptb results in the induction of apoptosis that is dependent on the effector YopJ [3,4,35]. Treatment of macrophages with LPS prior to infection with Yersinia has been shown to decrease YopJ-dependent apoptosis [33]. We confirmed that LPS pretreatment of macrophages (macrophage activation) [29] prior to infection with wild-type Yptb reduced macrophage cell death by approximately 50% as measured by release of cytosolic lactate dehydrogenase (LDH) (Figure 1A). In addition, macrophage activation increased LDH release from background levels to 30% during infection with a yopJ mutant. Both phenotypes were observed at LPS concentrations as low as 1 ng/ml (Figure 1A).
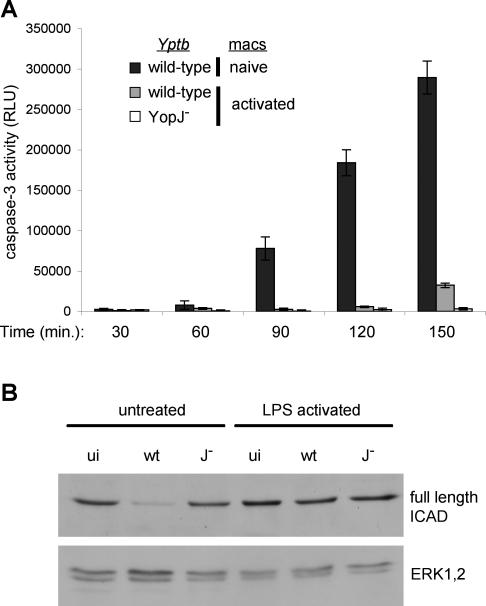
Figure 1. Activation of Macrophages Increases Susceptibility to Cell Death Induced by Infection with YopJ− Yptb .
BMDMs were treated with varying concentrations of LPS for 18 h prior to infection with wild-type or YopJ− Yptb.
(A) Host cell lysis was assessed by measuring release of cytosolic LDH into the supernatant at 3.5 h postinfection. Data shown are means and SDs calculated from three replicates and are representative of three experiments.
(B and C) Naïve macrophages and macrophages activated with 100 ng/ml LPS for 18 h were infected with GFP-expressing wild-type or YopJ− Yptb. The uniformity of host cell infection was assessed by confocal microscopy. The percentage of macrophages with associated Yptb immediately after (black bars) and 120 min (gray bars) postinfection was determined (B). Means and SDs were calculated from more than five fields with a minimum of 150 cells for each condition. GFP-expressing Yptb are shown interacting with host cells at 120 min postinfection (C). Host cells were visualized by staining actin with Texas Red-phalloidin; representative images are shown.
Altered sensitivity of activated macrophages to cell death induced by Yptb infection may result from a change in Yptb:macrophage interactions; therefore, the ability of Yptb to interact with naïve and activated macrophages was compared. Naïve and LPS-activated macrophages were infected with green fluorescent protein (GFP)-expressing wild-type or YopJ− Yptb and examined by microscopy. For all conditions, greater than 98% of macrophages had one or more associated bacteria immediately after infection (Figure 1B). At 2 h postinfection, over 94% of macrophages were associated with multiple bacteria (Figure 1B and 1C). Additionally, translocation of effector proteins into the cytosol of naïve and activated macrophages was measured during infection with Yptb expressing YopE fused to the Bordetella pertussis calmodulin-dependent adenylate cyclase (Cya), resulting in accumulation of cAMP when YopE-Cya reaches the host cell cytosol [36]. Activation of macrophages did not reduce the level of effector translocation (unpublished data). These results demonstrated that activation of macrophages did not affect the ability of Yptb to associate with macrophages or translocate effectors, but appeared to alter the cellular response to infection.
Yptb-Induced Cell Death in Activated Macrophages Results in Rapid Membrane Permeability Preceding DNA Damage
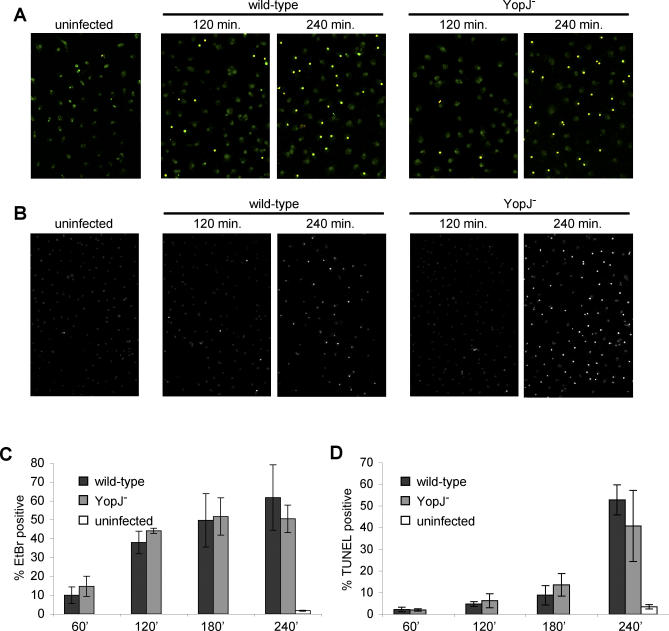
The similar levels of LDH release observed with wild-type and YopJ− Yptb infection of activated macrophages (Figure 1A) led us to hypothesize that the same process occurred during infection with both strains. We therefore assessed the kinetics of two nonspecific markers of cell death, membrane damage and DNA damage [37], during infection of activated macrophages with wild-type and YopJ− Yptb. Using uptake of a small membrane impermeant dye, ethidium bromide (EtBr, MW = 394 Da), allowed us to quantitatively examine membrane damage in individual cells during infection. EtBr uptake in uninfected macrophages was less than 2% (Figure 2A and 2C). Infection with wild-type or YopJ− Yptb resulted in very similar kinetics of EtBr uptake: 10%–15% cells were EtBr+ cells at 60 min postinfection, and this increased to 40%–45% EtBr+ cells by 120 min, and did not increase in the next 120 min of infection (Figure 2C). We used terminal deoxynucleotidyl transferase-nick end labeling (TUNEL) to examine DNA damage, and again, the kinetics were nearly identical during infection with both strains (Figure 2B and 2D). The percent of TUNEL-positive cells remained low until 180 min after infection; 15% of infected macrophages were TUNEL-positive at this time point. This increased to greater than 40% by 240 min, compared to less than 2% in uninfected macrophages (Figure 2B and 2D).
Figure 2. Wild-Type and YopJ− Yptb Infection of Activated Macrophages Stimulates Membrane Permeabilization Followed by DNA Damage.
The kinetics of cell death were examined in LPS-activated BMDMs infected with wild-type and YopJ− Yptb.
(A) Macrophages labeled with SYTO10 (green) were stained with membrane-impermeant EtBr (MW = 394 Da, red) and examined by confocal microscopy to assess increases in membrane permeability (EtBr-positive/SYTO10-labeled, yellow). Representative images are shown.
(B) DNA damage was also assessed by TUNEL and confocal microscopy in the same experiment. Representative images are shown.
(C) The percentage of EtBr-positive/SYTO10-labeled cells was determined; data shown are means and SDs from four or more fields with a minimum of 350 cells total for each time point. Results shown are representative of two experiments.
(D) The percentage of TUNEL-positive cells was determined; data shown are means and SDs from four or more fields with a minimum of 450 cells total for each time point. Results shown are representative of two experiments.
Similar kinetics of wild-type and YopJ− Yptb stimulated membrane and DNA damage during infection of activated macrophages is consistent with our hypothesis that both strains activate the same process. Importantly, membrane damage significantly preceded DNA damage (Figure 2). This observation is in contradistinction to YopJ-dependent apoptosis induced during wild-type Yptb infection of naïve macrophages, which has been previously described [3,4,11,16,35]. DNA damage began before membrane damage during wild-type Yptb induction of apoptosis in naïve macrophages (Figure S1A–S1D), and as shown in Figure 1A, YopJ− Yptb was unable to kill naïve macrophages. As expected, induction of apoptosis by wild-type Yptb activates apoptotic caspases-3 and −8 [9,16], a caspase-3 inhibitor correspondingly inhibits DNA damage, and also as shown previously [6,7], TLR4 signaling facilitates apoptosis during wild-type Yptb infection of naïve macrophages (Figure S1E–S1G). Finally, infection of naïve macrophages with wild-type Yptb results in the typical nuclear condensation characteristic of apoptosis (Figure S2A) [3,4,35], in contrast to the diffuse distribution of TUNEL-positive DNA observed in activated macrophages infected with wild-type or YopJ− Yptb (Figure S2B). Thus, the features of wild-type and YopJ− Yptb-induced cell death in activated macrophages, which include membrane permeability preceding DNA damage with morphological features excluding apoptosis [38], suggest an alternative mechanism of cell death.
