Abstract
It is advantageous to incorporate controlled growth factor delivery into tissue engineering strategies. The objective of this study was to develop a three-dimensional (3D) porous tissue engineering scaffold with the capability of controlled releasing recombinant human bone morphogenetic protein-7 (rhBMP-7) for enhancement of bone regeneration. RhBMP-7 was first encapsulated into poly(lactic-co-glycolic acid) (PLGA) nanospheres (NS) with an average diameter of 300 nm. Poly(L-lactic acid) (PLLA) scaffolds with interconnected macroporous and nano-fibrous architectures were prepared using a combined sugar sphere template leaching and phase separation technique. A post-seeding technique was then utilized to immobilize rhBMP-7 containing PLGA nanospheres onto prefabricated nano-fibrous PLLA scaffolds with well maintained 3D structures. In vitro release kinetics indicated that nanosphere immobilized scaffold (NS-scaffold) could release rhBMP-7 in a temporally controlled manner, depending on the chemical and degradation properties of the nanospheres which were immobilized onto the scaffold. In vivo, rhBMP-7 delivered from NS-scaffolds induced significant ectopic bone formation throughout the scaffold while passive adsorption of rhBMP-7 into the scaffold resulted in failure of bone induction due to either the loss of rhBMP-7 biological function or insufficient duration within the scaffold. We conclude that the interconnected macroporous architecture and the sustained, prolonged delivery of bioactive rhBMP-7 from NS immobilized nano-fibrous scaffolds actively induced new bone formation throughout the scaffold. The approach offers a new delivery method of BMPs and a novel scaffold design for bone regeneration.
1. Introduction
Bone morphogenetic proteins (BMPs) play an essential role in bone development and regeneration [1, 2]. They have been demonstrated to elicit new bone formation both at orthotopic and ectopic sites in experimental animal models [3–7]. Because of their osteogenic potentials [8], recombinant BMPs hold great promise for healing bone fractures, bridging bone nonunions, preventing osteoporosis, and treating periodontal defects. The use of BMP-7 (also known as osteogenic protein 1, OP-1) promotes periodontal regeneration, not only for osteogenesis, but also for cementogenesis and periodontal ligament reconstruction [9–11]. With the validation of the efficacy and safety for bone repair [12–15], recombinant human BMP-2 and 7 (rhBMP-2 and rhBMP-7) have recently obtained FDA approval for human clinical applications to stimulate spinal fusion (InFUSE®Bone Graft) and repair nonunion long bone defect (OP-1™ Implant), respectively.
Unfortunately, exogenous administration of BMPs in buffer solution does not always insure satisfactory new bone induction, especially in higher mammals [16, 17]. The major reasons are the rapid diffusion of BMPs away from application site and the loss of bioactivity, which lead to insufficient local induction and hence incomplete or failure of bone regeneration. Consequently, it is highly desired to develop an appropriate BMP delivery system to enable a prolonged duration, to reduce BMP proteolysis, denaturation and bioactivity loss and thereby to accelerate tissue healing and regeneration [18–20]. Delivery of BMPs from collagen matrices, while successful in preclinical and human clinical trials [15, 21, 22], demonstrate a number of disadvantages. It is still difficult to retain the BMPs formulated in a collagen matrix for a sufficient duration, which may explain the great loading and response variability in vivo [23]. In addition, the biodegradability and 3D structures of collagen matrix are difficult to control. Since BMPs are physically entrapped within collagen [24, 25], the capability of control over release kinetics from collagen matrix was limited and therefore collagen may not be appropriate for applications where varying release rate is needed. In addition, there are concerns over collagen in terms of immunogenicity and disease transmission [26, 27].
BMPs have also been delivered from porous hydroxyapatite (HAP) [28, 29], poly(lactide) (PLLA) [7, 30], poly(lactic-co-glycolic acid) (PLGA) [31, 32] for bone regeneration. However, these carriers have achieved no better success than collagen matrix and none of them has gained acceptance for human clinical investigation. Therefore, it remains a challenge to design an effective tissue engineering scaffold that adequately immobilizes BMPs, temporally and spatially controls release, presents interconnected porosity for vascularization and new bone induction, and ultimately degrades without soliciting unexpected side effects.
Previously, we have encapsulated bioactive polypeptides and proteins into biodegradable nanospheres for controlled delivery. Recombinant human parathyroid hormone (rhPTH) and platelet-derived growth factor (rhPDGF-BB) were successfully delivered from PLGA nanospheres in a well controlled pattern and were demonstrated bioactive to stimulate cellular activity [33, 34]. Notably, we have immobilized rhPDGF containing PLGA nanospheres onto a 3D PLLA scaffold from which rhPDGF was locally released with adjustable rates [34]. In this paper, we examined the release kinetics of rhBMP-7 from three types of PLGA nanospheres immobilized on a nano-fibrous PLLA scaffold in vitro and ectopic bone formation in vivo. The rhBMP-7 nanosphere incorporated scaffold offers both osteoinductivity and osteoconductivity for bone tissue engineering.
