Abstract
Intermittent (pulsatile) administration of parathyroid hormone (PTH) is known to improve bone micro-architecture, mineral density and strength. Therefore, daily injection of PTH has been clinically used for the treatment of osteoporosis. However, this regimen of administration is not convenient and is not a favorable choice of patients. In this study, an implantable delivery system has been developed to achieve pulsatile release of PTH. A well-defined cylindrical device was first fabricated with a biodegradable polymer, poly(lactic acid) (PLLA), using a reverse solid free form fabrication technique. Three-component polyanhydrides composed of sebacic acid, 1,3-bis(p-carboxyphenoxy) propane and poly(ethylene glycol) were synthesized and used as isolation layers. The polyanhydride isolation layers and PTH-loaded alginate layers were then stacked alternately within the delivery device. The gap between the stacked PTH-releasing core and the device frame was filled with PLLA to seal. Multi-pulse PTH release was achieved using the implantable device. The lag time between two adjacent pulses were modulated by the composition and the film thickness of the polyanhydride. The released PTH was demonstrated to be biologically active using an in vitro assay. Timed sequential release of multiple drugs has also been demonstrated. The implantable device holds promise for both systemic and local therapies.
Introduction
Parathyroid hormone (PTH) is the major hormone regulating calcium metabolism, and can have both catabolic and anabolic actions in bone [1–5]. Whereas continuous exposure to PTH results in bone resorption, intermittent administration (pulsatile delivery) of PTH improves bone micro-architecture, mineral density and strength [1, 2, 6]. The anabolic action makes PTH a particularly appealing agent to treat patients with osteoporosis [7–11]. In fact, the anabolic application of PTH is FDA-approved for stimulating bone formation, and it has been shown to reduce the risks of vertebral and non-vertebral fractures in postmenopausal women in clinical trials [3, 7, 12]. The current PTH therapy for osteoporosis requires daily subcutaneous injection of PTH. While daily injection of PTH is a feasible anabolic treatment, it is not a convenient mode of administration and is not a favorable choice of patients. In addition, injection of PTH is a systemic administration, which is not ideal for the treatment of localized bone defects. In contrast, controlled pulsatile release of PTH would be more convenient for administration and also suitable for local application. A local therapy, if indicated, has many potential advantages such as circumventing possible adverse side effects resulting from systemic administration, decreasing dose or number of dosages, and maintaining local agent levels within a desirable range. Therefore, pulsatile release of PTH is highly desirable for both systemic and local therapies, which may also expand the application of PTH to bone tissue engineering and regeneration [13].
There is no current successful pulsatile system of PTH release, although a variety of delivery strategies have been explored for the pulsatile release of other agents from polymeric implants [14–19]. Based on the triggering mechanisms, they can be classified as stimulus-induced pulsatile release systems and self-regulated pulsatile release systems. In stimulus-induced delivery systems, drugs are triggered to release by external stimuli, such as temperature [20–22], pH [23, 24], light [25], enzyme [26, 27], electric [28, 29] and magnetic [30] fields. While these stimulus-responsive devices have shown promising pulsatile release characteristics, many of the stimuli cannot be easily and safely utilized in patients. In addition, most of the systems in this category are not constructed using biodegradable polymers. Critical considerations have to be given to the biocompatibility, biodegradability and toxicity of these stimulus-responsive polymeric systems. In self-regulated pulsatile release systems, drugs are usually encapsulated in one way or another within a barrier material, which is composed of an erodible or biodegradable polymer [19, 31, 32]. Depending on the barrier material structure and thickness, different release times can be achieved. After the barrier material is dissolved, eroded or degraded, drugs are rapidly released from the inner reservoir core [17, 18, 33, 34]. These systems are usually biocompatible and biodegradable, but multiple barriers or coatings are necessary to achieve the needed multiple pulses of release, which often pose challenges in material properties and device fabrication technologies, often resulting in inconsistency.
By combining self-regulated release reservoir design with a microfabrication technique, a disk-shaped biodegradable polymeric chip (approximately 12 mm in diameter and 0.5 mm in thickness) has been fabricated to achieve multi-pulse drug release [35]. While this device achieved a modulated multi-pulse release profile and demonstrated the bioactivity of released heparin by using different polymer structures or molecular masses for the barrier membranes, the reservoirs in the device were parallel arranged, which is not a space-saving design and limits the miniaturization capacity. To date, an implantable delivery system that can release multi-pulses of PTH has yet to be developed.
In our previous work, we synthesized new biodegradable three-component polyanhydrides composed of sebacic acid (SA), 1,3-bis(p-carboxyphenoxy) propane (CPP) and poly(ethylene glycol) (PEG) [36]. The three-component PEG-containing polyanhydrides retain the surface erosion properties of the SA-CPP polyanhydrides while imparting a large range of tunable erosion rate. In this study, we designed and fabricated an implantable delivery device using these novel surface-erosion polymers to achieve controlled pulsatile PTH delivery. A well-defined hollow cylindrical device frame was first fabricated using a reverse solid free form (RSFF) fabrication technique. The PEG-containing polyanhydrides were selected as the isolation-layer materials, and stacked alternately with drug-loaded layers in the cylindrical delivery device, which was finally laminated and sealed with biodegradable poly(L-lactic acid) (PLLA). The multi-pulse release profiles of PTH and BSA were examined in vitro, and the bioactivity of the released PTH from the delivery system was demonstrated.
Materials and Methods
Materials
Three-component polyanhydrides composed of sebacic acid (SA), 1,3-bis(p-carboxyphenoxy) propane (CPP), and poly(ethylene glycol) (PEG, Mw=600) were synthesized and characterized using NMR, GPC and FTIR as previously reported [36]. The polyanhydride (weight ratio: SA/CPP/PEG = 80/20/1.6) with a viscosity-average molecular weight of 58.0 kDa and a compressive modulus of 58.1 MPa was used as isolation material for the pulsatile PTH release device. Lyophilized parathyroid hormone, PTH(1-34), was obtained from Bachem Bioscience Inc. (Torrance, CA). Bovine serum albumin (BSA, Fraction V) was purchased from Sigma (St. Louis, MO). Poly(L-lactic acid) (PLLA) with an inherent viscosity of approximately 1.6 was purchased from Boehringer Ingelheim (Ingelheim, Germany). Sodium alginate was obtained from Pronova Biopolymer (Drammen, Norway). Dichloromethane and 1,4-dioxane were purchased from Aldrich Chemical Company (Milwaukee, WI).