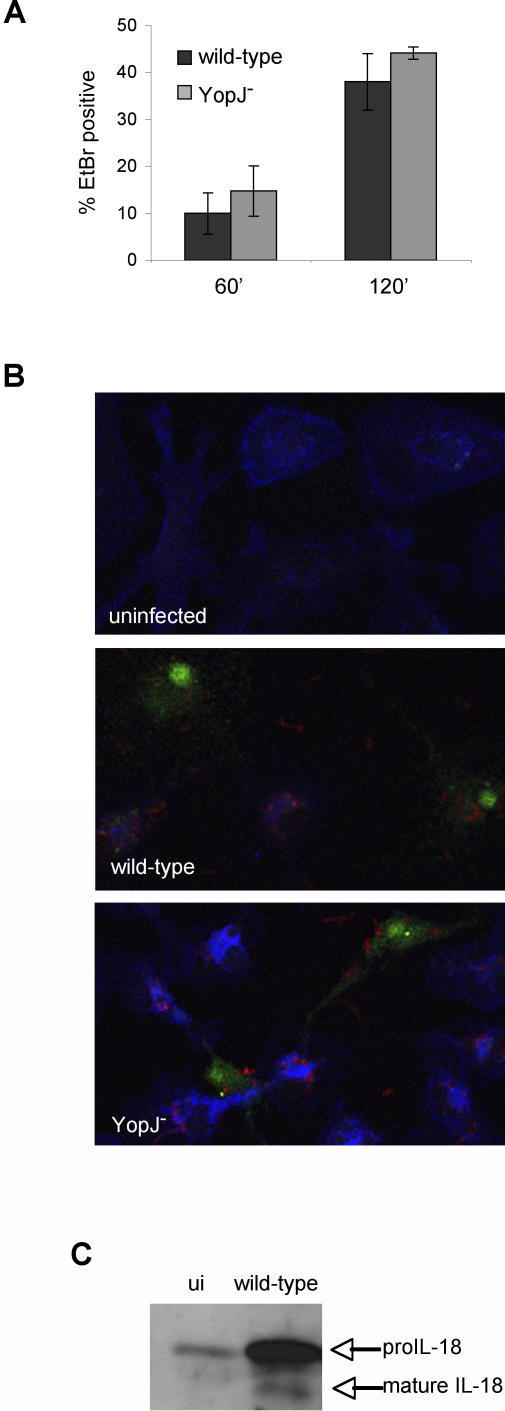
YopJ− Yptb-Induced Cell Death Requires Caspase-1
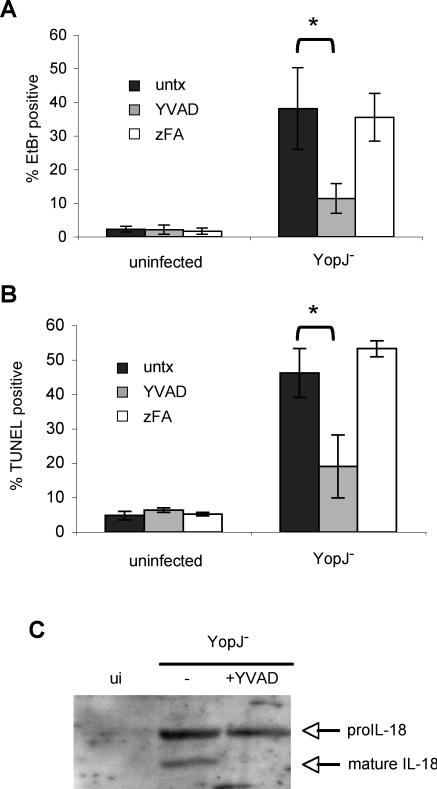
LPS activation renders macrophages susceptible to cell death induced by ATP treatment [32,39] or Francisella tularensis infection [40], and both processes involve caspase-1. The features of YopJ− Yptb-induced cell death, rapid membrane permeability preceding DNA damage, and the lack of nuclear condensation, also suggest caspase-1-dependent cell death or pyroptosis [38,41]. We therefore hypothesized that YopJ− Yptb-induced cell death was dependent on caspase-1. EtBr uptake by YopJ− Yptb infected macrophages was reduced by the specific caspase-1 inhibitor YVAD [42], but not by the negative control peptide zFA (Figure 3A), indicating caspase-1 is required for increased membrane permeability during infection. Additionally, the downstream events of membrane breakdown and release of LDH were inhibited by YVAD (unpublished data). DNA damage was caspase-1-dependent; the percentage of TUNEL positive cells was reduced in the presence of YVAD and unchanged by zFA (Figure 3B). Features of wild-type Yptb-induced apoptosis in naïve macrophages were not inhibited by YVAD (unpublished data), indicating YVAD was not nonspecifically inhibiting Yptb or apoptotic caspases. Finally, we examined supernatants collected from activated macrophages infected with YopJ− Yptb for the presence of the inflammatory cytokine interleukin (IL)-18, which is specifically cleaved and activated by caspase-1 [43]. Cleaved IL-18 was present in supernatants from macrophages infected with YopJ− Yptb, but not uninfected macrophages, and IL-18 processing was blocked by YVAD (Figure 3C). This demonstrated that the features of YopJ− Yptb induced cell death are caspase-1-dependent and accompanied by cleavage and release of the caspase-1 substrate IL-18.
Figure 3. YopJ− Yptb-Induced Membrane Permeability and DNA Damage Are Caspase-1-Dependent and Accompanied by Inflammatory Cytokine Processing.
LPS-activated BMDMs were treated with caspase-1 inhibitor (YVAD) or negative control (zFA) peptide (200 μM) during infection with YopJ− Yptb.
Membrane permeability was examined by SYTO10/EtBr staining (see Figure 2A legend) and confocal microscopy at 120 min postinfection (A). DNA damage was assessed using TUNEL and confocal microscopy at 240 min postinfection (B). The percentage of EtBr/TUNEL-positive cells was determined from four or more fields with a minimum of 1,000 cells total for each condition. Representative of two experiments. * p < 0.0005.
(C) Western blot analysis of mature IL-18 released into the supernatant by activated macrophages at 90 min postinfection with YopJ− Yptb confirms caspase-1 activation and cytokine processing. Representative of two experiments. ui, uninfected.
Induction of Pyroptosis Requires the Bacterial Type III Translocon, but None of the Known Yptb Effectors
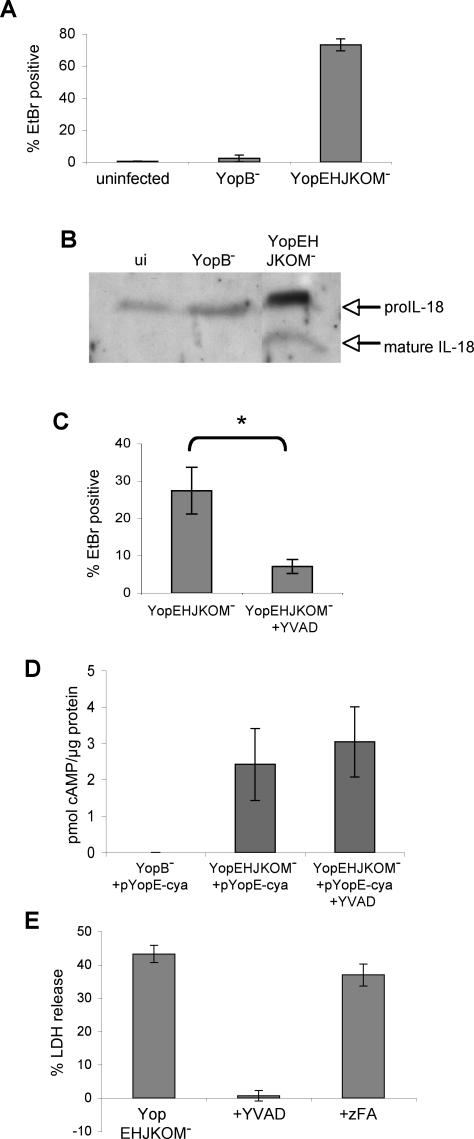
Pyroptosis induced by several other bacteria requires a functional T3SS [38,44–47]. YopB and YopD are structural components of the Yersinia type III translocon, and both are required for translocation of effector proteins into host cells [2]. To examine the requirement for the T3SS in Yptb-induced pyroptosis, activated macrophages were infected with YopB− Yptb; this mutant was unable to alter host membrane permeability (Figure 4A). In addition to YopJ, Yersinia translocates several other effectors into the macrophage cytosol (Yops E, H, O, and M) [2], and we examined the role of these effector proteins in inducing pyroptosis. Infection of activated macrophages with YopEHJKOM− Yptb, a mutant lacking all of the known translocated effectors but competent for type III translocation (YopBD+), increased macrophage membrane permeability and uptake of EtBr (Figure 4A). Furthermore, infection by YopEHJKOM− Yptb, but not YopB− Yptb, resulted in release of cleaved IL-18 (Figure 4B). Infection with Yersinia mutants lacking multiple effectors, but competent for type III translocation (YopBD+), results in pore formation in the host cell membrane and uptake of small molecules similar to EtBr in size [48,49]. This pore was thought to be the type III translocon composed of YopB and YopD. However, caspase-1 activation can lead to formation of membrane pores [41], and we hypothesized pore formation by YopEHJKOM− Yptb was instead a host-mediated process dependent upon caspase-1. Consistent with this idea, YVAD inhibited EtBr uptake by activated macrophages infected with YopEHJKOM− Yptb (Figure 4C). Importantly, we demonstrated the YopB/D translocation pore is formed and functional in the presence and absence of YVAD; a YopE-Cya fusion protein is translocated equally in both conditions (Figure 4D). Additionally, membrane breakdown and LDH release was completely inhibited by YVAD, but not zFA (Figure 4E). These data indicate pyroptosis induced by Yptb requires the T3SS, but none of its known translocated effector proteins, and host cell membrane damage and EtBr uptake are caspase-1-mediated processes stimulated by Yptb infection.