2. Materials and Methods
2.1 Materials
Recombinant human bone morphogenetic protein (rhBMP-7) was kindly provided by Stryker Biotech (Hopkinton, MA). Iodination of rhBMP-7 (125I-rhBMP-7) was carried out in the Assays and Reagent Facility (Department of Epidemiology) at the University of Michigan. Poly(lactic-co-glycolic acid) (PLGA) copolymers with LA/GA ratio of 50:50 (Medisorb®, PLGA50–6.5K, Mw=6.5 kDa; PLGA50–64K, Mw=64 kDa) and 75:25 (Medisorb®, PLGA75–113K, Mw=113 kDa) were purchased from Alkermes Inc. (Wilmington, OH). Poly(L-lactic acid) (PLLA) with inherent viscosity of 1.6 dl/g was purchased from Boehringer Ingelheim (Ingelheim, Germany). Other chemicals used were: poly(vinyl alcohol) (PVA) (88 mol% hydrolyzed, Mw=25,000) obtained from Polysciences Inc. (Warrington, PA); Trifluoroacetic acid (TFA), bovine serum albumin (BSA, Fraction V) and gelatin (type B from bovine skin) from Sigma (St. Louis, MO); dichloromethane, cyclohexane, hexane and tetrahydrofuran from Aldrich Chemical Company (Milwaukee, WI).
2.2 Preparation of nanosphere-immobilized nano-fibrous scaffolds (NS-scaffold)
Lyophilized rhBMP-7 powder was dissolved in 0.1% TFA with 0.1 wt% gelatin and BSA to form a clear aqueous solution. Three PLGA formulations: PLGA50–6.5K, PLGA50–64K and PLGA75–113K were used to encapsulate rhBMP-7 into nanospheres (NS) utilizing a double emulsion technique as described previously [33, 34]. For release kinetics evaluation, radio-labeled I125-rhBMP-7 was added during nanosphere preparation as a tracer (I125-rhBMP-7: unlabeled rhBMP-7=1:100, total 100 ng rhBMP-7 per mg polymer). RhBMP-7 (5 μg/mg polymer) was encapsulated into PLGA50–64K nanospheres for in vivo study. Gelatin/BSA-containing PLGA nanospheres (blank NS) were prepared as controls. Macroporous and nano-fibrous PLLA scaffolds were fabricated by the combination of phase separation and sugar-leaching techniques [35]. Highly porous scaffolds were cut into circular disks with dimensions of 7.2 mm in diameter and 2 mm in thickness. The scaffolds were sterilized using ethylene oxide (following the manufacturer’s protocol) for 24 h before the BMP nanospheres were immobilized.
PLGA nanospheres were immobilized onto nano-fibrous PLLA scaffolds using a post-seeding method [34]. Briefly, PLGA nanosphere suspension was seeded onto the prefabricated nano-fibrous PLLA scaffold and the scaffold was left in air to evaporate the solvent followed by vacuum drying. The protein amount in a scaffold was modulated by the concentration of protein encapsulated in NS and/or the amount of nanospheres incorporated into the scaffold. The NS-scaffolds were then subjected to a mixed solvent of hexane/THF (volume ratio of 90/10) to immobilize the nanospheres on the scaffold followed by vacuum-drying for 3 days to remove the solvent. Nanospheres containing gelatin/BSA were also immobilized onto scaffolds for morphological examination and as controls for release kinetics and animal implantation studies. The morphology of the scaffolds before and after nanosphere immobilization was examined using scanning electron microscopy (SEM, Philips XL30 FEG).
2.3 In vitro release study
RhBMP-7 release profiles from PLGA nanosphere-immobilized PLLA scaffolds were determined in vitro by radioactivity detection. One NS-scaffold was placed in 1.0 ml phosphate buffered saline (PBS, 10mM, pH=7.4 with 0.1% BSA) at 37 °C under orbital shaking at 60 rpm. Supernatant was collected and equal amount of fresh medium was added to each sample at the designated time points: 1, 3, 5, 7, 10, 14, 21, 28, 35, 42, 49, 56 days for PLGA50–6.5K NS-scaffolds; 1, 3, 5, 7, 10, 14, 21, 28, 35, 42, 49, 56, 63, 70 days for PLGA50–64K NS-scaffolds; and 1, 3, 7, 14, 21, 28, 35, 42, 49, 56, 63, 70 days for PLGA75–113K NS-scaffolds. The radioactivity of collected supernatant was analyzed using a gamma counter (Gamma 5500, Beckman) and converted to calculate the quantity of the released rhBMP-7. Scaffolds with gelatin/BSA containing nanospheres were used as controls.