Scanning Electron Microscopy (SEM) Examination of Erosion
Rod-shaped polyanhydride specimens (2 mm in diameter and 4 mm in length) with various chemical compositions were placed in LoBind tubes (Eppendorf AG, Germany) containing 1.0 ml of phosphate buffered saline (PBS, 0.1 M, pH 7.4). The tubes were kept in an incubator at 37°C for 24 h. The specimens were then taken out and vacuum-dried for 24 h. The specimens were cross-sectioned, gold-coated and examined with scanning electron microscopy (SEM, Philips XL30 FEG) at 10 kV.
Device frame design and fabrication
A cylindrical mold (3.2 mm in inner diameter, and 3.7 mm in outer diameter) with one end sealed was first designed and converted into a stereolithography (STL) file using Rhinoceros software (Robert McNeel & Associates, Seattle, WA), and then imported into Modelworks software (Solidscape) to convert the file for 3D printing [37]. To build the model, red wax and green wax were printed from two nozzles in a layer-by-layer fashion using a rapid prototyping machine (Modelmaker II, Solidscape) (Figure 1).
Figure 1.
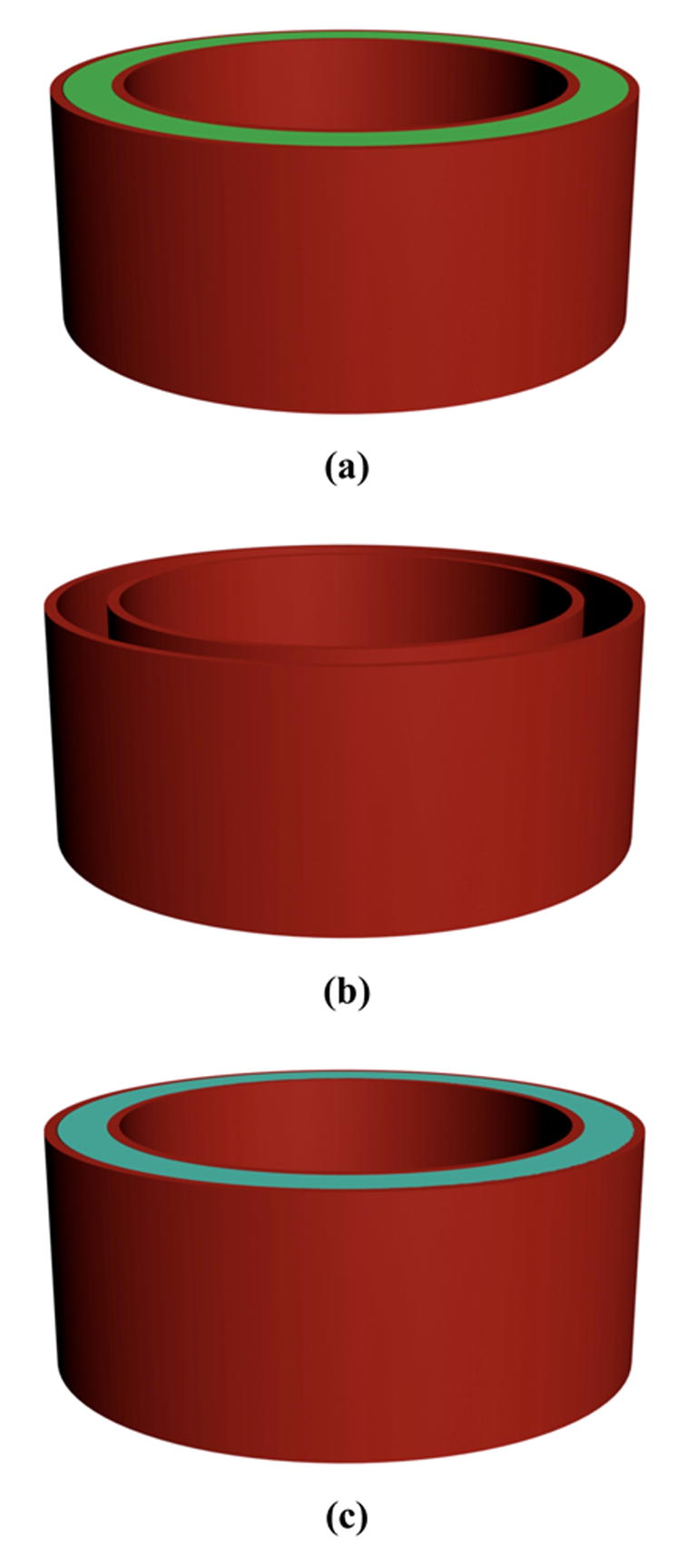
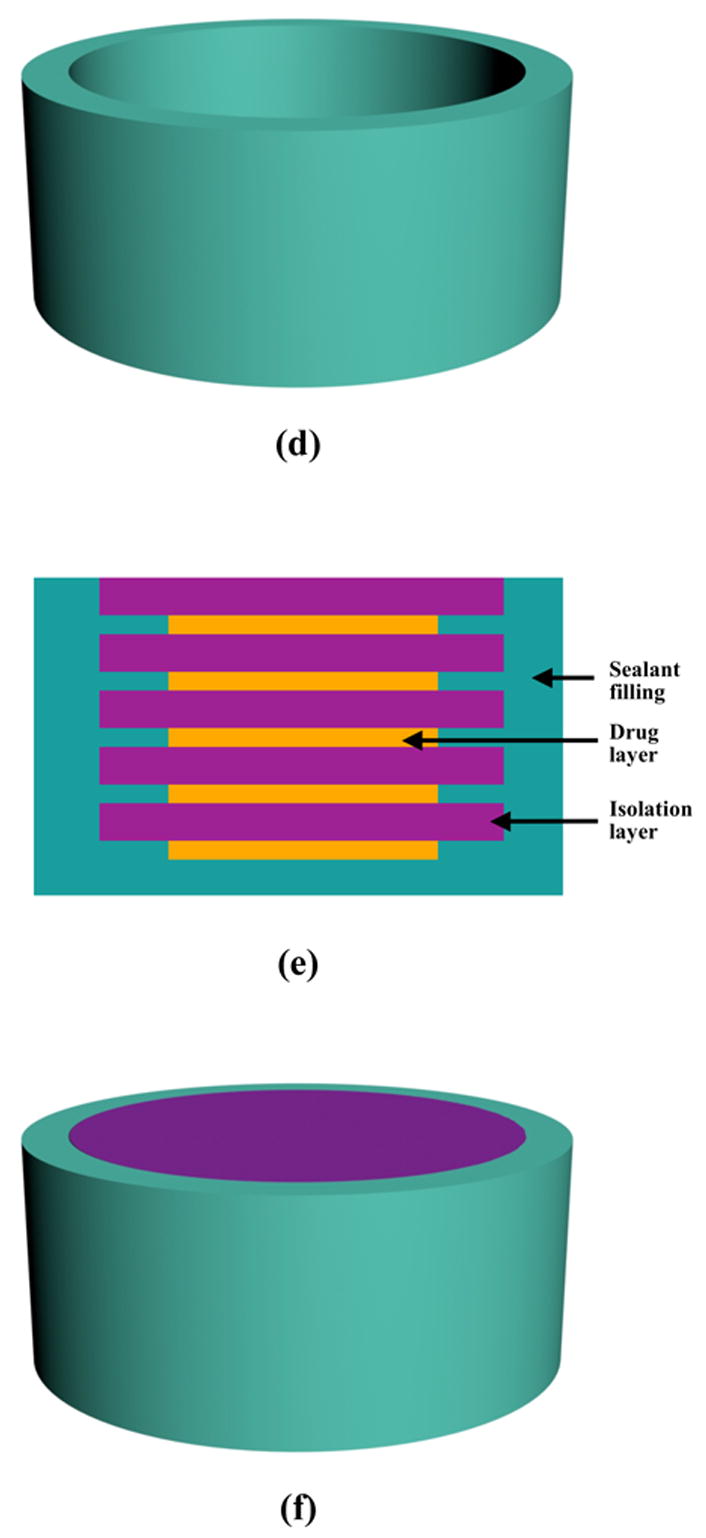
Schematic illustration of fabrication process of implantable pusatile delivery system. (a) device mold is printed using reverse solid free form fabrication technique (red and green wax materials were used); (b) green wax material is dissolved; (c) biodegradable PLLA solution is cast into the mold and air-dried; (d) red wax material is dissolved; (e) cross-section view of the device, showing that polyanhydride layers and drug layers are alternately stacked in the device; (f) overview of the completed device. The final device is a cylinder with a diameter of 3.6 mm and a thickness of about 1.6 mm.
After the cylindrical mold was printed, ethanol was used to dissolve the green wax of the mold. PLLA solution (10% wt/v) was then cast into the mold (taking the shape of the original green material) and air dried for 3 days. The mold containing the polymer was washed in 37°C cyclohexane to dissolve the red wax mold. The obtained PLLA cylinder was then washed with ethanol and water, and subsequently air-dried. The PLLA cylinder was stored in a desiccator before use.
Fabrication of PTH, BSA and polyanhydride films
PTH (1-34) (0.3 mg) and alginate (250 mg) were mixed and dissolved in 8 ml Milli-Q water (18.2 MΩ·cm) at 4°C. The solution was cast on a hexagonal polystyrene weigh boat (Fisher Scientific, USA) and vacuum dried at 4°C for 2 weeks. The thickness of the film was about 100 μm achieved by controlling the amount of casting solution on the weigh boat. PTH/alginate disks with a diameter of 2.0 mm were punched out from the PTH/alginate films.
In this work, BSA was first used as a model protein to study the pulsatile release profile of the delivery system. BSA/alginate solution was prepared by mixing BSA (0.15g) and alginate (0.25g) in Milli-Q water (10 ml). The BSA/alginate solution was then cast into thin films and punched into BSA/alginate disks with a diameter of 2.0 mm following the same procedure as for preparing the PTH/alginate thin disks.