Figure 4. Yptb-Induced Pyroptosis Is T3SS-Dependent.
LPS-activated BMDMs were infected with YopEHJKOM− (T3SS+, type III effector−) or YopB− (T3SS−) Yptb.
(A) Membrane permeability was examined at 90 min postinfection by EtBr/SYTO10 staining (See Figure 2A legend) and confocal microscopy. Data shown are from four or more fields with a minimum of 1,000 cells total for each condition. Representative of two experiments.
(B) Western blot analysis of mature IL-18 released into the supernatant by infected macrophages at 90 min postinfection confirms caspase-1 activation. Representative of two experiments. ui, uninfected.
(C) Membrane permeability was examined in infected macrophages treated with YVAD by SYTO10/EtBr staining and confocal microscopy at 45 min postinfection. Data shown are from four or more fields with a minimum of 1,000 cells for each condition. Representative of three experiments. * p < 0.0001.
(D) Translocation of a YopE-adenylate cyclase fusion protein into the cytoplasm of activated macrophages was assessed by quantifying cAMP levels at 60 min postinfection, and demonstrated equivalent levels of effector translocation by YopEHJKOM− Yptb in the presence of YVAD. Data shown are means and SDs calculated from three replicates.
(E) Membrane breakdown and LDH release from macrophages treated with YVAD or negative control peptide zFA 120 min postinfection with YopEHJKOM− Yptb. Data shown are means and SDs calculated from three replicates and are representative of three experiments.
Macrophage Activation Antagonizes Wild-Type Yptb-Induced Apoptosis and Stimulates Pyroptosis
We observed pyroptosis in activated macrophages infected with Yptb that lack YopJ but contain a functional T3SS. During infection of naïve macrophages with wild-type Yptb, YopJ and TLR4 signaling are required for maximal activation of caspase-3 and apoptosis [6,7,9]. However, macrophage activation can dampen future TLR4-mediated signaling events [50,51], and result in synthesis of gene products that inhibit the activation/activity of apoptotic caspases, including caspase-3 [10–12]. We therefore hypothesized that macrophage activation would decrease YopJ-dependent caspase-3 activation and apoptosis during subsequent infection with wild-type Yptb, and simultaneously stimulate pyroptosis. As expected, wild-type Yptb infection of naïve macrophages resulted in rapid caspase-3 activation (Figure 5A) and cleavage of the caspase-3 substrate inhibitor of caspase-activated DNase (ICAD) [52] (Figure 5B, left). Infection with YopJ− Yptb did not result in caspase-3 activity (Figure 5A) or degradation of ICAD (Figure 5B), regardless of the activation state of the macrophages. However, in activated macrophages infected with wild-type Yptb, caspase-3 activity was undetectable until 150 min postinfection (Figure 5A) and no ICAD degradation was detected (Figure 5B, right), which together confirm the lack of caspase-3 activity in these infected cells (Figure 5A).
Figure 5. Macrophage Activation Antagonizes Caspase-3 Activity and Apoptosis during Infection with Wild-Type Yptb .
Untreated and LPS-activated BMDMs were infected with wild-type or YopJ− Yptb.
(A) Kinetics of caspase-3 activation in infected macrophages. Data shown are means and SDs calculated from three replicates and presented as relative light units (RLU) in cell lysates from infected samples minus uninfected controls. Representative of three experiments. At 150 min postinfection, caspase-3 activity in naïve macrophages undergoing YopJ-dependent apoptosis during infection with wild-type Yptb (289,340 ± 20,466 RLU) is greatly reduced in activated macrophages infected with wild-type Yptb (32,315 ± 2,881 RLU). Activated macrophages infected with YopJ− Yptb fail to activate caspase-3 (3,231 ± 1,531 RLU).
(B) Cleavage of the caspase-3 substrate ICAD was examined in uninfected macrophages (ui) and at 120 min postinfection with wild-type (wt) or YopJ− (J−) Yptb by Western blot and confirmed the absence of caspase-3 activity in activated macrophages. ERK1/2 was used as a loading control.
An early feature of pyroptosis is permeability to EtBr (Figure 2A and 2B), and activated macrophages infected with wild-type or YopJ− Yptb became permeable to EtBr with identical kinetics (Figure 6A). Importantly, wild-type Yptb infection of activated macrophages resulted in EtBr uptake prior to any detectable increase in caspase-3 activity (Figure 5A), unlike infection of naïve macrophages, where caspase-3 activity precedes EtBr uptake (unpublished data). Activation of caspase-1 was examined by infecting macrophages in the presence of a fluorescently labeled inhibitor that irreversibly binds active caspase-1 (FAM-YVAD) [53]. Both wild-type and YopJ− Yptb infection resulted in FAM-YVAD staining (Figure 6B), and wild-type Yptb infection of activated macrophages also induced cleavage and release of the caspase-1 substrate IL-18 into the supernatant (Figure 6C). These data confirmed that activation of macrophages prior to infection alters host cell responses to wild-type Yptb, suppressing YopJ-dependent apoptosis and simultaneously enhancing pyroptosis, resulting in caspase-1 activation, increased membrane permeability, and release of bioactive IL-18 prior to any detectable caspase-3 activation. Activation of macrophages in vitro alters susceptibility to cell death, and redirects infected macrophages to utilize the inflammatory pyroptosis pathway.
Figure 6. Wild-Type Yptb Infection Induces Pyroptosis of Activated Macrophages.
LPS-activated BMDMs were infected with wild-type or YopJ− Yptb.
(A) The kinetics of membrane permeability were examined by EtBr/SYTO10 staining (see Figure 2B legend) and confocal microscopy. Data shown are from four or more fields with a minimum of 350 cells for each time point. Representative of three experiments.
(B) Macrophages were stained with FAM-YVAD (green) to identify cells with active caspase-1, Alexa633-phalloidin to visualize actin (blue), and anti-Yersinia antibodies (red) 90 min postinfection and examined by confocal microscopy. Representative images are shown.
(C) Western blot analysis of mature IL-18 released into the supernatant at 90 min postinfection confirmed caspase-1 activation by wild-type Yptb infection. Representative of two experiments. ui, uninfected.
Physiologically Relevant Stimuli Enhance Macrophage Susceptibility to Yptb-Induced Pyroptosis
In addition to LPS, other TLR ligands are present during Yptb infection in vivo, and may activate macrophages and increase their sensitivity to pyroptosis. Activation of macrophages with a TLR2 ligand (Pam3CSK) prior to infection with YopJ− Yptb increased pyroptosis to levels equivalent to LPS activation (Figure 7A). Pretreatment with whole heat-killed Yptb, which contain both TLR2 and TLR4 ligands [33], at ratios as low as one Yptb per macrophage also enhanced pyroptosis (Figure 7B). The TLR3 ligand poly(I:C) had a similar effect (unpublished data); although signaling through TLR3 may not be relevant in the context of Yptb infection, it supports the hypothesis that the redirection of macrophage death is a generalized host response to TLR stimulation.
