Abstract
The neuropeptides, arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP) are synthesized by neurons of the suprachiasmatic nucleus (SCN) of the hypothalamus and are important regulators of SCN function. Previous studies have demonstrated that acute exposure to stressors can disrupt circadian activity rhythms, suggesting the possibility of stress-related alterations in the expression of these neuropeptides within SCN neurons. In this study, we examined the effect of intermittent footshock stress on AVP mRNA and heterogeneous nuclear RNA (hnRNA) and VIP mRNA expression in neurons of the SCN. Young adult male Sprague/Dawley rats were subjected to 15 seconds of scrambled intermittent footshock (0.50 mA pulses, 1 pulse/second, 300 msec) every 5 minutes for 30 minutes. Animals were sacrificed 75 or 135 min after the onset of stress and brains examined for AVP mRNA and hnRNA, and VIP mRNA using in situ hybridization. Footshock stress increased AVP hnRNA levels at the 75 min timepoint whereas AVP mRNA was elevated at both the 75 and 135 min. timepoints. In contrast, footshock stress decreased the number of cells expressing VIP mRNA in the SCN without changing hybridization level per cell. These data indicate that the disruptive effect of stress on activity rhythms correlate with alterations in the expression of regulatory peptides within the SCN.
Introduction
The suprachiasmatic nuclei (SCN) of the hypothalamus function as an endogenous clock maintaining circadian rhythms under in vivo and in vitro conditions [1, 2, 3]. The endogenous rhythmicity of the SCN couples behavioral and physiological states, including activity levels, body temperature, autonomic regulation, and hormone secretion. While the SCN entrainment of these rhythms is strongly influenced by photic cues, there is increasing evidence that non-photic environmental cues also influence rhythm entrainment [4]. These include novelty-induced wheel running, food restriction, and behavioral stress. Among those stressors reported to alter rhythm entrainment are social defeat, conditioned emotional responses, controlled vs uncontrolled shock exposure, surgery, and chronic exposure to varying types of mild stressors [5 - 11].
Little is known about the mechanisms mediating stress-induced changes in circadian rhythmicity, or to what degree they overlap with those involved in photic entrainment. Photic regulation involves multiple elements that are both intrinsic and extrinsic to the SCN [12]. Arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) have been the most studied of the SCN's intrinsic regulatory proteins [13]. Under basal conditions, both maintain a circadian rhythm of expression within the SCN. AVP mRNA is elevated during the light phase of the light/dark cycle [14, 15], whereas VIP mRNA is elevated during the dark phase [15-17]. These diurnal changes in AVP are maintained under constant light conditions, whereas the VIP rhythm is abolished [18 – 20]. In animals housed under continuous dark conditions, photic stimuli decrease VIP mRNA [21].
AVP and VIP are expressed in the dorsomedial and ventrolateral cells of the SCN, respectively [22] and cooperate to regulate entrainment to photic and non-photic cues [23, 24]. Indirect evidence suggests that the expression of both peptides can be influenced by stress-related increases in plasma corticosterone (CORT). Although glucocorticoids decrease VIP expression in pituitary [25], this does not appear to be the case in brain. Adrenalectomy decreased VIP mRNA in the hippocampus [26] and the SCN, but the effect in SCN are not reversed by corticosterone [27] suggesting factors other than glucocorticoids influence SCN VIP expression. Nothing is known regarding the influence of CORT on AVP expression in the SCN, but various behavioral stressors increase AVP expression in the paraventricular nucleus (PVN) of the hypothalamus [28, 29]. Increases in AVP concentration within the SCN following exposure to a 10 minute forced swim [30] indicate that a similar effect occurs in the SCN.
Based on these observations, we have hypothesized that acute stressors may modulate the expression of regulatory neuropeptides within the SCN. Consequently, we examined the effect of footshock stress on the expression of AVP and VIP mRNAs within SCN neurons of male rats and compared this with the effect in the PVN and supraoptic nucleus (SON), two nuclei where neuropeptide mRNA expression has been shown to be stress sensitive [31, 32].