2.4 Preparation of implants
Three groups of scaffold implants were prepared for in vivo study on rats as listed in Table 1. Group I (Control scaffolds): PLLA scaffold with PLGA50–64K nanospheres containing gelatin/BSA; Group II (rhBMP-7 adsorbed scaffolds): sterilized PLLA scaffold with PLGA50–64K nanospheres containing gelatin/BSA, 40 μl rhBMP-7 buffer solution (sterile, 5 μg rhBMP-7) were added to the scaffold and air dried at 4 °C; Group III (rhBMP-7 NS-scaffolds): PLLA scaffold with PLGA50–64K nanospheres containing 5 μg rhBMP-7. The scaffolds (Groups I and III) were sterilized in 70% ethanol for 30 minutes, lyophilized under sterile conditions and stored at −20 °C until implantation.
Table 1.
Experiment design of BMP-delivering PLLA scaffolds for ectopic bone induction
| Scaffold group | BMP-7 (μg) | PLGA50-NS |
|---|---|---|
| I | 0 | 64K, with BSA encapsulated |
| II | 5 in buffer* | 64K, with BSA encapsulated |
| III | 5 in NS | 64K, with rhBMP-7 encapsulated |
Group I (control scaffold): PLLA scaffold with PLGA50–64K nanospheres containing gelatin/BSA;
Group II (rhBMP-7 adsorbed scaffold): PLLA scaffold with PLGA50–64K nanospheres containing gelatin/BSA, to which 40 ml rhBMP-7 buffer solution (5 mg rhBMP-7) was added and then air dried at 4 ° C;
Group III (rhBMP-7 NS-scaffold): PLLA scaffold with PLGA50–64K nanospheres containing gelatin/BSA and 5 mg rhBMP-7).
Three scaffold samples per group were implanted (n=3).
10 mM sodium acetate/acetic acid buffer, pH=4.5
2.5 Subcutaneous implantation
For implantation, male Sprague–Dawley rats with a weight range of 200–250 grams (Charles River Laboratories) were used in the study. Surgery was performed under general inhalation anesthesia with isofluorane. The back of the animals was shaved, washed and disinfected with povidone-iodine. Two midsagittal incisions were made on the dorsa and four subcutaneous pockets were created using blunt dissection. One scaffold was implanted subcutaneously into each pocket. Three samples were implanted for each group (n=3). After placement of scaffolds, the incisions were closed with staples. The scaffolds were placed alternately at different sites for each rat. At the end of each implantation period (3 or 6 weeks), the rats were sacrificed and the scaffolds were harvested. The animal procedures were performed according to the guidelines approved by the University of Michigan Committee of Use and Care of Laboratory Animals.
2.6 Radiographic and histological examination
The scaffolds were retrieved at 3 and 6 weeks after subcutaneous implantation. Radiographic analysis was performed for scaffolds using a microradiography system (Faxitron X-ray Corporation, Wheeling, IL) at conditions of 35 kV and 60 seconds. The percent radiopacity of scaffold specimen were measured using ImageJ software (NIH, Bethesda, MD) based on discrimination of gray-level density. In addition, the scaffold specimen was fixed in neutral buffered 10% formalin and then embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin (H & E) or von Kossa for light microscopic observation as previously described [10, 36].
3. Results
3.1 Characterization of BMP-7 nanosphere-immobilized scaffolds
RhBMP-7 encapsulated PLGA nanospheres had high encapsulation efficiency of 78–81% as determined using radioactivity detection. Despite the evaluation of three different PLGA formulations (PLGA50–6.5K, PLGA50–64K and PLGA75–113K) used, the nanospheres presented uniform spherical shapes and smooth non-porous surfaces (Fig. 1 A). The average diameter of the nanospheres was near 300 nm based on SEM observation.
Figure 1.

Characterization of PLGA50–64K nanospheres (NS) and nanosphere incorporated PLLA nano-fibrous scaffolds (NS-scaffolds). (A) Scanning electron micrograph of rhBMP-7 containing PLGA50–64K nanospheres; (B) Macroscopic photographs of PLLA scaffolds before (left) and after (right) nanosphere incorporation; (C, D) Scanning electron micrographs of PLLA nano-fibrous scaffolds before nanosphere incorporation at 100x (C) and 10,000x (D); (E, F) Scanning electron micrographs of PLLA nano-fibrous scaffolds after PLGA50–64K nanosphere incorporation at 100x (E) and 10,000x (F).
Three-dimensional PLLA macroporous and nano-fibrous scaffolds were prepared with a high porosity of 98%. The scaffold was characterized with 3D multi-level porous architectures: regular spherical macropores in diameter of 250–425 μm, interpore openings of ~100 μm, and nano fibers within the pore walls of the scaffold. Furthermore, the diameter of PLLA nano fibers ranged from 50 to 500 nm which is similar to type I collagen fibers in size (Fig. 1 B–D). The macropores were well interconnected from macro-, micro- to nano levels, which not only were important to be conducive for the cellular activity and tissue penetration into the scaffold, but also allowed for subsequent efficient incorporation of growth factor containing nanospheres.