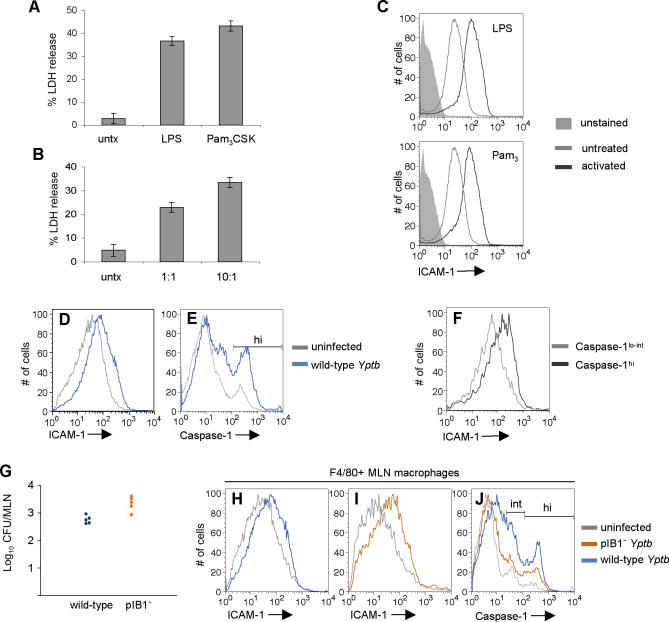
Figure 7. Macrophages Become Activated and Susceptible to Pyroptosis In Vivo during Murine Infection with Yptb .
(A and B) BMDMs were treated with 100 ng/ml LPS, 100 ng/ml Pam3CSK4 (A), or heat-killed Yptb at ratios of 10:1 or 1:1 Yptb:macrophage (B) for 18 h and infected with YopJ− Yptb. LDH release was measured after 3.5 h. Data shown are from three replicates and representative of three experiments.
(C) Macrophages were activated as in (A) and surface ICAM-1 expression was measured by flow cytometry.
(D–F) At 4–6 d postinfection, activated splenic macrophages from wild-type Yptb-infected mice were identified with anti-F4/80, and ICAM-1 expression and caspase-1 activity (FAM-YVAD) were measured by flow cytometry. Representative histograms of macrophage ICAM-1 expression (D), caspase-1 activity (E), and ICAM-1 expression of the caspase-1hi and caspase-1lo-int (F) macrophage populations from (E).
(G–J) Mice were infected with wild-type or pIB1− Yptb and tissues were examined 5 d post-infection. CFU in the MLNs were quantified (G). Macrophages from the MLNs were identified with anti-F4/80, and ICAM-1 expression and caspase-1 activity were examined by flow cytometry. Representative histograms of MLN macrophage ICAM-1 expression from wild-type Yptb-infected (H) and pIB1− Yptb-infected (I) mice; caspase-1 activity (J).
In Vivo Activation of Macrophages and Caspase-1 during Yptb Infection
We hypothesized that the abundance of activating ligands during Yptb infection would result in macrophage activation in vivo. Macrophages activated in vitro with LPS or Pam3CSK express increased levels of surface ICAM-1 (Figure 7C), which was used to monitor activation of F4/80+ macrophages and their susceptibility to pyroptosis in vivo. In mice infected orally with wild-type Yptb, splenic macrophages express increased surface ICAM-1 (Figure 7D), and this was observed in six of 14 infected mice (Figure S3). This was also observed in the mesenteric lymph nodes (MLNs); 16 of 18 mice with colonized MLNs contained macrophages with increased surface ICAM-1 expression (Figure S3).
The activation state of macrophages from wild-type Yptb infected mice suggested that these macrophages would be susceptible to pyroptosis; therefore, caspase-1 activation was examined by FAM-YVAD staining of splenocytes directly ex vivo. We observed an increase in the percentage of F4/80+ macrophages that were caspase-1hi (Figures 7E and S4; Table S1) from 12.2% (± 1.86%) in uninfected mice to 29.0% (± 5.76%) in infected mice with activated macrophages (p = 0.0009). Additionally, macrophages from Yptb-infected mice that had no detectable increase in surface ICAM-1 expression had caspase-1 activity similar to macrophages from uninfected mice (Figure S4; Table S1). Macrophages with high levels of active caspase-1 (caspase-1hi, Figure 7E) expressed greater surface ICAM-1 when compared to macrophages with lower levels of active caspase-1 from the same infected tissue (Figure 7F). This confirms the activation of macrophages during wild-type Yptb infection and demonstrates that macrophage activation is necessary for increased activation of caspase-1, and correlates with our in vitro data demonstrating that wild-type Yptb infection of activated macrophages results in caspase-1-dependent pyroptosis.
To address the T3SS dependence of caspase-1 activation in vivo, mice were infected with Yptb lacking the T3SS-encoding pIB1 virulence plasmid. pIB1− Yptb do not induce pyroptosis in vitro (unpublished data), but colonize the MLNs of infected mice as well as wild-type Yptb (Figure 7G) [28,54], and cause macrophage activation in vivo as measured by the increased expression of ICAM-1 (Figure 7H, wild-type; Figure 7I, pIB1−). However, the percentage of caspase-1int+hi macrophages from pIB1− Yptb-infected mice is significantly less than in wild-type Yptb infected mice (27.6% ± 4.57% versus 42.2% ± 2.18%, p < 0.003; Figure 7J), and macrophages from pIB1− Yptb-infected mice had levels of active caspase-1 similar to uninfected mice (27.6% ± 4.57% versus 23.0% ± 8.49%, p = 0.32). These results confirm the activation of caspase-1 during Yptb infection in vivo, and the requirement for the T3SS-encoding virulence plasmid to activate caspase-1, thereby implicating the bacterial T3SS in this process in vivo.
Y. pestis Induces Caspase-1 Activation
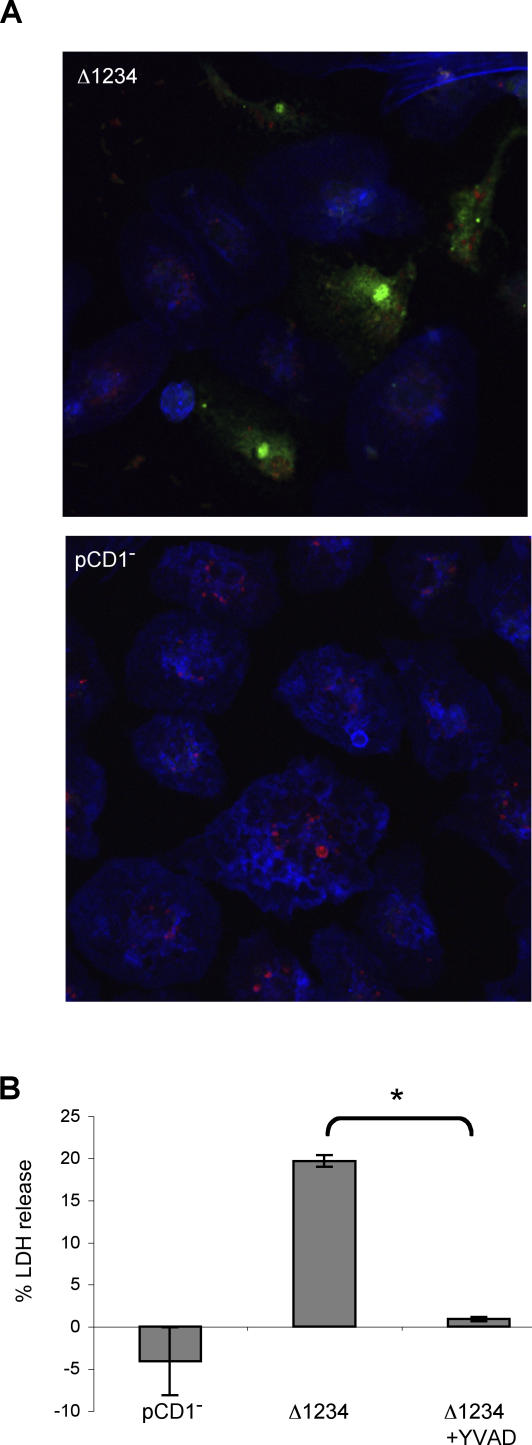
Yptb is closely related to Y. pestis, the causative agent of plague, and Yersinia spp. share several features including the plasmid encoded type III secretion apparatus [2]; therefore, we hypothesized that Y. pestis infection of activated macrophages would also result in pyroptosis. Activated macrophages were infected with a Y. pestis mutant competent for type III translocation, but lacking all translocated effectors (Δ1234) [55]. Like Yptb, Y. pestis causes activation of caspase-1 as demonstrated by staining with FAM-YVAD (Figure 8A, top). Y. pestis lacking the T3SS-encoding virulence plasmid (pCD1−) failed to induce FAM-YVAD staining in activated macrophages (Figure 8A, bottom), suggesting caspase-1 activation induced by Y. pestis also requires the T3SS. Infection of activated macrophages with Y. pestis also resulted in LDH release that was blocked by the caspase-1 inhibitor YVAD (Figure 8B); indicating Y. pestis contains the ligand responsible for caspase-1 activation, and this leads to caspase-1-dependent lysis of activated macrophages.