Materials and Methods
Animals
60 day old male Sprague-Dawley rats (Charles River, Portage MI) were used in these studies. Animals were group housed in climate-controlled rooms under a 12:12 light-dark cycle (lights on at 0600). Food and water were available ad libitum. On the day of experiment, animals were placed in a stainless steel cage with a grid floor. Animals were stressed by 15 seconds of scrambled intermittent shock (0.50 mA pulses, 1 pulse/second, 300 msec duration) applied every 5 minutes for 30 minutes. Control animals were placed in the shock cages for the same duration, but received no shock. Subsequently, animals were returned to their home cage. The stress and control procedures were conducted during a single day between 0930 and 1100 hrs. Animals were killed by decapitation at 75 or 135 minutes after the onset of the shock-stress session. These times where chosen to capture the increases of AVP mRNA and hnRNA which reportedly peak in the PVN about 2 hrs following stress [31]. Subsequently, brains were removed and frozen in 2-methylbutane at -30°C, transferred to dry ice and stored at -70°C. All experimental protocols were approved by the San Diego State University IACUC.
In situ Hybridization
Coronal sections (16μm) through the hypothalamus were cut in a cryostat at -20°C. Sections were thaw-mounted onto Superfrost-plus Slides (Fisher Scientific), dried briefly on a slide warmer, and stored at -70°C. Just prior to hybridization, sections were warmed to room temperature, fixed in 4% formaldehyde in phosphate buffered saline (pH 7.0; 5 min), acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0; 10 min), rinsed in 2× standard saline citrate (SSC); transferred through ascending ethanols and delipidated in 100% chloroform.
Hybridization was performed using synthetic oligonucleotide probes and all tissues were processed concurrently. The probe for AVP mRNA was a 48 base oligomer complimentary to the nucleotides encoding the last 16 amino acids of AVP (20). The oligonucleotide probe for VIP corresponded to nucleotides 367 to 408 of the rat VIP mRNA.
The probe for AVP heterogeneous nuclear RNA (hnRNA) was a 48 base oligomer complementary to the 5′ end of intron I. For these studies, hnRNA was measured to estimate primary transcript levels. Advantages to measuring hnRNA rather than mature mRNA include: hnRNA expression is a relatively accurate representation of rate of transcription, as compared to measuring steady state levels of mRNA, which are influenced, not only by transcription rate, but also by rate of turnover. Subtle changes in transcription can be detected since hnRNA is found at low levels in the basal state and thus, large pools of mRNA do not camouflage changes. The measurement of hnRNA allows discrimination of changes in transcription at a single cell level [31,32]. Corresponding VIP hnRNA was not measured in these studies. Because the VIP gene contains 6 introns and 7 exons, a suitable probe for measuring VIP hnRNA levels has yet to be described.
All probes were 3′ end-labeled with 35S dATP (Perkin Elmer) using terminal deoxynucleotidyl transferase (Boehringer Mannheim). Probes were dissolved in buffer containing 600 mM NaCI, 20 mM Tris-HCI, 0.04% Denhart's, 2 mM EDTA, 0.2% denatured DNA, 0.1% total yeast RNA, 20% dextran sulfate and 50% deionized formamide containing 0.1% sodium thiosulfate, 100 mM dithiothreitol and 0.5% sodium dodecyl sulfate. 1 × 106 cpm of probe in 80ul was applied to each slide. Sections were coverslipped and hybridization performed at 40°C overnight. After hybridization, slides weree washed 3×20 min in 2× SSC/50% formamide at 40°C and 2×30 min each in 1× SSC RT. Slides were dehydrated in increasing ethanols, air-dried and exposed to Hyperfilm Bmax (Amersham) for three days (AVP mRNA), four days (hnRNA) and 3 days (VIP mRNA). Subsequently, slides were dipped in Kodak NTB2 photographic emulsion and exposed for 4 days (AVP mRNA) to 5 ½ days for VIP mRNA. Slides were counterstained with cresyl violet.
