Abstract
What is already known about this subject
Although placebo effects have been shown on subjective continuous variables such as pain, placebo effects on objective continuous variables remain uncertain.
The present, pilot, follow-up investigation represents the first to assess a placebo effect on the objective continuous measurement of acute postprandial plasma glucose.
What this study adds
Placebo effects may be operating on postprandial plasma glucose outcomes.
Cornstarch sources of placebo may decrease the plasma glucose response to a 75-g oral glucose tolerance test, rendering them as positive controls when assessing postprandial outcomes.
Other carbohydrate sources used as placebos in research may show similar effects.
Aims
Placebo effects in clinical trials remain uncertain. To investigate a placebo effect on acute postprandial plasma glucose, we conducted a follow-up investigation on a previous study.
Methods
The effect of placebo (9 g encapsulated cornstarch +500 ml water, taken at −40 min) on the plasma glucose response to a 75-g oral glucose tolerance test (OGTT) was assessed in a previous study in 12 healthy subjects (gender, five male, seven female; age 27 ± 6 years; body mass index 24 ± 3.4 kg m−2). This was compared with the effect of a water control (500 ml water taken alone at −40 min) on the same outcome in the same subjects in a follow-up study.
Results
Cornstarch placebo decreased plasma glucose area under the curve during the 75-g OGTT by 28% [Δ (95% confidence interval) −63.3 min mmol−1 l−1 (−218.33, 91.66), P < 0.02] compared with the water control (P< 0.05).
Conclusions
Postprandial plasma glucose outcomes may be vulnerable to placebo effects.
Keywords: CAM, diabetes, ginseng, glucose, placebo effect, postprandial
Introduction
Placebo effects in clinical trials have been debated since the introduction of the placebo-controlled clinical trial over half a century ago. The debate was initiated by Beecher in 1955 with his observation that ∼35% of subjects receiving placebo experienced a therapeutic benefit based on the pooled aggregate of 15 clinical trials [1]. Although this analysis was later determined to be flawed [2], placebo effects have remained a focus of investigation. A recent, rigorously conducted meta-analysis found that placebo had only a small effect on subjective continuous variables such as pain. No effect was observed for objective continuous variables such as blood pressure, cholesterol and weight [3, 4]. It remains unclear whether there is an effect of placebo on objective continuous variables.
Acute postprandial plasma glucose may be sensitive to placebo effects. We recently conducted an acute, randomized, double-blind, double-placebo-controlled, multiple-crossover study in 14 healthy subjects, to identify a source of American ginseng with the greatest efficacy in lowering the postprandial plasma glucose response to a 75-g oral glucose tolerance test (OGTT). Consistent with our ginseng testing programme [5], efficacy was assessed as the effect of active treatment compared with the mean effect of placebo administered on two separate occasions. The mean effect of the two placebos on the postprandial plasma glucose response to the 75-g OGTT seemed unusually low. The objective of the present pilot investigation was to explore this effect of placebo by comparing it with a follow-up water control in the same subjects.
Methods
A follow-up, nonrandomized, crossover design was used in which the results of the original study were compared with the results of a second study performed in the same subjects. The study was approved by the St Michael's Hospital research ethics board. Twelve [gender, five male, seven female; age 27 ± 6 years; body mass index (BMI) 24 ± 3.4 kg m−2] of the original 14 subjects agreed and gave informed consent to participate in the follow-up study. The placebo treatments in the original study consisted of 9 g of encapsulated ground cornstarch taken with 500 ml of water. To achieve a more representative placebo, these placebo treatments were administered on two separate occasions with their effects averaged for comparisons. The water control in the follow-up study was administered once and consisted of 500 ml water taken alone. The wash-out period between the two studies was > 2 weeks. A 75-g OGTT protocol was followed in both studies, in which the treatments were administered 40 min before (−40 min) a 75-g OGTT and venous blood samples were drawn at −40, 0, 15, 30, 45, 60, 90 and 120 min.