The newly synthesized polyanhydride copolymers were heated until melted. The polyanhydride melt was then cooled down to room temperature and compressed into films of various thickness with a pressure of 5×107 Pa for 5 min using Carver® Presses (Carver Inc., IN). The polyanhydride films were then punched into disks with a diameter of 3.0 mm.
Drug delivery device fabrication
The inner side of PLLA cylinder was first pre-wetted with ethanol. PTH or BSA disks and polyanhydride disks were alternately introduced into the PLLA cylinder (Figure 1e). The loaded amount of PTH in each layer was 50% higher than that in the layer immediately above to overcome the additional adsorption and diffusive losses of the released PTH in lower layers. After the PTH or BSA and polyanhydride layers were loaded, the laminated device was compressed with a pressure of 5×107 Pa for 10 min. PLLA solution (10% wt/v) was carefully added to fill the spaces between loaded drug (PTH or BSA) layers and isolation layers and the gap between the stacked films and the PLLA cylinder. The device was then air purged for 30 min to accelerate the solvent evaporation and then vacuum dried for 24 h.
In vitro BSA/PTH release
The drug-loaded devices were immersed in 2 ml PBS (0.1 M, pH = 7.4) and incubated at 37°C. After designated times, the medium was collected and replaced with equal amount of fresh PBS. The collected medium was stored at −20°C until analysis. The concentration of PTH in the released medium was measured using PTH (1-34) ELISA assay (Immutopics Inc., San Clemente, CA) [38]. The amount of released BSA was measured using a MicroBCA protein assay (Pierce, Rockford, IL) [38].
In vitro PTH (1-34) bioactivity assay
The in vitro bioactivity of released PTH was determined by adenylate cyclase stimulation assay and cAMP binding protein assay as previously described [38, 39]. Briefly, rat osteosarcoma cells (ROS 17/2.8) were treated with PTH (1-34) of known concentrations or with eluent from the PTH delivery device for designated times in calcium- and magnesium-free hanks’ balanced salt solution (Invitrogen, Carlsbad, CA) containing 0.1% BSA and 1 mM isobutylmethylxanthine (IBMX). After incubation of the treated cells at 37°C for 10 min, the cAMP in cells was extracted by adding cold perchloric acid. The cAMP extracts were then neutralized by adding KOH solution and were centrifuged to remove the precipitate. The [3H]-cAMP (ICN, Irvine, CA) was incubated with standards or unknowns and cAMP binding protein for 90 min on ice. The unbound [3H]-cAMP was removed by adding dextran-coated charcoal. The samples were then centrifuged and the supernatant of each tube was decanted to a scintillation tube. The radioactivity of the supernatants was determined using a liquid scintillation counter (Wallacs 1410; Wallac, Gaithersburg, MD) and cAMP levels were calculated by the loglogit method using a standard curve [38].