Analysis
Semiquantitative analysis of film autoradiograms was conducted using NIH Image 1.54 (NIH, Bethesda, MD). Sections were anatomically matched and optical densities in SCN, SON and PVN were obtained using a template of fixed size. Background was taken from an adjacent non-hybridized area and subtracted from the total hybridization to obtain specific hybridization. All data were expressed in terms of arbitrary density units. Data were analyzed using two-tailed Student's t-tests comparing unstressed values with the post stress time points.
Estimations of grain density over individual neurons were conducted in SCN using a computer assisted image analysis system with a macro written by Dr. Karl Beykirch (UCLA). The program identifies silver grains based upon a user-defined threshold and estimates grain density based upon the pixel numbers above threshold in a user-defined area. Grain density over a random sampling of cells in the SCN was determined for each animal. Grains over 4-5 adjacent cell-sized areas were also counted in each section and used to calculate background level. An SCN neuron was considered labeled if the number of silver grains over its soma was greater than three-times the average background of surrounding neuropil. The specific hybridization level per cell was defined as the ratio of the total grains/cell minus the mean background silver grains for that section. The percentage of labeled SCN neurons for each animal was calculated by dividing the number of labeled neurons by the total number of labeled and unlabelled neurons and multiplying the result by 100. The range of labeled cells measured per animals was 54-88 neurons/SCN.
Results
Effect of stress on VIP mRNA expression in the SCN
Film densitometry of the hybridization signal revealed that VIP mRNA expression was significantly reduced by stress at 75 (t[8]=4.18; p<0.01) and 135 minute (t[8]=4.69; p<0.001) timepoints compared to controls. Analysis of VIP mRNA over individual neurons revealed no significant difference in cellular level of hybridization (data not shown), but there was a significant reduction in the percentage of labeled SCN neurons (Figure 1).
Figure 1.
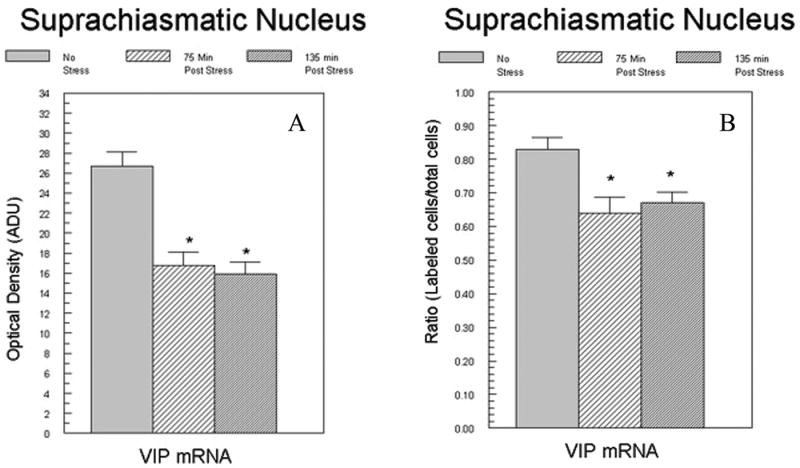
Effect of footshock stress on VIP mRNA in the SCN. Adult male rats were sacrificed either 75 min. or 135 min. after receiving 30 min of intermittent footshock. Each bar represents the group means (± SEM). Figure 1A depicts the data obtained following density analysis of film autoradiograms. Figure 1B depicts the results of VIP mRNA levels following grain density estimates from emulsion coated slides expressed as the ratio of labeled cells to unlabeled cells. Ratios for individual animals were derived from analyses performed on 55-74 neurons. No differences in the density of the individual labeled neurons were observed between. N=5/group. NS = nonstressed. *p<0.05 from control group.