Plasma glucose concentrations were measured by the St Michael's core laboratory, by the rate oxygen consumption method [6] using a Synchron LX system with a Beckham Oxygen electrode. The intra-assay coefficient of variation (CV) for this method is 2.9% at a mean glucose concentration of 43.7 ± 1.3 mmol l−1 and 0.4% at a mean glucose concentration of 397.1 ± 1.7 mmol l−1.
Statistical analyses were performed using NCSS 2000 (NCSS statistical software, Kaysville, UT, USA). Plasma glucose was expressed as the incremental change from baseline and the positive incremental area under the curve (AUC) was calculated [7]. Responses to the two placebo treatments were averaged. The main effects of treatment (cornstarch placebo vs. water control), time (−40, 0, 15, 30, 45, 60, 90 and 120 min) and their interaction on incremental plasma glucose were assessed by repeated-measures two-way anova. Significant interactions of treatment × time on incremental plasma glucose were explored with repeated-measures one-way anova. This same test also assessed differences in peak and AUC plasma glucose. Data were expressed as mean ± SEM. Ninety-five percent confidence intervals (95% CI) were provided for differences.
Results
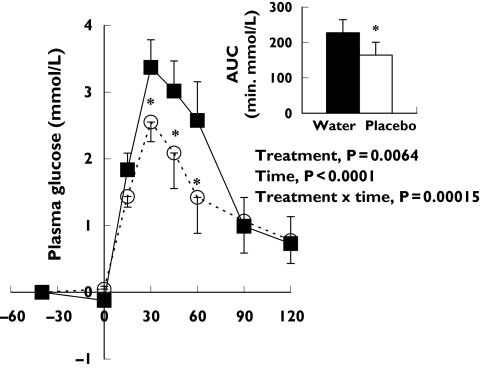
A significant placebo effect was observed. Figure 1 shows the mean effect of the two cornstarch placebos compared with the water control on the incremental plasma glucose response to a 75-g OGTT. Repeated-measures two-way anova applied to these data showed that the main effect of treatment [Δ (95% CI) −0.5 mmol l−1 (−1.54, 0.52), P = 0.0064] (cornstarch placebo vs. water control), the main effect of time (P< 0.001) (−40, 0, 15, 30, 45, 60, 90 and 120 min) and their interaction were significant (P< 0.001). The significant interaction between treatment and time was explored by repeated-measures one-way anova. Incremental plasma glucose at 30, 45 and 60 min during the 75-g OGTT was significantly lower for the mean of the two cornstarch placebos than for the water control [Δ (95% CI) −1.2 mmol l−1 (−3.68, 1.29), −1.1 mmol l−1 (−3.73, 1.60), −1.3 mmol l−1 (−3.95, 1.44), respectively, P < 0.05]. Peak plasma glucose was also shown to be lower [7.5 ± 0.4 vs. 8.6 ± 0.5 mmol l−1, Δ (95% CI) −1.1 mmol l−1 (−3.07, 0.80), P = 0.0018]. These changes were reflected in a 28% decrease in AUC plasma glucose [Δ (95% CI) −63.3 min mmol−1 l−1 (−218.33, 91.66), P < 0.02] during the 75-g OGTT by the two placebos, compared with the water control.
Figure 1.
Effect of placebo on postprandial plasma glucose to a 75-g oral glucose tolerance test (OGTT). The line plots and bars represent the plasma glucose incremental change and area under the curve (AUC) for the mean of two placebo treatments (9 g cornstarch with 500 ml water; ○) performed on separate occasions or one water control (500 ml water alone; ▪) each taken 40 min before a 75-g OGTT (−40 min) in 12 healthy normoglycaemic subjects (gender, five male, seven female; age 27 ± 6 years; body mass index 24 ± 3.4 kg m−2). P-values reported in the base of the plot area are for the main effects of treatment, time and their interaction by repeated-measures two-way anova. The significant interaction of treatment × time was explored with repeated-measures one-way anova at each level of time. *Points or bars are significantly different from water control (P ≤ 0.05, repeated-measures one-way anova). Data are mean ± SEM
Discussion
The present follow-up, pilot study indicates that postprandial plasma glucose indices may represent objective continuous variables that are vulnerable to placebo effects. The mean effect of the two cornstarch placebos decreased incremental, peak and AUC plasma glucose responses to the 75-g OGTT compared with a water control.