Figure 8. Y. pestis Infection Induces Pyroptosis in Activated Macrophages.
LPS-activated BMDMs were infected with Y. pestis Δ1234 (T3SS+, type III effector−) or pCD1− (T3SS−).
(A) Macrophages were stained with FAM-YVAD (green) to identify cells with active caspase-1, Alexa633-phalloidin to visualize actin (blue), and anti-Yersinia antibodies (red) 90 min postinfection and examined by confocal microscopy. Representative of two experiments.
(B) Membrane breakdown and LDH release from infected macrophages was inhibited by YVAD. Data shown are from three replicates and representative of two experiments. * p < 0.0001.
Discussion
Our results demonstrate the ability of macrophage activation to fundamentally alter the host response to Yptb infection. In naïve macrophages, the YopJ-mediated inhibition of proinflammatory signaling [13–15] and induction of apoptosis [3,4,35] have been well described. However, in activated macrophages YopJ no longer functions in this capacity, and activation of the apoptotic executioner, caspase-3, is suppressed such that activation of macrophages results in susceptibility to caspase-1-dependent pyroptosis. The features of pyroptosis in Yptb-infected macrophages included early membrane permeabilization followed by DNA damage, and inflammatory cytokine processing and release. Macrophage activation may enhance sensitivity to pyroptosis by increasing synthesis of host proteins involved in triggering the activation of caspase-1 in response to Yersinia; pyroptosis is not observed during infection of naïve macrophages with YopJ− Yptb. Alternatively, macrophage activation may overcome the ability of translocated Yersinia effector proteins to inhibit the activation of caspase-1 [56]. This is the first report of proinflammatory pyroptosis induced by wild-type Yersinia, bacteria previously thought to neutralize macrophages exclusively by noninflammatory apoptosis.
In vitro, Yersinia species are capable of suppressing inflammatory cytokine production in response to bacterial products [13–15], and during the early phase of infection in vivo, there is a marked lack of inflammation and inflammatory cytokine production [23,24]. This suggests that the majority of macrophages interacting with Yersinia in vivo would have a naïve phenotype, and this is consistent with the YopJ-dependent macrophage death observed [19,21], and the lack of caspase-1 activation when the bacterial burden is low and macrophages are not activated (Figure S4; Table S1). However, as infection progresses, histological examination of Yersinia-infected tissues reveals extensive inflammation and inflammatory cytokine production [24–28]. We have demonstrated that the inflammatory nature of Yptb infection leads to macrophage activation and up-regulation of surface ICAM-1, indicating that macrophages become resistant to YopJ and sensitive to pyroptosis. This result was confirmed by the finding that activated macrophages from Yptb-infected mice also contain active caspase-1, and this process required the T3SS-encoding virulence plasmid, and therefore was likely dependent on the T3SS. In vivo, we have not formally excluded the involvement of plasmid-encoded gene products that are not part of the T3SS in caspase-1 activation; however, we feel this is unlikely considering our results confirming the T3SS-dependence of pyroptosis in vitro. T3SS-dependent pyroptosis and inflammatory cytokine production may help explain the ability of T3SS+ Yptb to induce greater levels of tissue necrosis than T3SS− Yptb [28], even in the presence of type III effectors capable of suppressing inflammation [2]. Our results suggest that during Yersinia infection in vivo macrophages encounter TLR or other activating ligands that trigger a host-mediated switch from YopJ-dependent apoptosis to pyroptosis. In addition, we predict macrophage populations that are continuously encountering bacterial products, like those in the Peyer's patches (PPs), would be refractory to the effects of YopJ. Consistent with this hypothesis, YopJ does not confer a replicative advantage in the PPs; YopJ− Yptb replicate as well as wild-type in the PPs of infected mice [19,21]. PP macrophages may also be inherently susceptible to pyroptosis; unfortunately, we were unable to analyze caspase-1 activation in the PPs due to the low numbers of cells present.
Superficially, Yersinia infection of activated or naïve macrophages simply results in host cell death; however, the responses of other host cells to apoptosis and pyroptosis are quite different. Apoptotic cells often display surface markers that facilitate their uptake by neighboring cells [57] and prevent release of inflammatory intracellular contents from dying cells. Phagocytes that encounter apoptotic cells produce anti-inflammatory cytokines TGF-β and IL-10 and produce lower levels of several inflammatory cytokines [58,59] and costimulatory molecules [60]. This potent anti-inflammatory response is able to modulate the adaptive immune response by reducing the ability of antigen-presenting cells to stimulate T cells [61]. The anti-inflammatory nature of apoptosis is consistent with the ability of YopP (functionally equivalent to YopJ) to delay priming of T cells during Y. entercolitica infection [62].
In contrast, pyroptosis is intrinsically inflammatory, as the cell death process is linked to maturation and release of inflammatory cytokines. Pyroptosis also results in rapid lysis and release of intracellular contents [38,41] that can act as “danger signals” and promote the immune response [61,63]. Yersinia-induced macrophage death could result in drastically different outcomes depending on the activation state of the macrophage, even though the immediate consequence in both naïve and activated macrophages is simply cell death. The role of IL-18 and IL-1β in enhancing immune responsiveness has been thoroughly demonstrated. Both induce inflammatory cytokine production and increased expression of adhesion molecules, recruiting neutrophils and lymphocytes to sites of infection [64]. Correspondingly, Yersinia-infected mice have increased numbers of neutrophils in colonized tissues [26,28]. IL-18 also plays a major role, in conjunction with IL-12, in stimulating interferon gamma production [43]. Depending on the cytokine milieu, IL-18 can stimulate CD4+ T cell differentiation to Th1 or Th2 phenotype [65]. Both IL-18 and T cell responses are critical in controlling Yersinia infection in vivo, as IL-18–deficient mice [66] and mice lacking T cells [67] are unable to resolve the infection, and adoptive transfer of Yersinia-specific T cells confers partial protection against challenge [68]. Thus, redirecting macrophages to undergo pyroptosis appears to play an important role in generating an appropriate and effective immune response to Yersinia.
The activation of caspase-1 is initiated by recognition of cytosolic ligands by members of the NOD-leucine-rich repeat family of proteins. This recognition triggers formation of a multiprotein complex called the inflammasome, which then acts as a platform for the activation of caspase-1 [69]. Induction of pyroptosis by Yptb requires the bacterial T3SS but none of its known effectors. We hypothesized that the Yersinia T3SS actively or passively transports a caspase-1–activating ligand into the macrophage cytosol. Recent studies with Salmonella and Legionella have implicated cytosolic flagellin in activating caspase-1 through the NOD-like receptor family member Ipaf [70–73]. Y. pestis strains have a mutation inactivating flhD [74] that results in suppression of flagellin subunit production, and the observed lack of motility and flagella [75]. Our observation that Y. pestis also induces caspase-1 activation suggests the delivery of an alternative caspase-1 activating ligand to the host cytosol, and experiments to identify the ligand(s) produced by Yersinia species are ongoing.
Bacterial pathogens are often capable of modulating host cell processes, including cell death. Pathogens prevent cell death to maintain a protective intracellular environment or replicative niche [76,77], or induce cell death to eliminate host cells and suppress immune function [5,78]. In addition, activation of caspase-1-dependent inflammatory programmed cell death, or pyroptosis, in response to cytosolic bacterial ligands may serve as a host defense mechanism [70–73]. This study demonstrates host-mediated redirection of Yersinia-induced cell death; recognition of host inflammatory mediators and bacterial products results in inhibition of apoptosis, a noninflammatory process thought to benefit the bacteria, and primes macrophages to die by pyroptosis, potentially benefiting the host by shifting host cell responses toward inflammation.
Materials and Methods
Bacterial strains and growth conditions.
Yptb strains used in the present study were wild-type (YPIII) and the following mutants derived from this strain: YP26 YopJ− [15], YP18 YopB− [15], and YP37 YopJOEHKM− [79] (a gift from Dr. James Bliska). A plasmid expressing green fluorescent protein was generated by inserting the LacZ promoter (bases 246–575) from pBluescriptSK− into the EcoRI and BamHI sites of pDW1 [80]. A yopE::cyaA fusion was constructed as described [36] and inserted into the HindIII and BamHI sites of pBR322. pIB1− Yptb were generated as described [81] and screened by PCR to confirm loss of multiple yop genes. Bacteria were routinely cultured in LB at room temperature.