Effect of stress on the expression of AVP mRNA and AVP hnRNA
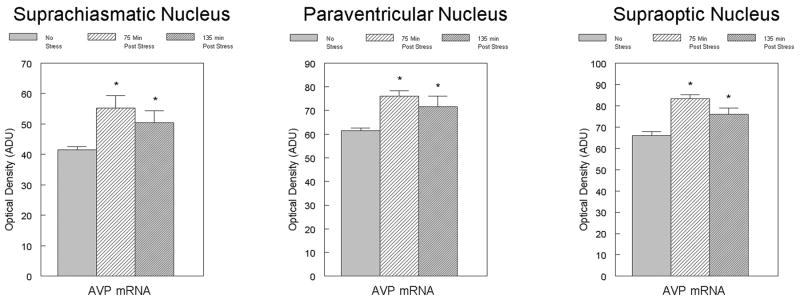
As shown in figure 2, specific hybridization for the AVP mRNA directed probe was observed in the SCN, PVN and SON. Computer-assisted film analysis showed that the hybridization density was significantly increased in the SCN at both 75 and 135 minutes timepoints (t[8]=2.48; p<0.05; t[8]=1.89; p<0.05). Similar increases were also detected in the SON (t[8]=4.78; p<0.001; t[8]=2.13; p<0.05). In the PVN, there was a significant increase at 75 (t[8]=4.01 p<0.001), but not at 135 minutes (P<0.10).
Figure 2.
Effect of footshock stress on AVP mRNA expression in the SCN, PVN and SON of adult male rats. mRNA levels were measured using optical density of film autoradiograms. Each bar represents the mean +/- SEM of 5 animals/group. *p<0.05 from control group.
Analysis of emulsion coated slides also indicated that AVP mRNA increased in SCN at both time-points (figure 3). There were no significant differences in the percentage of labeled cells in the SCN following stress (data not shown). Unfortunately, cellular levels of AVP mRNA in PVN and SON were not possible to measure in these sections because the photographic emulsion over these brain areas was saturated at the exposure duration that produced a measurable signal in the SCN.
Figure 3.
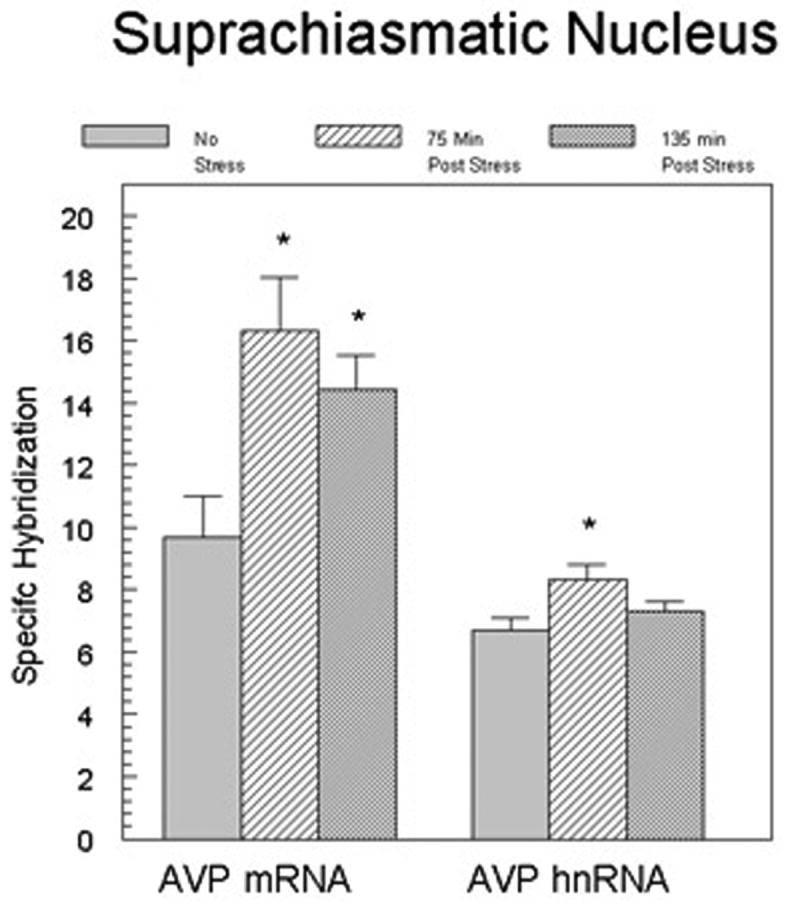
AVP mRNA and heteronuclear RNA levels in individual neurons of the SCN. Data shown are the mean (± SEM) number of grains (minus background) over individual SCN neurons. The analysis measured 60-75 cells per animal with 5 animals representing each group. *p<0.05 from control group.