Results
Polyanhydrides surface erosion
Three-component (SA, CPP and PEG) polyanhydrides showed surface erosion characteristics (Figure 2). Polyanhydrides containing PEG segments exhibited faster erosion rate compared to those without PEG. The erosion depth of the polyanhydride rods without PEG segments was about 100 μm after 24 h in PBS at 37°C, while the erosion depths were about 380 μm and 650 μm for polyanhydrides with 2.5% and 7.5% PEG in the copolymers, respectively. There were evident intervening spaces between the eroded and un-eroded portions of all surface-eroded samples. For polyanhydride with 2.5% PEG, a small portion of the eroded polyanhydride was separated from the polyanhydride rod. As PEG content of the copolymer increased to 7.5%, virtually the entire eroded portion was detached from the un-eroded portion of the polyanhydride rod. The eroded surfaces were floppy and porous. The surface roughness increased with increasing PEG content of the polyanhydride.
Figure 2.
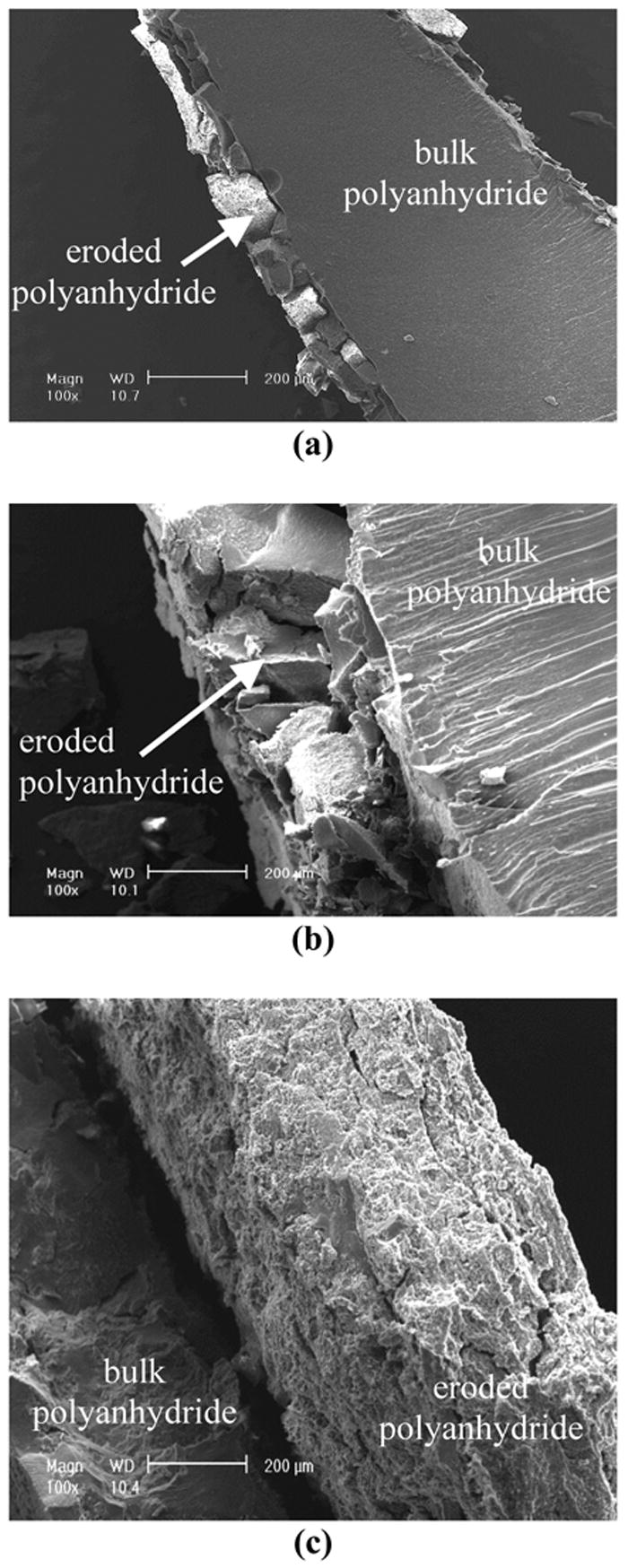
SEM micrographs of polyanhydrides specimens with different compositions after erosion in 0.1M PBS at 37°C for 24 h. (a) PEG/SA/CPP = 0/20/80; (b) PEG/SA/CPP = 2.5/20/80; (c) PEG/SA/CPP/= 7.5/20/80
Pulsatile protein release from the device
The pulsatile delivery device was fabricated using a reverse solid free form fabrication and layer-by-layer stacking techniques (Figure 1). The release profile of the laminate device was first investigated using BSA as a model protein. Figure 3 shows four well-defined pulses with an interval of 24 h between two adjacent pulses. The released amount of protein was almost the same for each pulse. The released amounts of protein between pulses were very low.
Figure 3.
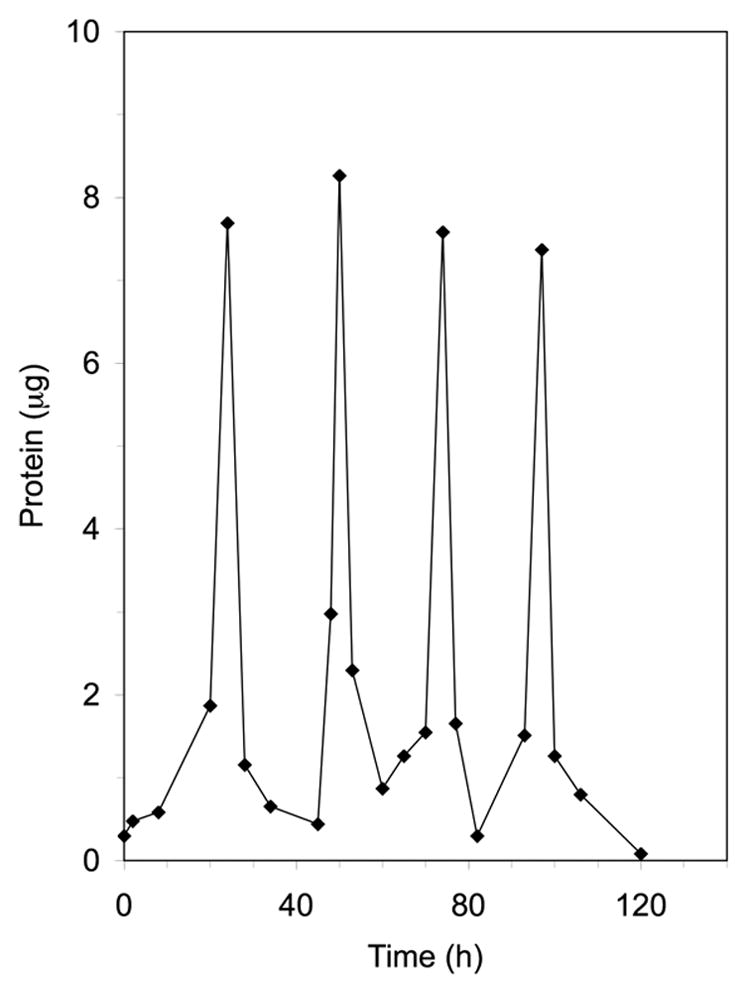
Pulsed release profile of BSA from the laminated device. Four layers of BSA were loaded into the device. The thickness of each polyanhydride layer (with PEG amount of 1.6% in the polyanhydride copolymer) was 230±20 μm (n=3).