For macrophage infections, overnight cultures were back-diluted 1:40 into LB containing 20 mM sodium oxalate and 20 mM magnesium chloride and grown at room temperature with shaking for 1 h followed by incubation at 37 °C with shaking for 2 h. Bacteria were harvested and resuspended in PBS for infection.
Yersinia pestis strains used in the present study were KIM8 Δ1234 [55] and pCD1− plasmid-cured (a gift from Dr. Greg Plano). Y. pestis was grown as described for Yptb and sonicated briefly prior to infection to reduce clumping.
Heat-killed YPIII were prepared by growing the bacteria as for infection, washing cells and resuspending in PBS, and incubating at 65 °C for 1 h.
Macrophages and infection.
Bone marrow-derived macrophages (BMDMs) were isolated from the femur exudates of C57BL/6 mice (Jackson Laboratories) and cultured at 37 °C in 5% CO2 in Dulbecco's minimal essential medium (DMEM, Invitrogen) supplemented with 10% FCS, 5 mM HEPES, 0.2 mg/ml L-glutamine, 0.05 mM β-mercaptoethanol, 50 mg/ml gentamicin sulfate, and 10,000 U/ml penicillin and streptomycin with 30% L-cell-conditioned medium [82]. After 6–7 d of incubation, macrophages were collected by washing with ice-cold PBS containing 1 mM EDTA, resuspended in supplemented antibiotic-free DMEM containing 5% FCS, and allowed to adhere for 18–24 h before infection. Macrophages were activated with ultrapure LPS from Salmonella minnesota (List Biologicals) at a final concentration of 100 ng/ml unless otherwise indicated, 100 ng/ml Pam3CSK (EMC microcollections), or heat-killed wild-type YPIII for 18 h prior to infection. Medium was replaced 1 h before infection, and contained 200 μM YVAD.cmk and zFA.fmk (Calbiochem) when indicated. Bacteria were added at a multiplicity of infection of 20 and spun briefly at 200 g to bring bacteria into contact with macrophages. Gentamicin sulfate was added to 100 μg/ml at 2 h.
Efficiency of infection was confirmed by infection with GFP-expressing Yptb followed by incubation for 5 min or 2 h. Macrophages were stained using Texas Red-phalloidin or Alexa 633-phalloidin per the manufacturers instructions and examined by confocal microscopy using the BioRad MRC-600 or Leica SL confocal microscope in the W. M. Keck Center for Advanced Studies in Neural Signaling (University of Washington, Seattle, WA). The number of macrophages with associated bacteria was determined from multiple fields.
Lactate dehydrogenase release.
Macrophages grown in 96-well plates were infected with Yptb, and supernatants were evaluated for the presence of the cytoplasmic enzyme LDH using the Cytotox 96 kit (Promega) as directed by the manufacturer's instructions. Percentage cytotoxicity was calculated as 100 × (experimental LDH − spontaneous LDH) ÷ (maximum LDH release − spontaneous LDH).
CyaA-based translocation assay.
Macrophages were infected with Yptb containing pYopE::cyaA at an MOI of 20 for 1–2 h. 2 × 104 macrophages were lysed in 0.1 M HCl, and cAMP levels were determined by using the Direct cAMP Correlate-EIA Kit (Assay Designs) and normalized for protein content determined by the Bradford Protein Assay (Bio-Rad).
Caspase-3 activity.
Macrophages were infected with Yptb for the indicated length of time and the Caspase-3/7 Glo Assay (Promega) was performed according to the manufacturers instructions. Briefly, 60 μl of Caspase-3/7 Glo reagent was added to 1 × 104 macrophages in 60 μl of medium and incubated at room temperature for 1 h. Luminescence was measured using a TECAN GENios Pro. Caspase-3/7 activity is reported as relative light units (RLU) of infected samples minus uninfected control.
For ICAD immunobloting, 1.5 × 106 macrophages were harvested at 2 h postinfection and lysed in sample buffer. Proteins were separated by 15% SDS-PAGE and transferred to nitrocellulose membranes. ICAD cleavage was assessed by Western blotting using anti-ICAD antibodies and peroxidase conjugated secondary antibodies (BDPharMingen). Immunoblots were developed with and enhanced chemiluminescence system (Amersham Biosciences). Anti-p44/p42 antibodies were used to confirm equal loading.
Ethidium bromide/SYTO10 staining.
Macrophages grown on glass coverslips were infected with Yptb for the indicated length of times. Media was removed and adherent cells were stained with SYTO 10 (Molecular Probes) and ethidium bromide at 25 μg/ml (Sigma-Aldrich) in HBSS for 5 min. Coverslips were analyzed using a BioRad MRC-600 or Leica SL confocal microscope in the W. M. Keck Center for Advanced Studies in Neural Signaling. The means and standard deviations (SDs) were derived from counting three fields for uninfected samples and six fields for infected samples.
DNA fragmentation assays.
Macrophages grown on glass coverslips were infected with Yptb, and DNA strand breaks were detected using terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) using the In Situ Cell Death Detection Kit as directed by the manufacturer's instructions (Roche Applied Science). Coverslips were mounted using ProLong antifade (Molecular Probes) and analyzed using a BioRad MRC-600 or Leica SL confocal microscope in the W. M. Keck Center for Advanced Studies in Neural Signaling. The means and SDs were derived from counting three fields for uninfected samples and six fields for infected samples.
Caspase-1 staining in vitro.
Macrophages grown on glass coverslips were infected with Yptb for 90 min total and carboxyfluorescein-YVAD-fluoromethyl ketone (FAM-YVAD; Immunochemistry Technologies) was added to 1× at 30 min postinfection. Macrophages were washed thoroughly to remove unbound FAM-YVAD and then stained with Alexa 633 phalloidin (Molecular Probes) per the manufacturers instructions. Bacteria were labeled using anti-Yptb and anti-rabbit PE (Abcam) antibodies. Coverslips were mounted using ProLong antifade (Molecular Probes) and analyzed using Leica SL confocal microscope in the W. M. Keck Center for Advanced Studies in Neural Signaling.
IL-18 secretion.
Macrophages were infected in serum-free media, and at the indicated time points the supernatant was removed, sterilized using a 0.22 μm filter, and concentrated using a 10,000 MWCO Centricon Plus-20 centrifugal filter device (Millipore). Supernatant from 2.4 × 106 macrophages was separated by 15% SDS-PAGE, transferred to nitrocellulose membranes, and cytokine processing and release was analyzed by western blot using anti-IL-18 M19 and peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). Immunoblots were developed with an enhanced chemiluminescence system (Amersham Biosciences).
Mouse infections.
Female C57BL/6 mice aged 6–8 wk (Jackson Laboratories) were infected orogastrically with 6−8 × 108 wild-type or pIB1− Yptb in 100μl of PBS. Mice were sacrificed on days 4, 5, and 6 postinfection, and spleen and MLNs were removed and placed in cold PBS. Organs were homogenized between frosted glass slides. An aliquot was removed and lysed in 1% triton for CFU determination by plating dilutions on cefulosodin-irgasan-novobiocin (CIN) agar. The remaining cells were processed for staining: red blood cells were lysed in 17 μM Tris (pH 7.4), 140 μM NH4Cl for 5 min at room temperature, washed once in cold PBS, and passed through a 70 μM filter to create a single cell suspension. Cell numbers were determined by Trypan blue exclusion. To identify activated macrophages, 2 × 106 cells were stained with anti-F4/80-PE antibodies (Caltag), anti-ICAM1-biotin antibodies, and streptavidin-APC (BD Pharmingen) on ice for 30 min. Cells were fixed and analyzed by flow cytometry using a BD LSR 6 color analyzer. Isotype control antibodies resulted in an MFI equivalent to that of unstained cells. To identify macrophages with active caspase-1, 2 × 106 cells were incubated for 30 min at 37 °C with 5% CO2 with 1× FAM-YVAD in PBS supplemented with 5 mM glycine to reduce cell breakdown [41]. Cells were then washed thoroughly to remove unbound FAM-YVAD and labeled with anti-F4/80-PE antibodies, fixed, and analyzed by flow cytometry. Increased FAM-YVAD staining was not due to cross reactivity with caspases activated during Yptb-induced apoptosis; naïve macrophages infected with wild-type Yptb did not have increased FAM-YVAD staining.
Animal experiments were approved by the University of Washington Institutional Animal Care and Use Committee, Seattle, WA.
Supporting Information
Naïve BMDMs were infected with wild-type or YopJ− Yptb in the same experiment reported in Figure 2.
(A) Macrophages labeled with SYTO10 (green) were stained with membrane-impermeant EtBr (MW = 394 Da, red) and examined by confocal microscopy to assess increases in membrane permeability (EtBr-positive/SYTO10-labeled, yellow). Representative images are shown.