For AVP hnRNA, grain density analysis showed a significant increase in hybridization level in individual SCN neurons at 75 min (t[8]=2.69; p<0.05), but not at 135 min after the onset of stress (Figure 3). We observed no significant increase between groups in the total number of cells that expressed AVP hnRNA (data not shown).
Discussion
In this study, we show that footshock stress can alter the expression of regulatory neuropeptides within the SCN. Because previous results showred disruptive effects of stress on physiological and behavioral rhythms, we anticipated a stress-induced inhibition of the normal circadian expression of one or both peptides. This was the case for VIP, but not AVP. Exposure to intermittent footshock stress decreased the number of SCN cells expressing VIP mRNA. In contrast, expression of AVP mRNA within individual SCN neurons was significantly enhanced without changes in AVP mRNA expressing SCN cell numbers. This was accompanied by a corresponding increase in AVP hnRNA. Since AVP hnRNA levels reliably predict AVP primary transcript levels, this finding indicates that the intracellular changes in AVP mRNA reflected alterations in transcription. Therefore, stress appears to have amplified the differential pattern of expression that is normally present during this phase of the circadian cycle in the rat SCN. However, any interpretation of the response pattern is limited since the present study applied stress at only a single time point in the circadian cycle.
The stress-related increase that we observed in AVP expression appears to be consistent with a previous report that AVP in the SCN, PVN and SON is increased following acute exposure to swim stress [30]. The rise is also consistent with other studies showing a stress-induced rise in AVP expression in the SON and PVN [28, 33]. However, no other studies to our knowledge have examined the content or expression of VIP in the SCN subsequent to non-photic stimuli.
Acute stress can induce both short (1-2 days) and longer-term effects (> 7 days) on a number of physiological systems. This raises the question about the degree to which these changes are associated with changes in expression of SCN AVP and AVP mRNA following the stressor. Factors that influence the pacemaker functions of the SCN are generally divided in photic and non-photic classes, with stress falling into the latter. But distinct pathways have been identified for different types of stressors [e.g., 34]. Further, feedback systems can influence regional clock cells that control tissue specific rhythmic outputs [35]. Thus, stress-induced changes in the SCN may not reflect the extent of regulatory disruption in rhythm entrainment control.
The mechanism for stress induced changes in SCN function is unknown. Data suggest that this may not be partially due to stress-induced elevations in circulating glucocorticoids. Glucocorticoids inhibit AVP expression in the human SCN [36], as well as the rat and human PVN [37, 38]. Although these studies did not examine glucocorticoid effects on AVP expression in the rat SCN, glucocorticoids inhibit AVP release both in vitro and in vivo [39]. In contrast, glucocorticoid effects appear to be negligible for VIP mRNA. Corticosterone administered to adrenalectomized animals, does not depress the expression of VIP in the SCN [27]. Furthermore, a direct action of stress activated PVN neurons on SCN function is not indicated since CRH containing fibers are not found in the SCN [40].