The morphology of nano-fibrous PLLA scaffolds after PLGA50–64K nanosphere incorporation is shown in Figure 1 (E&F). Compared to original PLLA scaffolds before NS incorporation, the spherical macropores (250–425 μm) and interpore openings (~ 100 μm) of the scaffolds were well-preserved. The pore walls of the scaffolds presented both nano fibers and nanospheres which adhered on nano-fibers and were distributed uniformly throughout the scaffolds.
3.2 In vitro BMP-7 release kinetics
Figure 2 shows the rhBMP-7 release profiles from NS-scaffolds in varying temporal patterns because of different PLGA nanosphere immobilization. RhBMP-7 was rapidly released from the PLGA50–6.5K NS-scaffold with a 48% initial burst release in day 1 and 78% of rhBMP-7 released within 2 weeks. PLGA50–64K NS-scaffolds released rhBMP-7 in a multi-phasic release pattern. After an initial burst release of 23% and fast release (1.5% per day) during the first two weeks, rhBMP-7 was subsequently released more slowly (0.3% per day) during the next 3 weeks. From week 5 to week 8, there was a second rapid release of 20% rhBMP-7 (0.98% per day) followed by another slow release. The second rapid release was in accordance with the significant mass loss and disintegration of PLGA nanospheres at that time (data not shown). PLGA75 NS-scaffold showed a sustained slow release (0.13% per day) for the whole time period investigated after the initial burst release (9.5%). With the increase of molecular weight and/or LA/GA ratio in PLGA copolymer NS, the initial burst release was reduced significantly. By comparing the release profiles of rhBMP-7 from NS-scaffold with those from nanospheres alone, it was found that the overall release patterns were similar in trend (data not shown). These results indicated that the release kinetics of protein from a scaffold is mainly controlled by the release from nanospheres, which can be controlled by tailoring the chemical and physical properties of copolymers.
Figure 2.

In vitro release kinetics of rhBMP-7 from nanospheres immobilized on nano-fibrous scaffolds: In 10 mM PBS with a BMP-7 loading of 200 ng/scaffold. Each data point represents a mean ± standard deviation (n=3).
3.3 Radiographic Analysis
Three weeks after subcutaneous implantation, radiographic examination showed increased radiodensity in the rhBMP-7 NS immobilized scaffolds, but not in control and rhBMP-7 adsorbed scaffolds. This indicates that ectopic bone formation was induced by rhBMP-7 released from PLGA nanospheres which were immobilized within the porous scaffolds, as shown in Figure 3A. At 6 weeks, increased radiopacity consistent with newly formed bone was noted throughout the rhBMP-7 NS-scaffolds, while bone formation was scant to non-existent in control and BMP adsorbed scaffolds. The radiopacity in rhBMP-7 NS-scaffolds was significantly higher than that formed in control and rhBMP-7 adsorbed scaffolds (Fig. 3B).
Figure 3.

Radiographic results of retrieved scaffold samples: (A,B) 3 weeks and (C,D) 6 weeks after implantation. In Figures A & C, I: control scaffolds; II: scaffolds with 5 μg adsorbed rhBMP-7; and III: scaffolds with PLGA50–64K nanospheres containing 5 μg rhBMP-7. In Figures B & D, Each data point represents a mean ± standard deviation (n=3). *, p<0.05; **, p<0.01.
3.4 Histologic Analysis
Although cells and tissues penetrated throughout the scaffolds for all groups (A–C) at three weeks, the histological micrographs showed no bone formation in control and rhBMP-7 adsorbed scaffolds (Fig. 4 A–D). These cells were mainly fibroblasts and some multinucleated giant cells. In contrast, new bone consistently formed on the surface of BMP-7 nanosphere immobilized scaffolds (Fig. 4 E&F). The neo-bone was stained pink with H & E staining. After 6 weeks, no bone formation was observed in control scaffolds, and macrophages accumulated around the polymer matrix (nano-fibrous pore walls) (Fig. 5 A&B). RhBMP-7 adsorbed scaffolds also failed to induce significant ectopic bone formation (Fig. 5 C&D). However, robust bone formation was achieved throughout the rhBMP-7 NS-scaffolds and the presence of macrophages was minimal (Fig. 5 E&F). The newly formed bone in rhBMP-7 NS-scaffolds was mineralized as noted by von Kossa staining (Fig. 6 E,F). The results suggest that incorporation of rhBMP-7 into nanospheres which were then immobilized onto scaffolds protected the biological activity of rhBMP-7 and delivered locally with prolonged duration to induce ectopic bone formation throughout the scaffold. In contrast, simple adsorption of rhBMP-7 onto the scaffolds failed to induce bone formation in the scaffolds likely due to significant loss of the biological activity of the BMP-7.
Figure 4.