The observed placebo effect may have important implications for research. The 28% reduction in the AUC plasma glucose response to the 75-g OGTT by the mean of the two cornstarch placebos is comparable to the reductions in the postprandial plasma glucose AUC reported for oral antihyperglycaemic agents such as the sulphonylureas, glipizide and glibenclamide (18–22%) and the nonsulphonylurea repaglinide (26%) [8]. It is possible that the use of a cornstarch placebo may bias research comparisons related to acute postprandial data. Cornstarch placebos may act as positive controls, leading to a clinically significant underestimation or nullification of the effect of active treatments on postprandial plasma glucose.
There are several explanations for the placebo effect on plasma glucose. Regression to the mean, subject–investigator expectations, conditioning, symbolism, self-perception and investigator care have all been proposed as factors [9]. A metabolic explanation may be more viable. A ‘second meal’ effect on the plasma glucose response to carbohydrate has been described, such that manipulating the glycaemic index of a carbohydrate preload can reduce the glycaemic response to a second meal [10]. It is possible that the cornstarch acted as such a preload. This mechanism may apply more broadly to other carbohydrate sources.
Design limitations must be considered as a source of variability. The follow-up, nonrandomized design meant that placebo treatments were received first and the water control was received second. It is possible that the placebo effect observed was confounded by a sequence effect, in which the participants responded higher to the follow-up 75-g OGTT. This interpretation, however, is disputed. The opposite directional bias has been noticed, in which 75-g OGTT outcomes have a tendency to be lower during subsequent tests [11]. Another possibility is that the placebo effect is an artefact of the normal biological variability in the responses to oral glucose, irrespective of sequence. The high variability of 75-g OGTT outcome is well established. We have shown previously that the intrasubject CV of postprandial plasma glucose following multiple 75-g OGTTs is 7–22% for individual time points and 20–43% for AUC [12, 13]. This range in variability overlaps with the differences observed. That being said, a ‘double placebo’ was used to minimize biological variability, in which subjects received the same placebo on two different occasions and the responses to the two treatments were averaged for comparisons with the water control. The variability in 75-g OGTT outcome was also accounted for in the power (1-β) calculations. Post hoc analyses of the present study showed that the power for differences in the main effects of treatment (cornstarch placebo vs. water control), time (−40, 0, 15, 30, 45, 60, 90 and 120 min) and their interaction was 86%, 99% and 100%, respectively.
Timing effects on placebo must also be considered. In the present study, placebo was administered at −40 min relative to the 75-g OGTT. Second meal effects, however, have been shown for administration times up to 5 h before the second meal [14]. The implication is that these data may apply more broadly to administration times from −40 min to −300 min relative to the 75-g OGTT. Nevertheless, caution should be exercised when extrapolating these findings to different timing schedules, as timing interactions have been identified in other studies investigating placebo effects [15]. In this regard, these data apply only to a cornstarch placebo administered at −40 min relative to a 75-g OGTT.
In conclusion, cornstarch sources of placebo administered at −40 min may decrease the plasma glucose response to a 75-g OGTT. This may have the undesired effect of rendering them as positive controls when assessing postprandial outcomes. If the mechanism of action on postprandial carbohydrate metabolism is mediated by a ‘second meal’ effect, then other carbohydrate sources and times of administration used for placebo may show similar effects. To address this possibility, the reproducibility of these pilot findings needs to be verified using better designed, randomized studies with adequate power calculations and repeated controls to mitigate confounding by the biological variability in postprandial plasma glucose. Comparisons should be made across different carbohydrate sources of placebo such as cornstarch, lactose, glucose, lactulose and wheat bran and times of administration ranging from −300 min to 0 min before the 75-g OGTT.