Although neither AVP nor VIP is essential to rhythm generation by the SCN, the two peptides cooperate in modulating the entrainment and amplitude of circadian rhythms by the SCN [41]. VIP containing cells are prevalent in the ventrolateral subdivision and receive direct and indirect photic information, as well as modulatory input from the raphe. In contrast, AVP neurons are confined to the dorsomedial subdivision, which is the area most critical for rhythmic output. Photic information is projected to the dorsomedial area from VIP neurons [23]. The dorsomedial SCN also receives input from other areas of the hypothalamus, limbic forebrain and brainstem that are involved in homeostatic regulation [42,43]. It has few reciprocal connections with the ventrolateral subdivision, but sends efferents to hypothalamic and extrahypothalamic sites that integrate physiological and hormonal responses, including those related to stress [44–47].
Leak et al [42,47] have described a model of pacemaker organization that proposes that the two segregated subdivisions allow the SCN to integrate and balance information from different types of external and internal stimuli important for homeostasis. Photic input to the ventrolateral subdivision is serially conveyed to the dorsomedial subdivision to insure that light is the dominant influence on rhythm generation and modulation. Inputs to the dorsomedial region from areas outside of the SCN allow non-photic influences to be integrated into modulation of amplitude and phase of rhythms under SCN control. Current behavioral, physiological, and anatomical data related to photic influences on SCN regulation strongly support the model. However, the effect of stress that we observed indicates that the VIP neurons in the ventrolateral subdivision are sensitive to non-photic cues as well.
Acknowledgments
This work was supported by NIH AA011600 (RFM), NS039951(RJH) and AA014974(RJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore RY, Card JP. Visual pathways and the entrainment of circadian rhythms. Ann NY Acad Sci. 1985;453:123–133. doi: 10.1111/j.1749-6632.1985.tb11805.x. [DOI] [PubMed] [Google Scholar]
- 2.DeCoursey PJ, Buggy J. Circadian rhythmicity after neural transplant to hamster third ventricle: specificity of suprachiasmatic nuclei. Br Res. 1989;500:263–275. doi: 10.1016/0006-8993(89)90322-3. [DOI] [PubMed] [Google Scholar]
- 3.Lehman MN, Silver R, Gladstone WR, Khan RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant: immunocytochemical characterization of the graft and its integration with the host. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 5.Amir S, Stewart J. Conditioned fear suppresses light-induced resetting of the circadian clock. Neuroscience. 1998;86:345–351. doi: 10.1016/s0306-4522(98)00172-9. [DOI] [PubMed] [Google Scholar]
- 6.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacol Biochem Behav. 1996;54:229–234. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 7.Harper DG, Tornatzky W, Micek KA. Stress induced disorganization of circadian and ultradian rhythms: Comparisons of effects of surgery and social stress. Physiology and Behavior. 1996;59:409–419. doi: 10.1016/0031-9384(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 8.Kant GJ, Bauman RA, Pastel RH, Myatt CA, Closser-Gomez E, D'Angelo CP. Effects of controllable vs uncontrollable stress on circadian temperature rhythms. Physiology and Behavior. 1991;49:625–630. doi: 10.1016/0031-9384(91)90289-z. [DOI] [PubMed] [Google Scholar]
- 9.Meerlo P, de Boer SF, Koolhaas JM, Daan S, van den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiology and Behavior. 1996;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- 10.Meerlo p, Overkamp GJF, Benning MA, Koolhaas JM, van den Hoofdakker RH. Long-term changes in open filed behavior following a single social defeat in rats can be reversed by sleep deprivation. Physiology and Behavior. 1996;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- 11.Tornatzky W, Micek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiology and Behavior. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- 12.Herzog ED, Schwartz WJ. A neural clockwork for encoding circadian time. J Appl Physiol. 2002;92:401–408. doi: 10.1152/japplphysiol.00836.2001. [DOI] [PubMed] [Google Scholar]