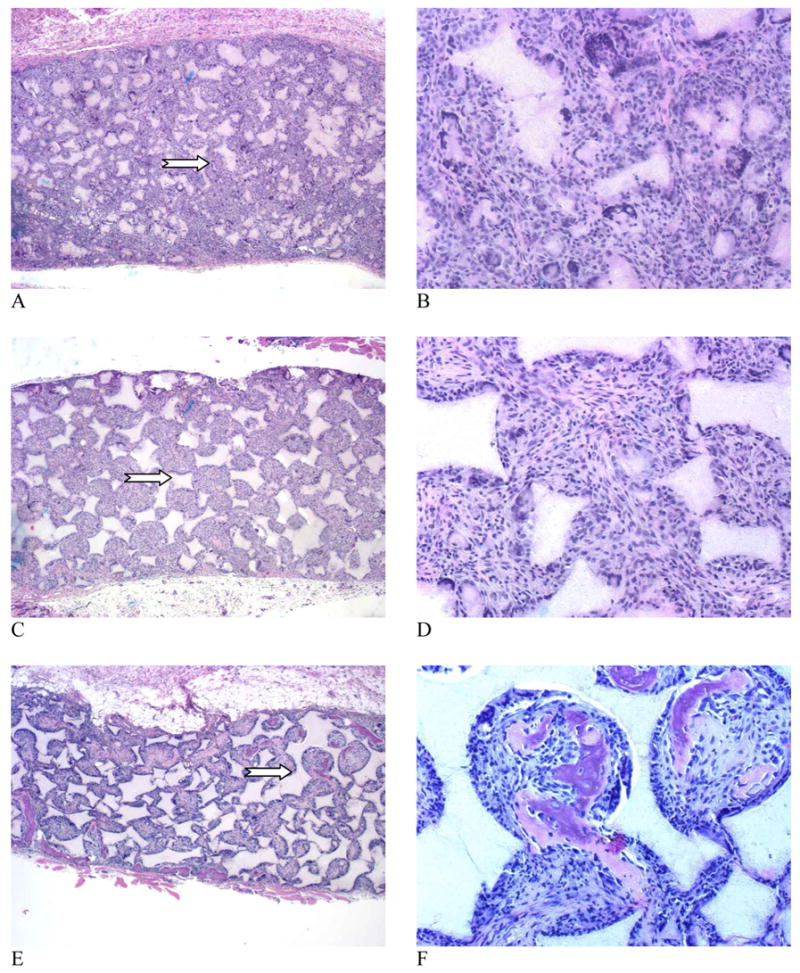
Microscopic observations of the H & E stained tissue sections of scaffolds retrieved 3 weeks after implantation. (A, B) Control scaffold; (C, D) 5 μg rhBMP-7 adsorbed to scaffold; (E, F) 5 μg rhBMP-7 incorporated in NS-scaffold. Original magnifications: (A, C, E) 40x for full cross sections, and (B, D, F) 200x for high magnification views of selected representative areas (arrows point to the selected areas in A, C, and E).
Figure 5.
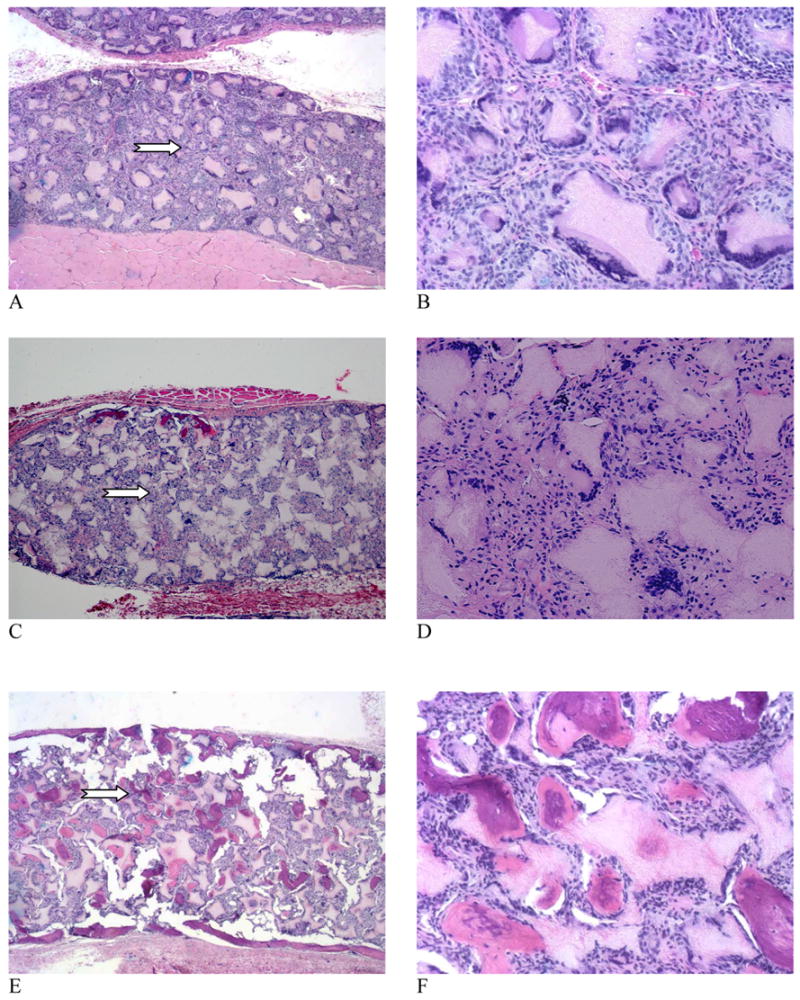
Microscopic observations of the H & E stained tissue sections of scaffolds retrieved 6 weeks after implantation. (A, B) Control scaffold; (C, D) 5 μg rhBMP-7 adsorbed to scaffold; (E, F) 5 μg rhBMP-7 incorporated in NS-scaffold. Original magnifications: (A, C, E) 40x full cross sections, and (B, D, F) 200x for high magnification views of selected representative areas (arrows point to the selected areas in A, C, and E). Note: In order to conduct von Kossa staining (Figure 6), the engineered tissue samples were not decalcified and the sectioning resulted in some artifacts, which appeared in the H&E histology (multiple cracks in the highly mineralized constructs, i.e., E & F).