(B) The percentage of EtBr-positive/SYTO10-labeled cells was determined; data shown are means and SDs from multiple fields. Representative of two experiments.
(C) DNA damage was assessed by TUNEL and confocal microscopy. Representative images are shown.
(D) The percentage of TUNEL-positive cells was determined; data shown are means and SDs from multiple fields. Representative of two experiments. Increased DNA damage was observed at 60 min (D) *p = 0.0002, prior to any significant increase in membrane permeability (B) ^p = 0.1 (no significant difference), and these features were dependent on YopJ. This result differs from infection of activated macrophages, in which wild-type and YopJ− Yptb-induced membrane permeability precedes DNA damage by 120 min (Figure 2C and 2D).
(E) YopJ-dependent apoptosis in naïve macrophages was accompanied by activation of apoptotic caspases-8 and −3 [9,16]. Data presented are fold changes in caspase activity in cell lysates of infected macrophages compared with uninfected controls at 120 min. Means and SDs were calculated from three replicates and are representative of two experiments.
(F) YopJ-dependent DNA fragmentation in naïve macrophages was dependent on the apoptotic executioner caspase-3. Naïve BMDMs were treated with the caspase-3 inhibitor DEVD (200 μM) and infected with wild-type Yptb. DNA damage was assessed by TUNEL and confocal microscopy. The percentage of TUNEL-positive cells was determined; data shown are means and SDs from multiple fields. Representative of two experiments. * p = 0.0001.
(G) TLR4KO macrophages have reduced susceptibility to YopJ-dependent apoptosis as previously described [6,7]. Naïve wild-type and TLR4KO C57BL/6 macrophages were infected with wild-type and YopJ− Yptb, and LDH release was measured 4 h after infection. Data shown are means of LDH release from three replicates and are representative of two experiments. *p = 0.0007.
(2.1 MB TIF)
We examined nuclear morphology of cells immediately after the appearance of damaged DNA (TUNEL positivity) during infection of naïve and activated macrophages with Yptb. During YopJ-dependent apoptosis, TUNEL-positive nuclei undergo nuclear condensation and fragmentation characteristic of apoptosis [3,4,35].
(A) Naïve macrophages were infected with wild-type Yptb; TUNEL-positive nuclei demonstrating typical apoptotic condensation indicated by open arrowheads.
(B) In contrast, activated macrophages infected with wild-type or YopJ− Yptb became TUNEL positive without marked nuclear condensation, and damaged DNA remained evenly distributed within the nucleus, suggestive of the previously reported morphology associated with caspase-1-dependent cell death [38].
(973 KB TIF)
At 4, 5, and 6 d postinfection, mesenteric lymph nodes (A) and spleens (B) were harvested from mice infected with wild-type Yptb and CFUs were quantified. In parallel, macrophage activation was assessed by ICAM-1 expression on F4/80+ macrophages from infected tissues. A greater than 2-fold increase in the percentage of ICAM-1hi macrophages relative to macrophages from uninfected mice was scored as activated (red circles, activated; black circles, not activated). Data in (A) and (B) are from two independent experiments. ND, none detected.
(C) ICAM-1 expression on F4/80+ macrophages from MLNs (top row) and spleen (bottom row) and corresponding colony-forming units (CFU) from one time point represented in (A) and (B).
(1.8 MB TIF)
Spleens were harvested from mice infected with wild-type Yptb, and macrophage activation was analyzed by ICAM-1 expression (top row with corresponding CFU). Caspase-1 activity (bottom row) was examined in parallel by FAM-YVAD staining. Red histograms were scored as Yptb-infected with activated macrophages and black histograms as Yptb-infected without activated macrophages. ND, none detected. Caspase-1hi macrophages have higher levels of surface ICAM-1 than caspase-1lo macrophages (see Figure 7E and 7F).
(1.5 MB TIF)
Percentage of active caspase-1hi macrophages from uninfected and infected macrophage populations averaged from two independent experiments, including Figure S4.
(25 KB DOC)
Accession Numbers
The GenBank (http://www.ncbi.nlm.nih.gov/Genbank/) GI numbers for the genes and gene products discussed in this paper are: Yptb YopJ (56405330); Yptb YopB (549791); caspase-1 (86198305); TLR4 (10946594); IL-18 (6680413); caspase-3 (118129865); ICAD (141802948); ICAM-1 (30172560).
Acknowledgments
We thank James Bliska and Greg Plano for providing bacterial strains, Kelly Smith for TLR4 knock-out mice, Susan Fink for helpful discussions, and Matt Johnson for technical assistance.
Abbreviations
- BMDM
bone marrow-derived macrophage
- CFU
colony-forming units
- Cya
calmodulin-dependent adenylate cyclase
- EtBr
ethidium bromide
- IL
interleukin
- GFP
green fluorescent protein
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MLN
mesenteric lymph node
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor kappa B
- PP
Peyer's patch
- SD
standard deviation
- T3SS
type III secretion system
- TLR
Toll-like receptor
- TUNEL
terminal deoxynucleotidyl transferase-nick end labeling
- Yptb
Yersinia pseudotuberculosis
Footnotes
Author contributions. TB and BTC conceived and designed the experiments. TB performed the experiments. TB and BTC analyzed the data. TB and BTC wrote the paper.
Funding. This work was supported by NIH grants AI47242 and P50 HG02360 (BTC) and in part by PHS NRSA T32 GM07270 from NIGMS (TB).
Competing interests. The authors have declared that no competing interests exist.
References
- Cornelis GR, Boland A, Boyd AP, Geuijen C, Iriarte M, et al. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis GR. Yersinia type III secretion: send in the effectors. J Cell Biol. 2002;158:401–408. doi: 10.1083/jcb.200205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, et al. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci U S A. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bliska JB. Immunol Role of macrophage apoptosis in the pathogenesis of Yersinia. Curr Top Microbiol. 2005;289:151–173. doi: 10.1007/3-540-27320-4_7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bliska JB. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect Immun. 2003;71:1513–1519. doi: 10.1128/IAI.71.3.1513-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase R, Kirschning CJ, Sing A, Schrottner P, Fukase K, et al. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171:4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, et al. Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol. 2004;173:3320–3328. doi: 10.4049/jimmunol.173.5.3320. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ting AT, Marcu KB, Bliska JB. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol. 2005;174:7939–7949. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
- Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis—CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Boland A, Cornelis GR. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser K, Spiik AK, Dukuzumuremyi JM, Neurath MF, Pettersson S, et al. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- Palmer LE, Hobbie S, Galan JE, Bliska JB. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- Denecker G, Declercq W, Geuijen CA, Boland A, Benabdillah R, et al. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J Biol Chem. 2001;276:19706–19714. doi: 10.1074/jbc.M101573200. [DOI] [PubMed] [Google Scholar]
- Autenrieth IB, Heesemann J. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol (Berl) 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- Galyov EE, Hakansson S, Wolf-Watz H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre N, Sebbane F, Long D, Hinnebusch BJ. Yersinia pestis YopJ suppresses tumor necrosis factor alpha induction and contributes to apoptosis of immune cells in the lymph node but is not required for virulence in a rat model of bubonic plague. Infect Immun. 2006;74:5126–5131. doi: 10.1128/IAI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley SC, Bowmer WS. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulzsch K, Sporleder T, Igwe EI, Russmann H, Heesemann J. Contribution of the major secreted yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect Immun. 2004;72:5227–5234. doi: 10.1128/IAI.72.9.5227-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Brubaker RR. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci U S A. 2005;102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci U S A. 2001;98:10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SA, Dube PH, Revell PA, Miller VL. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect Immun. 2004;72:1645–1656. doi: 10.1128/IAI.72.3.1645-1656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth IB, Hantschmann P, Heymer B, Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993;187:1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- Balada-Llasat JM, Mecsas J. Yersinia has a tropism for B and T cell zones of lymph nodes that is independent of the type III secretion system. PLoS Pathog. 2006;2:e86. doi: 10.1371/journal.ppat.0020086. doi: 10.1371/journal.ppat.0020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Kuwabara T, Mimuro H, Kuwae A, Imajoh-Ohmi S. Shigella-induced necrosis and apoptosis of U937 cells and J774 macrophages. Microbiology. 2003;149:2513–2527. doi: 10.1099/mic.0.26341-0. [DOI] [PubMed] [Google Scholar]
- Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 2002;277:3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K, Richter K. Lipopolysaccharide desensitization of macrophages provides protection against Yersinia enterocolitica-induced apoptosis. Infect Immun. 2002;70:5259–5264. doi: 10.1128/IAI.70.9.5259-5264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- Mills SD, Boland A, Sory MP, van der Smissen P, Kerbourch C, et al. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci U S A. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory MP, Cornelis GR. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- Griffiths RJ, Stam EJ, Downs JT, Otterness IG. ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol. 1995;154:2821–2828. [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, et al. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- Chen LM, Kaniga K, Galan JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- Stockbauer KE, Foreman-Wykert AK, Miller JF. Bordetella type III secretion induces caspase 1-independent necrosis. Cell Microbiol. 2003;5:123–132. doi: 10.1046/j.1462-5822.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Sun GW, Lu J, Pervaiz S, Cao WP, Gan YH. Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell Microbiol. 2005;7:1447–1458. doi: 10.1111/j.1462-5822.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- Neyt C, Cornelis GR. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol Microbiol. 1999;33:971–981. doi: 10.1046/j.1365-2958.1999.01537.x. [DOI] [PubMed] [Google Scholar]
- Viboud GI, Bliska JB. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 2001;20:5373–5382. doi: 10.1093/emboj/20.19.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Smolewski P, Bedner E, Du L, Hsieh TC, Wu JM, et al. Detection of caspases activation by fluorochrome-labeled inhibitors: multiparameter analysis by laser scanning cytometry. Cytometry. 2001;44:73–82. doi: 10.1002/1097-0320(20010501)44:1<73::aid-cyto1084>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Sato T, Nagasako R, Takeda I. Acute mesenteric lymphadenitis due to Yersinia pseudotuberculosis lacking a virulence plasmid. J Clin Microbiol. 1991;29:1271–1275. doi: 10.1128/jcm.29.6.1271-1275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra SS, Jackson MW, Ross JA, Plano GV. Calcium-regulated type III secretion of Yop proteins by an Escherichia coli hha mutant carrying a Yersinia pestis pCD1 virulence plasmid. Infect Immun. 2006;74:1381–1386. doi: 10.1128/IAI.74.2.1381-1386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte P, Denecker G, Van Den Broeke A, Vandenabeele P, Cornelis GR, et al. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J Biol Chem. 2004;279:25134–25142. doi: 10.1074/jbc.M401245200. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- Trulzsch K, Geginat G, Sporleder T, Ruckdeschel K, Hoffmann R, et al. Yersinia outer protein P inhibits CD8 T cell priming in the mouse infection model. J Immunol. 2005;174:4244–4251. doi: 10.4049/jimmunol.174.7.4244. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Fantuzzi G. Interleukin-18 and host defense against infection. J Infect Dis. 2003;187(Suppl 2):S370–384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, et al. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30:3147–3156. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hein J, Sing A, Di Genaro MS, Autenrieth IB. Interleukin-12 and interleukin-18 are indispensable for protective immunity against enteropathogenic Yersinia. Microb Pathog. 2001;31:195–199. doi: 10.1006/mpat.2001.0458. [DOI] [PubMed] [Google Scholar]
- Autenrieth IB, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth IB, Tingle A, Reske-Kunz A, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Deng W, Burland V, Plunkett G, 3rd, Boutin A, Mayhew GF, et al. Genome sequence of Yersinia pestis KIM. J Bacteriol. 2002;184:4601–4611. doi: 10.1128/JB.184.16.4601-4611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Elberg SS. Scanning electron microscopic study of virulent Yersinia pestis and Yersinia pseudotuberculosis type 1. Infect Immun. 1977;15:972–977. doi: 10.1128/iai.15.3.972-977.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne GI, Ojcius DM. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol. 2004;2:802–808. doi: 10.1038/nrmicro1007. [DOI] [PubMed] [Google Scholar]
- Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P, Ricci V, Galmiche A, Gauthier NC. Gastric cell apoptosis and H. pylori: has the main function of VacA finally been identified? Trends Microbiol. 2003;11:410–413. doi: 10.1016/s0966-842x(03)00211-7. [DOI] [PubMed] [Google Scholar]
- Viboud GI, So SS, Ryndak MB, Bliska JB. Proinflammatory signalling stimulated by the type III translocation factor YopB is counteracted by multiple effectors in epithelial cells infected with Yersinia pseudotuberculosis. Mol Microbiol. 2003;47:1305–1315. doi: 10.1046/j.1365-2958.2003.03350.x. [DOI] [PubMed] [Google Scholar]
- Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol. 2006;61:795–809. doi: 10.1111/j.1365-2958.2006.05271.x. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Smith JL. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Naïve BMDMs were infected with wild-type or YopJ− Yptb in the same experiment reported in Figure 2.
(A) Macrophages labeled with SYTO10 (green) were stained with membrane-impermeant EtBr (MW = 394 Da, red) and examined by confocal microscopy to assess increases in membrane permeability (EtBr-positive/SYTO10-labeled, yellow). Representative images are shown.
(B) The percentage of EtBr-positive/SYTO10-labeled cells was determined; data shown are means and SDs from multiple fields. Representative of two experiments.
(C) DNA damage was assessed by TUNEL and confocal microscopy. Representative images are shown.
(D) The percentage of TUNEL-positive cells was determined; data shown are means and SDs from multiple fields. Representative of two experiments. Increased DNA damage was observed at 60 min (D) *p = 0.0002, prior to any significant increase in membrane permeability (B) ^p = 0.1 (no significant difference), and these features were dependent on YopJ. This result differs from infection of activated macrophages, in which wild-type and YopJ− Yptb-induced membrane permeability precedes DNA damage by 120 min (Figure 2C and 2D).
(E) YopJ-dependent apoptosis in naïve macrophages was accompanied by activation of apoptotic caspases-8 and −3 [9,16]. Data presented are fold changes in caspase activity in cell lysates of infected macrophages compared with uninfected controls at 120 min. Means and SDs were calculated from three replicates and are representative of two experiments.
(F) YopJ-dependent DNA fragmentation in naïve macrophages was dependent on the apoptotic executioner caspase-3. Naïve BMDMs were treated with the caspase-3 inhibitor DEVD (200 μM) and infected with wild-type Yptb. DNA damage was assessed by TUNEL and confocal microscopy. The percentage of TUNEL-positive cells was determined; data shown are means and SDs from multiple fields. Representative of two experiments. * p = 0.0001.
(G) TLR4KO macrophages have reduced susceptibility to YopJ-dependent apoptosis as previously described [6,7]. Naïve wild-type and TLR4KO C57BL/6 macrophages were infected with wild-type and YopJ− Yptb, and LDH release was measured 4 h after infection. Data shown are means of LDH release from three replicates and are representative of two experiments. *p = 0.0007.
(2.1 MB TIF)
We examined nuclear morphology of cells immediately after the appearance of damaged DNA (TUNEL positivity) during infection of naïve and activated macrophages with Yptb. During YopJ-dependent apoptosis, TUNEL-positive nuclei undergo nuclear condensation and fragmentation characteristic of apoptosis [3,4,35].
(A) Naïve macrophages were infected with wild-type Yptb; TUNEL-positive nuclei demonstrating typical apoptotic condensation indicated by open arrowheads.
(B) In contrast, activated macrophages infected with wild-type or YopJ− Yptb became TUNEL positive without marked nuclear condensation, and damaged DNA remained evenly distributed within the nucleus, suggestive of the previously reported morphology associated with caspase-1-dependent cell death [38].
(973 KB TIF)
At 4, 5, and 6 d postinfection, mesenteric lymph nodes (A) and spleens (B) were harvested from mice infected with wild-type Yptb and CFUs were quantified. In parallel, macrophage activation was assessed by ICAM-1 expression on F4/80+ macrophages from infected tissues. A greater than 2-fold increase in the percentage of ICAM-1hi macrophages relative to macrophages from uninfected mice was scored as activated (red circles, activated; black circles, not activated). Data in (A) and (B) are from two independent experiments. ND, none detected.
(C) ICAM-1 expression on F4/80+ macrophages from MLNs (top row) and spleen (bottom row) and corresponding colony-forming units (CFU) from one time point represented in (A) and (B).
(1.8 MB TIF)
Spleens were harvested from mice infected with wild-type Yptb, and macrophage activation was analyzed by ICAM-1 expression (top row with corresponding CFU). Caspase-1 activity (bottom row) was examined in parallel by FAM-YVAD staining. Red histograms were scored as Yptb-infected with activated macrophages and black histograms as Yptb-infected without activated macrophages. ND, none detected. Caspase-1hi macrophages have higher levels of surface ICAM-1 than caspase-1lo macrophages (see Figure 7E and 7F).
(1.5 MB TIF)
Percentage of active caspase-1hi macrophages from uninfected and infected macrophage populations averaged from two independent experiments, including Figure S4.
(25 KB DOC)