Acknowledgments
Competing interests: None declared.
Contributions of authors
J.L.S. was the Postdoctoral Fellow assigned to the project. He cowrote the grant, participated in the design, coordinated clinical testing and laboratory analyses, performed the statistical analyses and wrote the paper. A.E. and A.D. participated in the collection of data and made critical revision of intellectual content. V.V. was the senior supervisor and principal investigator of the project. He secured the funding for the project and supervised all aspects of the research directly and provided final input into design, analyses, interpretation and critical revision of intellectual content.
The funding for this study was provided by a grant from the Canadian Institutes of Health Research (CIHR).
References
- 1.Beecher HK. The powerful placebo. JAMA. 1955;159:1602–6. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 2.McDonald CJ, Mazzuca SA. How much of the placebo effect is really statistical regression? Star Med. 1983;2:417–27. doi: 10.1002/sim.4780020401. [DOI] [PubMed] [Google Scholar]
- 3.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–602. doi: 10.1056/NEJM200105243442106. Erratum in. 2001; 345: 304. [DOI] [PubMed] [Google Scholar]
- 4.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256:91–100. doi: 10.1111/j.1365-2796.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 5.Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis. 2005;15:149–60. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Kadish AH, Hall DA. A new method for the continuous monitoring of blood glucose by measurement of dissolved oxygen. Clin Chem. 1965;11:869–75. [PubMed] [Google Scholar]
- 7.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54:846–54. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- 8.Cozma LS, Luzio SD, Dunseath GJ, Langendorg KW, Pieber T, Owens DR. Comparison of the effects of three insulinotropic drugs on plasma insulin levels after a standard meal. Diabetes Care. 2002;25:1271–6. doi: 10.2337/diacare.25.8.1271. [DOI] [PubMed] [Google Scholar]
- 9.Kaptchuk TJ. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351:1722–5. doi: 10.1016/S0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins DJ, Jenkins AL, Wolever TM, Vuksan V, Rao AV, Thompson LU, Josse RG. Low glycemic index: lente carbohydrates and physiological effects of altered food frequency. Am J Clin Nutr. 1994;59(Suppl. 3):706S–709S. doi: 10.1093/ajcn/59.3.706S. [DOI] [PubMed] [Google Scholar]
- 11.Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, Heine RJ. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39:298–305. doi: 10.1007/BF00418345. [DOI] [PubMed] [Google Scholar]
- 12.Sievenpiper JL, Jenkins DJA, Josse RG, Leiter LA, Vuksan V. Dilution of the 75g-glucose tolerance test improves overall tolerability but not reproducibility in subjects with different body composition. Diabetes Res Clin Pract. 2001;51:87–95. doi: 10.1016/s0168-8227(00)00209-6. [DOI] [PubMed] [Google Scholar]
- 13.Sievenpiper JL, Leiter LA, Vuksan V. Intrasubject coefficient-of-variation corresponds to diagnostic reproducibility in diabetes screening. Can J Diabetes Care. 2002;26:105–12. [Google Scholar]
- 14.Rostami-Hodjegan A, Abdul-Manap R, Wright CE, Tucker GT, Morice AH. The placebo response to citric acid-induced cough: pharmacodynamics and gender differences. Pulm Pharmacol Ther. 2001;14:315–9. doi: 10.1006/pupt.2001.0301. [DOI] [PubMed] [Google Scholar]
- 15.Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJ, Vantini I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83:817–22. doi: 10.1093/ajcn/83.4.817. [DOI] [PubMed] [Google Scholar]