The time interval between two pulses (lag time) could be easily controlled by the thickness of polyanhydride films, which acted as isolation layers (Figure 4). The lag time increased approximately linearly with the thickness of isolation layer. As showed in Figure 4, the lag time increased from 24 h to 40 h when the thickness of the polyanhydride layer (with 1.6% PEG in the polyanhydride) increased from 230 μm to 400 μm.
Figure 4.
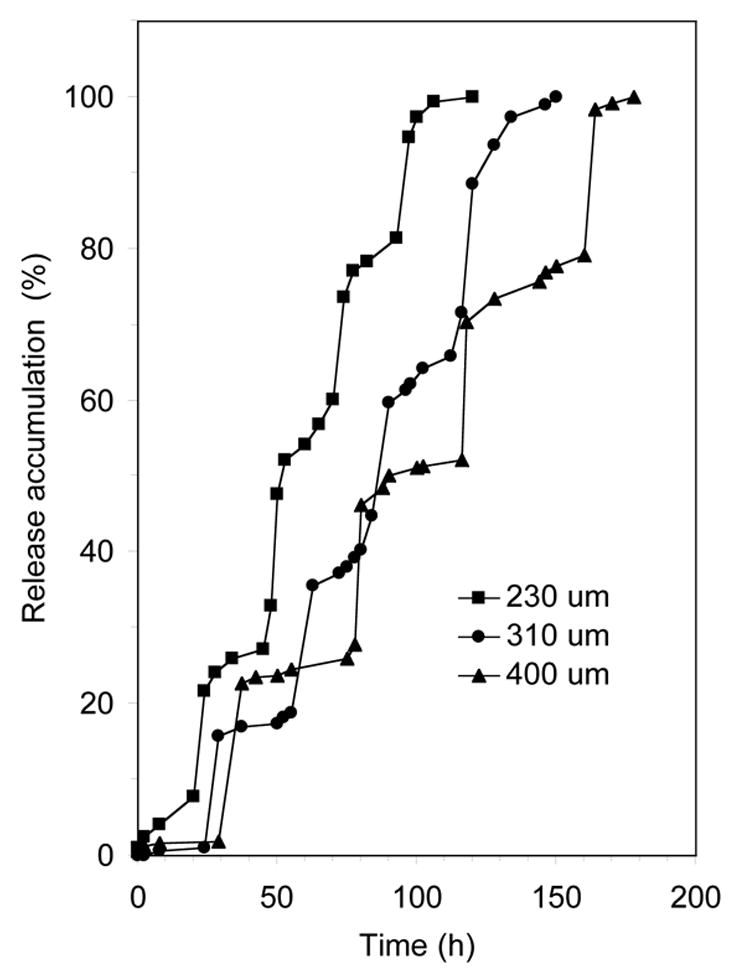
Release accumulation of BSA from laminated devices with different thickness of isolation layers. Four layers of BSA were loaded into each device (n=3).
The lag time could also be controlled by polyanhydride composition (Figure 5). Three drug layers were loaded into each release device with isolation layers of the same thickness but different PEG contents. Three pulses of BSA release were observed on each cumulative release curve. The lag times of BSA release were about 24 h and 32 h for the devices with PEG contents of 1.1% and 2.5%, respectively. As the PEG content was further increased to 7.5% and 10.0%, BSA was released much faster and the lag times were about 14 h and 12 h, respectively. At these two higher PEG contents, the pulses became less defined to nearly un-identifiable; BSA was released almost at constant rate when PEG content in the polyanhydride was 10.0%.
Figure 5.
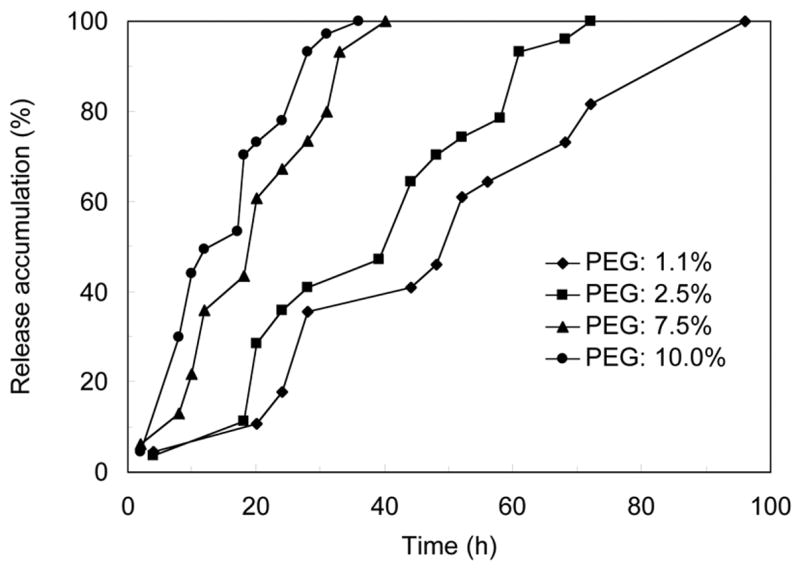
Cumulative release of BSA from laminated devices using polyanhydrides of different composition as isolation layer materials. Three layers of BSA were loaded into each device (n=3).