Figure 6.

Microscopic observations of the von Kossa stained tissue sections of scaffolds retrieved 6 weeks after implantation. (A, B) Control scaffold; (C, D) 5 μg rhBMP-7 adsorbed to scaffold; (E, F) 5 μg rhBMP-7 incorporated in NS-scaffold. Original magnifications: (A, C, E) 40x and (B, D, F) 100x (arrows point to the selected areas in A, C, and E).
4. Discussion
In the experimental studies that validate the effectiveness of BMPs for the stimulation of bone formation, the need of an efficient delivery system is recognized [16, 21]. The properties of carriers, including material type, geometry, porosity, and pore size, are critical determinants both for the delivery of BMPs and the subsequent success of bone regeneration [37–39]. In bone tissue engineering, the ideal delivery system should serve two primary roles: as a delivery carrier to maximize the osteogenic effect of BMPs by maintaining the bioactivity and duration of BMPs at implantation site with an optimal release profile; and as an osteoconductive scaffold with suitable pore structure for vascularization and bone formation. In the present study, the nanosphere immobilized scaffold was designed to serve these two functions. RhBMP-7 containing PLGA nanospheres were successfully immobilized onto 3D macroporous and nano-fibrous PLLA scaffolds using a post-seeding technique. By varying the composition and molecular weight of PLGA nanospheres which were immobilized onto the scaffold, rhBMP-7 release times from weeks to months were achieved from the 3D porous tissue engineering scaffold. The rhBMP-7 delivering NS-scaffold has been demonstrated to induce ectopic bone formation throughout the scaffold after subcutaneous implantation in rats. This new system offers several advantages over other scaffold delivery systems. First, both PLGA and PLLA used are biodegradable and biocompatible polymers that have been widely used for biomedical applications with minimal immunogenicity. Second, PLGA copolymers are commercially available with a variety of LA/GA ratios, molecular weights and end groups, which offers great flexibility to adjust release kinetics of encapsulated growth factors from a scaffold. The NS-scaffold system can provide varying BMP release rates to satisfy the needs of bone healing and regeneration at different sites and under different conditions. Third, the porous scaffold provides a suitable microenvironment for cellular activity and tissue formation. After immobilization with rhBMP-7 PLGA nanospheres, the NS-scaffolds retain high porosity (98%) and well-interconnected macroporous structures (Fig. 1 B&E). The maintenance of interconnected macroporosity provides sufficient spaces for rhBMP-7 induced new bone formation throughout the scaffold (Fig. 5&6, E,F). Fourth, the pore walls are nano-fibrous. In our previous studies, the nano-fibrous structures have been demonstrated to improve bone cell attachment and differentiation [40–42] due to the structural similarity to type I collagen fibers which is a major extracellular matrix (ECM) component of natural bone. In addition, PLLA macroporous and nano-fibrous scaffolds allow uniform bone-like apatite growth in simulated body fluid (SBF), which may provide superior osteoconductivity for bone repair [35].
Because of interconnected porous structures, it was observed that cells and tissues penetrated into all control, rhBMP-7 adsorbed, and rhBMP-7 NS immobilized scaffolds 3 weeks post subcutaneous implantation (Fig. 3&4). There was substantial de novo bone formation in rhBMP-7 NS-scaffold while there was little bone formation in control and rhBMP-7 adsorbed scaffolds. The rhBMP-7 NS-scaffold delivery system released and localized the rhBMP-7 for a desired duration at the implantation site, ensuring the differentiation of invaded cells into osteoblasts for bone formation. It was confirmed in our study that not only the initial level of rhBMP-7 but also the sustained local release of rhBMP-7 was important for achieving adequate bone induction and mineralization. RhBMP-7 adsorbed NS-scaffold provided either a bolus or a pulse delivery of rhBMP-7 with substantial loss of bioactivity, leading to the failure of bone formation. In contrast, nanosphere incorporation protected bioactive growth factors from denaturation which has commonly occured in passive adsorption of growth factors onto biodegradable scaffolds/implants due to conformational changes or degradation under physiological environment [43, 44]. Encapsulation of growth factors into nanospheres which are subsequently immobilized onto scaffolds, has been demonstrated to be a unique and successful strategy to achieve prolonged release of bioactive growth factors from scaffolds for tissue engineering applications.