- 13.van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res Brain Res Rev. 2000;33:34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- 14.Jac M, Kiss A, Sumova A, Illnerova H, Jezova D. Daily profiles of arginine vasopressin mRNA in the suprachiasmatic, supraoptic and paraventricular nuclei of the rat hypothalamus under various photoperiods. Brain Res. 2000;887:472–476. doi: 10.1016/s0006-8993(00)03050-x. [DOI] [PubMed] [Google Scholar]
- 15.Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998;139:4189–4196. doi: 10.1210/endo.139.10.6259. [DOI] [PubMed] [Google Scholar]
- 16.Albers HE, Stopa EG, Zoeller RT, Kauer JS, King JC, Fink JS, Mobtaker H, Wolfe H. Day-night variation in prepro vasoactive intestinal peptide/peptide histidine isoleucine mRNA within the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1990;7:85–89. doi: 10.1016/0169-328x(90)90077-q. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto S, Okamura H, Miyake M. A diumal variation of vasoactive intestinal peptide (VIP) mRNA under a daily light-dark cycle in the rat suprachiasmatic nucleus. Histochemistry. 1991;95:525–528. doi: 10.1007/BF00315750. [DOI] [PubMed] [Google Scholar]
- 18.Larsen PJ, Vrang N, Moller M, Jessop DS, Lightman SL, Chowdrey HS, Mikkelsen JD. The diumal expression of genes encoding vasopressin and vasoactive intestinal peptide within the rat suprachiasmatic nucleus is influenced by circulating glucocorticoids. Mol Br Res. 1994;27:342–6. doi: 10.1016/0169-328x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 19.Noto T, Hashimoto H, Doi Y, Nakajima T, Kato N. Biorhythm of arginine-vasopressin in the paraventricular, supraoptic, and suprachiasmatic nuclei of rats. Peptides. 1983;4:875–878. doi: 10.1016/0196-9781(83)90084-0. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi Y, Okamura H, Tanaihara N, Hamada S, Fujita S, Ibata Y. Vasoactive intestinal peptide immunoreactive neurons in the rat suprachiasamtic nucleus demonstrate diumal variations. Br Res. 1989;479:374–377. doi: 10.1016/0006-8993(89)90283-7. [DOI] [PubMed] [Google Scholar]
- 21.Shinohara K, Tominaga K, Inouye ST. Phase dependent response of vasoactive intestinal polypeptide to light and darkness in the suprachiasmatic nucleus. Neuroscience Research. 1999;33:105–110. doi: 10.1016/s0168-0102(98)00122-9. [DOI] [PubMed] [Google Scholar]
- 22.Van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus of the rat: Immunoctochemical analysis of 25 neuronal antigens. Neuroscience. 1985;46:859–879. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 23.Ibata Y, Okamura H, Tanaka M, Taamada Y, Hatashi S, Iijima T, Matsuda T, Munekawa K, Tahamatsu T, Hisa Y, Shigeyoshi Y, Amaya F. Functional morphology of the suprachiasmatic nucleus. Frontiers in Neuroendocrinology. 1999;20:241–268. doi: 10.1006/frne.1999.0180. [DOI] [PubMed] [Google Scholar]
- 24.Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res. 1998;119:351–64. doi: 10.1016/s0079-6123(08)61580-0. [DOI] [PubMed] [Google Scholar]
- 25.Lam KS, Srivastava G, Tarn SP. Divergent effects of glucocorticoid on the gene expression of vasoactive intestinal peptide in the rat cerebral cortex and pituitary. Neuroendocrinol. 1992;56:32–37. doi: 10.1159/000126205. [DOI] [PubMed] [Google Scholar]
- 26.Rotsztejn WH, Besson J, Briaud B, Gagnant L, Rosselin G, Kordon C. Effect of steroids on vasoactive intestinal peptides in discrete brain regions and peripheral tissues. Neuroendocrinol. 1980;31(4):287–291. doi: 10.1159/000123090. [DOI] [PubMed] [Google Scholar]