The release of different drugs at different time points was easily realized by using this implantable delivery system. In Figure 6, both PTH and BSA were loaded in the same device (the first two layers were loaded with PTH, and the third and fourth layers were loaded with BSA). As predicted, two pulses of PTH were first released between 0 and 50 h, followed by two pulses of BSA (protein) released between 50 h and 100 h. Because the level of BSA was more than two orders of magnitude higher than that of PTH, the protein assay actually reflected the BSA and not the PTH amounts. The released pulses appeared at about every 24 h as designed.
Figure 6.
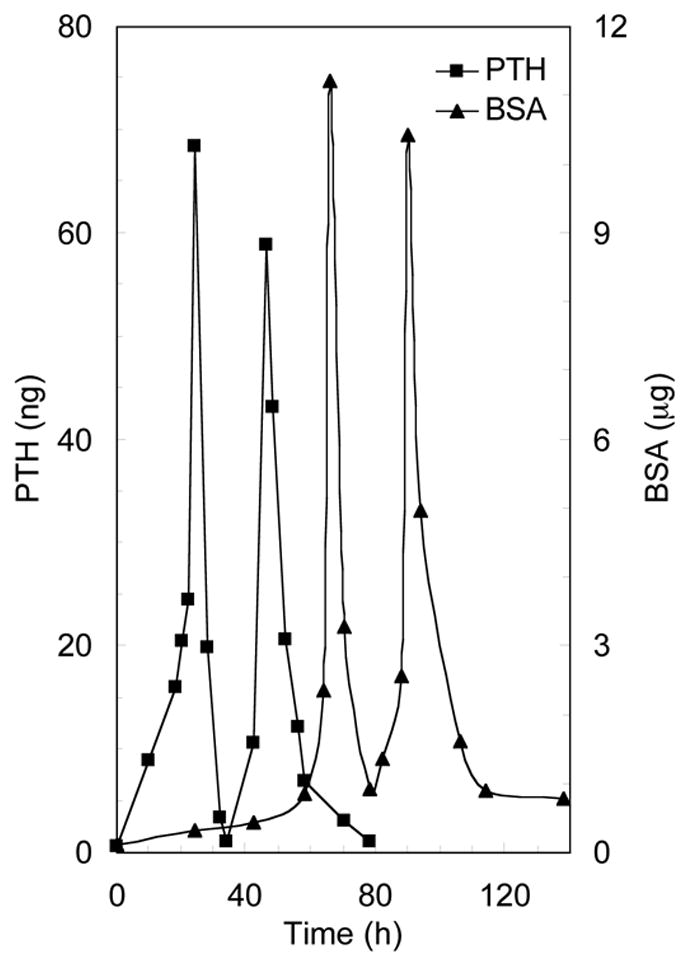
Pulsed release profiles of PTH and BSA from the laminated devices (n=3). In each device, the first and second drug layers were loaded with PTH, while the third and fourth drug layers were loaded with BSA. The average thickness of each polyanhydride layer (with PEG amount of 1.6% in the polyanhydride copolymer) was 230 μm.
In vitro PTH release and bioactivity
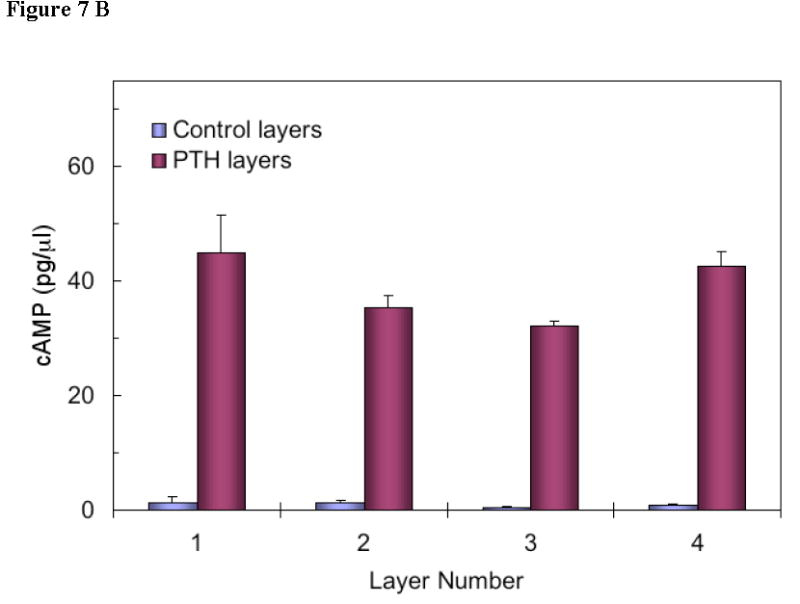
Multi-pulse release of PTH from the device was also examined. Figure 7a shows the amount of PTH released from each layer in the device (every pulse of release is associated with one PTH-containing layer). The bioactivity of PTH released from each layer was measured in vitro (Figure 7b). Based on the standard curve of PTH (positive control), the bioactivity of PTH released from the last layer was equivalent to 47.5 pg/μl PTH. Compared with the released amount of PTH from the last layer in Figure 7a, it was calculated that 90.9% PTH was bioactive after it was released from the device. Similar results of released PTH from other layers were obtained. Therefore, it was concluded that the released PTH from the delivery system retained high bioactivity.
Figure 7.
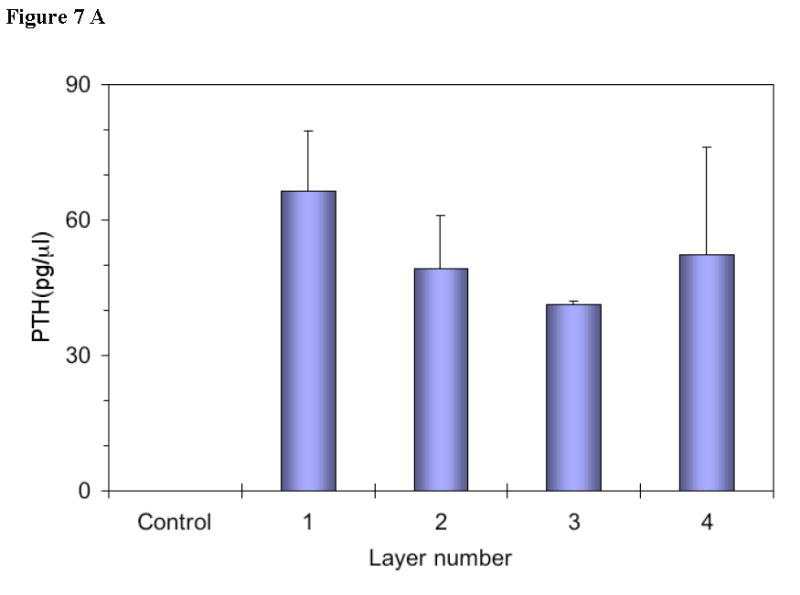
(a) PTH concentration in device eluent measured using PTH (1-34) ELISA kit with PTH antibody coated wells (n=3). Four layers of PTH were loaded into the device with each layer of PTH predicted to be released at every 24h. PTH devices were incubated in PBS at 37°C and the eluent was collected at 24h, 48h, 72h, and 96h, respectively (for bioactivity evaluation, not for release profiles). The concentration of PTH in the eluent of the control devices (no PTH loaded) was less than 1 pg/μl.