The technique developed in this present work is an effective method to control the delivery of bioactive factors from a 3D porous scaffold. It is a versatile approach and can be expanded to many other growth factors, cytokines and bioactive molecules. The combination of controlled growth factor delivery and 3D biomimetic scaffold design provides a new strategy for tissue engineering.
5. Conclusions
We have developed a rhBMP-7 delivering nano-fibrous scaffold from which rhBMP-7 was released in a temporally controlled fashion with resultant biological functions to induce bone formation at ectopic sites. Both the highly interconnected porous structure of the scaffold and the sustained release of bioactive rhBMP-7 appear to be important for inducing ectopic bone formation throughout the scaffold. The NS-scaffold can be used as a delivery system for multiple bioactive factors or as inductive tissue engineering scaffold for various tissue regeneration applications.
Acknowledgments
This study was supported by NIH/NIDCR grants DE15384 & DE14755 (MPX), and DE13397 (WVG). The authors thank Stryker Biotech for kindly supplying the rhBMP-7. The authors also wish to thank Dr. David Rueger for critically reviewing the manuscript and his valuable input before submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urist MR. Bone - Formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Groeneveld EHJ, Burger EH. Bone morphogenetic proteins in human bone regeneration. European Journal of Endocrinology. 2000;142(1):9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 3.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clinical Orthopaedics and Related Research. 1998;(346):26–37. [PubMed] [Google Scholar]
- 4.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22(7):669–671. [PubMed] [Google Scholar]
- 5.Ripamonti U, VandenHeever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13(3–4):273. doi: 10.3109/08977199609003228. [DOI] [PubMed] [Google Scholar]
- 6.Welch RD, Jones AL, Bucholz RW, Reinert CM, Tjia JS, Pierce WA, Wozney JM, Li XJ. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goal tibial fracture model. Journal of Bone and Mineral Research. 1998;13(9):1483–1490. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 7.Zegzula HD, Buck DC, Brekke J, Wozney JM, Hollinger JO. Bone formation with use of rhBMP-2 - (Recombinant human bone morphogenetic protein-2) Journal of Bone and Joint Surgery-American Volume. 1997;79A(12):1778–1790. doi: 10.2106/00004623-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Wang EA, Rosen V, Dalessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, Lapan P, Luxenberg DP, Mcquaid D, Moutsatsos IK, Nove J, Wozney JM. Recombinant Human Bone Morphogenetic Protein Induces Bone-Formation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(6):2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ripamonti U, Heliotis M, Rueger DC, Sampath TK. Induction of cementogenesis by recombinant human osteogenic protein-1 (hOP-1/BMP-7) in the baboon (Papio ursinus) Archives of Oral Biology. 1996;41(1):121–126. doi: 10.1016/0003-9969(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 10.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. Journal of Periodontology. 2003;74(2):202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TCK. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. Journal of Periodontology. 1998;69(2):129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 12.Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR (TM) allograft ring and the ATLANTIS (TM) anterior cervical plate. Spine. 2003;28(12):1219–1224. doi: 10.1097/01.BRS.0000065486.22141.CA. [DOI] [PubMed] [Google Scholar]
- 13.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans -A prospective, randomized clinical pilot trial - 2002 Volvo Award in clinical studies. Spine. 2002;27(23):2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions - A prospective, randomized clinical trial comparing rhOP-1 with fresh bone autograft. Journal of Bone and Joint Surgery-American Volume. 2001;83A:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 15.Geesink RGT, Hoefnagels NHM, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. Journal of Bone and Joint Surgery-British Volume. 1999;81B(4):710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 16.Wang EA. Bone Morphogenetic Proteins (Bmps) - Therapeutic Potential in Healing Bony Defects. Trends in Biotechnology. 1993;11(9):379–383. doi: 10.1016/0167-7799(93)90096-R. [DOI] [PubMed] [Google Scholar]
- 17.Sykaras N, Opperman LA. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci. 2003;45(2):57–73. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 18.Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58(2):197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Rose FRAJ, Hou QP, Oreffo ROC. Delivery systems for bone growth factors -the new players in skeletal regeneration. Journal of Pharmacy and Pharmacology. 2004;56(4):415–427. doi: 10.1211/0022357023312. [DOI] [PubMed] [Google Scholar]