- 27.Gozes I, Avidor R, Giladi E, Shani Y, McEwen BS, Dussaillant M, Rostene W. Adrenalectomy decreases vasoactive intestinal peptide mRNA levels in the rat suprachiasmatic nucleus. Neurosci Lett. 1994;176:24–28. doi: 10.1016/0304-3940(94)91019-7. [DOI] [PubMed] [Google Scholar]
- 28.Lightman SL, Young SW., III Corticotropin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J Physiol (London) 1988;403:511–523. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palkovits M. Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur J Pharmacol. 2000;405:161–166. doi: 10.1016/s0014-2999(00)00549-5. [DOI] [PubMed] [Google Scholar]
- 30.Engelmann M, Ebner K, Landgraf R, Wotjak CT. Swim stress triggers the release of vasopressin within the suprachiasmatic nucleus of male rats. Brain Res. 1998;792:343–347. doi: 10.1016/s0006-8993(98)00243-1. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs KJ, Sawchenko PE. Seqeunce of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma XM, Levy A, Lightman SL. Rapid changes in heteronuclear RNA for corticotropin-releasing hormone and arginine vasopressin in response to acute stress. J Endocrinol. 1997;152:81–89. doi: 10.1677/joe.0.1520081. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs KJ. Functional neuroanatomy of the parvocellular vasopressinergic system: transcriptional responses to stress and glucocorticoid feedback. Prog Brain Res. 1998;119:31–43. doi: 10.1016/s0079-6123(08)61560-5. [DOI] [PubMed] [Google Scholar]
- 34.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 35.Amir S, Lamont EW, Robinson B, Stewart J. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci. 2004;24:781–790. doi: 10.1523/JNEUROSCI.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu RY, Unmehopa UA, Zhou JN, Swaab DF. Glucocorticoids suppress vasopressin gene expression in human suprachiasmatic nucleus. J Steroid Biochem Mol Biol. 2006;98:248–253. doi: 10.1016/j.jsbmb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Br Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.04.005. In Press. [DOI] [PubMed] [Google Scholar]
- 38.Shibata M, Fujihara H, Suzuki H, Ozawa H, Kawata M, Dayanithi G, Murphy D, Ueta Y. Physiological studies of stress responses in the hypothalamus of vasopressin-enhanced green fluorescent protein transgenic rat. J Neuroendocrinol. 2007;19:285–292. doi: 10.1111/j.1365-2826.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 39.Isobe Y, Torii T, Kawaguchi T, Nishino H. Dexamethasone induces different wheel running activity than corticosterone through vasopressin release from the suprachiasmatic nucleus. Brain Res. 2004;1028:219–224. doi: 10.1016/j.brainres.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Reuss S, Hurlbut EC, Speh JC, Moore RY. Immunohistochemical evidence for the presence of neuropeptidesin the hypothalamic suprachiasmatic nucleus of ground squirrels. Anat Rec. 1989;225:341–346. doi: 10.1002/ar.1092250410. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 2000;877:361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- 42.Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 43.Watts AG. The efferent projections of the suprachiasmatic nucleus: Anatomical insights into the control of circadian rhythms. In: Klein DC, Moore RY, Reppert SM, editors. The Suprachiasmatic Nucleus: The Mind's Clock. Oxford Univ Press; New York: 1991. pp. 77–106. [Google Scholar]
- 44.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 45.Buijs RM. The anatomical basis for the expression of circadian rhythms: The efferent projections of the suprachiasmatic nucleus. In: Buijs RM, et al., editors. Hypothalamic Interegulation of Circadian Rhythms, Prog Br Res. 229. Vol. 111. 1996. p. 240. [DOI] [PubMed] [Google Scholar]
- 46.Cui LN, Saeb-Parsy K, Dyball REJ. Neurones in the supraoptic nucleus of the rat are regulated by a projection from the suprachiasmatic nucleus. J Physiology. 1997;502:149–159. doi: 10.1111/j.1469-7793.1997.149bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res. 1999;819:23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]