(b) Biological activity of eluent from devices as described in Figure 7. PTH receptor-mediated adenylate cyclase was stimulated by treating ROS (17/2.8) cells with known concentrations of PTH (1-34) or with device eluent (control or PTH) of different layers. The cAMP in the cells was extracted and a cAMP binding protein assay was performed to determine cAMP levels. All values are the mean for triplicate samples. Note the level of cAMP in all the control devices (no PTH loaded) was less than 2 pg/μl.
Discussion
PTH is well known for its catabolic action resulting in bone resorption and the increase of calcium concentration in the blood. Interestingly, PTH also stimulates bone formation with a net anabolic effect under certain conditions, which makes PTH a desirable therapeutic agent for the treatment of osteoporosis [1, 6, 8, 9]. The catabolic or anabolic action of PTH depends on the pattern of delivery: continuous exposure to PTH results in bone resorption, whereas intermittent administration (pulsatile release) of PTH increases bone formation. Therefore, the development of a pulsatile delivery system that can precisely modulate PTH release is critical for its successful therapeutic utility in the clinical treatment of osteoporosis, overcoming limitations of the current treatment regimen (daily injection). The objective of this work was to develop an implantable delivery system to achieve PTH pulsatile release. The computer-assisted design (CAD) and a reverse solid free form fabrication technique were utilized to aid the successful fabrication of such a device in this work. One of the advantages of using these techniques is that devices of various shapes and sizes can be accurately fabricated, ensuring repeatability. The novel design and fabrication techniques allow us to achieve the small device size in this study, and will allow us to further miniaturize such devices as needed.
The implantable delivery system consists of three components: drug layers, isolation layers and sealant filling. Alginate was used in the drug layers as a carrier for PTH or BSA because of its biocompatibility and suitable processing properties. A series of new polyanhydrides with surface erosion characteristics were synthesized and used as the materials of isolation-layers. PEG segments were incorporated into polyanhydride copolymers to modulate the erosion rate and to improve processing properties of the polyanhydride films. With increasing PEG content, the polyanhydride erosion rate increased. Our data shows that the polyanhydride with 1.6% PEG was excellent to achieve both suitable surface erosion rate and favorable processing properties. The structural tunability of such polyanhydrides will facilitate the accomplishment of a broad range of lag times and various device sizes. PLLA was selected as the sealant filling material and external frame material of the device because of its well-known biocompatibility and biodegradability as well as its favorable mechanical properties. Hence an advantage is that there is no need for retrieval of empty devices after the completion of drug release.
The main difficulty in successful fabrication of the delivery system is the prevention of device leakage. If the isolation layer or sealant filling has defective pores connecting or increasing diffusion between drug layers, body fluid (water) will penetrate inside the drug layer before the isolation layer is eroded, leading to unpredictable drug release patterns. Three measures were taken to prevent the device leakage in this work. First, the size of drug layers was designed to be smaller than that of isolation layers (Figure 1e), so that the contact area between drug layers and isolation layers near the outer edges (the area of a higher defective probability) decreased. Second, a sealant material was used to fill the gap between the device core and outer frame and possible defective pores. Third, the device was continually compressed with constant pressure during the period of sealing process to reduce the possibility of air pocket formation. Our results demonstrated the advantages of these leakage-prevention measures.
This implantable delivery system has been demonstrated to release drugs in multiple pulses (Figure 3). The release characteristics (lag time and release pattern) were tailored by the polyanhydride composition and the thickness of polyanhydride films. The lag time between two adjacent pulses changes approximately linearly with the thickness of polyanhydride isolation layer, which will provide great flexibility to tailor for specific clinical applications. The bioactivity assay also showed that PTH retains high biological activity after it is released from the delivery system.
This implantable delivery system possesses many advantageous characteristics. First, all three-layers of materials (alginate, polyanhydride and PLLA) of the device are biocompatible and biodegradable, thus the device could be implanted in vivo with minimum immune-reaction. Second, devices of various shapes and sizes could be accurately fabricated using the reverse solid free form fabrication technique. Third, this device system is able to deliver more than one drug from a single device. Fourth, the load of therapeutics can be easily tailored over a broad range in the drug layers. Last, such devices could be implanted in the vicinity of blood vessels to achieve systemic delivery or adjacent to defective tissues for local delivery.
Conclusions
An implantable delivery system to administer pulsatile release of PTH (and other therapeutics) has been developed in this work. Polyanhydrides with three components of SA, CPP and PEG were synthesized and used as isolation material in the delivery system. The delivery system was fabricated by using reverse solid free form fabrication and layer-by-layer stacking techniques. Typical multi-pulse release of BSA or PTH was demonstrated using this delivery system. The lag time between two adjacent pulses and the overall release pattern can be modulated by the polyanhydride composition and the thickness of polyanhydride films. Furthermore, the delivery of more than one protein from the same device with a pulsatile profile was demonstrated in this work. The released PTH from the delivery system retained high bioactivity, which implicates the potential of this system for release of biologically functional therapeutics in clinical applications.