- 20.Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27(16):S16–S23. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 21.Cook SD, Wolfe MW, Salkeld SL, Rueger DC. Effect of Recombinant Human Osteogenic Protein-1 on Healing of Segmental Defects in Nonhuman-Primates. Journal of Bone and Joint Surgery-American Volume. 1995;77A(5):734–750. doi: 10.2106/00004623-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC, Whitecloud TS. The Effect of Recombinant Human Osteogenic Protein-1 on Healing of Large Segmental Bone Defects. Journal of Bone and Joint Surgery-American Volume. 1994;76A(6):827–838. doi: 10.2106/00004623-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lane JM. BMPs: Why Are They Not in Everyday Use? J Bone Joint Surg Am. 2001;83(Supp 1):161–162. [PubMed] [Google Scholar]
- 24.Uludag H, D'Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46(2):193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Uludag H, Friess W, Williams D, Porter T, Timony G, D'Augusta D, Blake C, Palmer R, Biron B, Wozney J. rhBMP-collagen sponges as osteoinductive devices: Effects of in vitro sponge characteristics and protein pI on in vivo rhBMP pharmacokinetics. Ann N Y Acad Sci. 1999;875:369–378. doi: 10.1111/j.1749-6632.1999.tb08519.x. [DOI] [PubMed] [Google Scholar]
- 26.Delustro F, Dasch J, Keefe J, Ellingsworth L. Immune-Responses to Allogeneic and Xenogeneic Implants of Collagen and Collagen Derivatives. Clinical Orthopaedics and Related Research. 1990;(260):263–279. [PubMed] [Google Scholar]
- 27.Bach FH, Fishman JA, Daniels N, Proimos J, Anderson B, Carpenter CB, Forrow L, Robson SC, Fineberg HV. Uncertainty in xenotransplantation: Individual benefit versus collective risk. Nat Med. 1998;4(2):141–144. doi: 10.1038/nm0298-141. [DOI] [PubMed] [Google Scholar]
- 28.Zhang RW, Xu DZ, Landeryou T, Toth C, Dimaano N, Berry J, Evans J, Hawkins M. Ectopic bone formation using osteogenic protein-1 carried by a solution precipitated hydroxyapatite. J Biomed Mater Res. 2004;71A(3):412–418. doi: 10.1002/jbm.a.30151. [DOI] [PubMed] [Google Scholar]
- 29.Ripamonti U, Ma SS, Reddi AH. Induction of Bone in Composites of Osteogenin and Porous Hydroxyapatite in Baboons. Plastic and Reconstructive Surgery. 1992;89(4):731–739. [PubMed] [Google Scholar]
- 30.Hollinger JO, Leong K. Poly(alpha-hydroxy acids): Carriers for bone morphogenetic proteins. Biomaterials. 1996;17(2):187–194. doi: 10.1016/0142-9612(96)85763-2. [DOI] [PubMed] [Google Scholar]
- 31.Lee SC, Shea M, Battle MA, Kozitza K, Ron E, Turek T, Schaub RG, Hayes WC. Healing of Large Segmental Defects in Rat Femurs Is Aided by Rhbmp-2 in Plga Matrix. Journal of Biomedical Materials Research. 1994;28(10):1149–1156. doi: 10.1002/jbm.820281005. [DOI] [PubMed] [Google Scholar]
- 32.Kirker-Head CA, Gerhart TN, Armstrong R, Schelling SH, Carmel LA. Healing bone using recombinant human bone morphogenetic protein 2 and copolymer. Clinical Orthopaedics and Related Research. 1998;(349):205–217. doi: 10.1097/00003086-199804000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Wei GB, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25(2):345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 34.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112(1):103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei GB, Ma PX. Macro-porous and nano-fibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res. 2006;78:306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 36.Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly(alpha- hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54(2):284–293. doi: 10.1002/1097-4636(200102)54:2<284::aid-jbm16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 37.Stenport VF, Roos-Jansaker AM, Renvert S, Kuboki Y, Irwin C, Albrektsson T, Claffey N. Failure to induce supracrestal bone growth between and around partially inserted titanium implants using bone morphogenetic protein (BMP): an experimental study in dogs. Clinical Oral Implants Research. 2003;14(2):219–225. doi: 10.1034/j.1600-0501.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 38.Jin QM, Takita H, Kohgo T, Atsumi K, Itoh H, Kuboki Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. Journal of Biomedical Materials Research. 2000;51(3):491–499. [PubMed] [Google Scholar]
- 39.Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. Journal of Biochemistry. 1997;121(2):317–324. doi: 10.1093/oxfordjournals.jbchem.a021589. [DOI] [PubMed] [Google Scholar]
- 40.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res. 2003;67A(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 41.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27(21):3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 42.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, Kim GS, Somerman MJ, Ma PX. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2006 doi: 10.1016/j.biomaterials.2006.06.013. in press. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto S, Takaoka K, Okada T, Yoshikawa H, Hashimoto J, Suzuki S, Ono K. Evaluation of Polylactic Acid Homopolymers as Carriers for Bone Morphogenetic Protein. Clinical Orthopaedics and Related Research. 1992;(278):274–285. [PubMed] [Google Scholar]
- 44.Ziegler J, Mayr-Wohlfart U, Kessler S, Breitig D, Gunther KP. Adsorption and release properties of growth factors from biodegradable implants. Journal of Biomedical Materials Research. 2002;59(3):422–428. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]