Acknowledgments
We acknowledge grant support from NASA (NNC04AA21A, NASA Bioscience and Engineering Institute) and NIH (DE015384 & DE017689: PXM; DK053904: LKM). The authors thank Sijian Hou for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic Actions of Parathyroid-Hormone on Bone. Endocrine Reviews. 1993;14(6):690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- 2.Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, Hock JM. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33(3):372–379. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 3.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends in Endocrinology and Metabolism. 2004;15(2):60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. Journal of Clinical Investigation. 1999;104(4):439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnero P, SornayRendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. Journal of Bone and Mineral Research. 1996;11(3):337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 6.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. Journal of Bone and Mineral Research. 1999;14(6):960–968. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 7.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang OH, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. New England Journal of Medicine. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 8.Reeve J. PTH: A future role in the management of osteoporosis? Journal of Bone and Mineral Research. 1996;11(4):440–445. doi: 10.1002/jbmr.5650110404. [DOI] [PubMed] [Google Scholar]
- 9.Rosen CJ. What’s new with PTH in osteoporosis: where are we and where are we headed? Trends in Endocrinology and Metabolism. 2004;15(5):229–233. doi: 10.1016/j.tem.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Morley P, Whitfield JF, Willick GE. Parathyroid hormone: An anabolic treatment for osteoporosis. Current Pharmaceutical Design. 2001;7(8):671–687. doi: 10.2174/1381612013397780. [DOI] [PubMed] [Google Scholar]
- 11.Lane NE, Sanchez S, Modlin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid Hormone Treatment can reverse corticosteroid-induced osteoporosis - Results of a randomized controlled clinical trial. Journal of Clinical Investigation. 1998;102(8):1627–1633. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: A paired biopsy study. Journal of Bone and Mineral Research. 2001;16(10):1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 13.Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA, Goldstein SA, McCauley LK. Anabolic actions of PTH (1-34): Use of a novel tissue engineering model to investigate temporal effects on bone. Bone. 2005;36(6):959–970. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Langer R. New Methods of Drug Delivery. Science. 1990;249(4976):1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Advanced Drug Delivery Reviews. 2002;54(1):53–77. doi: 10.1016/s0169-409x(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 16.Bussemer T, Dashevsky A, Bodmeier R. A pulsatile drug delivery system based on rupturable coated hard gelatin capsules. Journal of Controlled Release. 2003;93(3):331–339. doi: 10.1016/j.jconrel.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Iskakov RM, Kikuchi A, Okano T. Time-programmed pulsatile release of dextran from calcium-alginate gel beads coated with carboxy-n-propylacrylamide copolymers. Journal of Controlled Release. 2002;80(1–3):57–68. doi: 10.1016/s0168-3659(01)00551-x. [DOI] [PubMed] [Google Scholar]
- 18.Jimoh AG, Wise DL, Gresser JD, Trantolo DJ. Pulsed Fsh Release from an Implantable Capsule System. Journal of Controlled Release. 1995;34(2):87–95. [Google Scholar]
- 19.Jiang HL, Zhu KJ. Pulsatile protein release from a laminated device comprising of polyanhydrides and pH-sensitive complexes. International Journal of Pharmaceutics. 2000;194(1):51–60. doi: 10.1016/s0378-5173(99)00336-1. [DOI] [PubMed] [Google Scholar]
- 20.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Advanced Drug Delivery Reviews. 1998;31(3):197–221. doi: 10.1016/s0169-409x(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 21.Bae YH, Okano T, Hsu R, Kim SW. Thermosensitive Polymers as On-Off Switches for Drug Release. Makromolekulare Chemie-Rapid Communications. 1987;8(10):481–485. [Google Scholar]
- 22.Dong LC, Hoffman AS. Synthesis and application of thermally reversible heterogels for drug delivery. Journal of Controlled Release. 1990;13(1):21–31. [Google Scholar]
- 23.Dong LC, Hoffman AS. A novel approach for preparation of pH-sensitive hydrogels for enteric drug delivery. Journal of Controlled Release. 1991;15(2):141–152. [Google Scholar]
- 24.Siegel RA, Falamarzian M, Firestone BA, Moxley BC. PH-controlled release from hydrophobic poly-electrolyte copolymer hydrogels. Journal of Controlled Release. 1988;8(2):179–182. [Google Scholar]
- 25.Mathiowitz E, Cohen MD. Polyamide Microcapsules for Controlled Release.5. Photochemical Release. Journal of Membrane Science. 1989;40(1):67–86. [Google Scholar]
- 26.Fischelghodsian F, Brown L, Mathiowitz E, Brandenburg D, Langer R. Enzymatically Controlled Drug Delivery. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(7):2403–2406. doi: 10.1073/pnas.85.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podual K, Doyle FJ, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. Journal of Controlled Release. 2000;67(1):9–17. doi: 10.1016/s0168-3659(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 28.Kwon IC, Bae YH, Kim SW. Electrically Erodible Polymer Gel for Controlled Release of Drugs. Nature. 1991;354(6351):291–293. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 29.Santini JT, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 30.Edelman ER, Kost J, Bobeck H, Langer R. Regulation of Drug Release from Polymer Matrices by Oscillating Magnetic-Fields. Journal of Biomedical Materials Research. 1985;19(1):67–83. doi: 10.1002/jbm.820190107. [DOI] [PubMed] [Google Scholar]
- 31.Wuthrich P, Ng SY, Fritzinger BK, Roskos KV, Heller J. Pulsatile and Delayed Release of Lysozyme from Ointment-Like Poly(Ortho Esters) Journal of Controlled Release. 1992;21(1–3):191–200. [Google Scholar]
- 32.Krogel I, Bodmeier R. Pulsatile drug release from an insoluble capsule body controlled by an erodible plug. Pharmaceutical Research. 1998;15(3):474–481. doi: 10.1023/a:1011940718534. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi A, Kawabuchi M, Sugihara M, Sakurai Y, Okano T. Pulsed dextran release from calcium-alginate gel beads. Journal of Controlled Release. 1997;47(1):21–29. doi: 10.1016/s0168-3659(98)00141-2. [DOI] [PubMed] [Google Scholar]
- 34.Krogel I, Bodmeier R. Floating or pulsatile drug delivery systems based on coated effervescent cores. International Journal of Pharmaceutics. 1999;187(2):175–184. doi: 10.1016/s0378-5173(99)00189-1. [DOI] [PubMed] [Google Scholar]
- 35.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nature Materials. 2003;2(11):767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 36.Hou SJ, McCauley LK, Ma PX. Synthesis and erosion properties of PEG-containing polyanhydrides. Macromolecule bioscience. 2007 doi: 10.1002/mabi.200600256. [DOI] [PubMed] [Google Scholar]
- 37.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27(21):3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 38.Wei GB, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25(2):345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 39.Chen HL, McCauley LK, D’Silva NJ. cAMP binding protein assay for widespread use in cell signaling studies. Biotechniques. 2002;33(1):66–68. 70, 72. doi: 10.2144/02331st03. [DOI] [PubMed] [Google Scholar]


