Abstract
What is already known about this subject
Atomoxetine is a cytochrome P450 2D6 (CYP2D6) substrate and its pharmacokinetics has been characterized in a predominantly White population during clinical development.
There are scant East Asian pharmacokinetic data available.
The CYP2D6*10 allele is particularly prevalent in East Asian populations and may contribute to the known ethnic differences in CYP2D6 metabolic capacity.
What this study adds
The pharmacokinetics of multiple-dose 80 mg daily atomoxetine observed in Chinese healthy subjects appears comparable to previous data in other ethnic populations.
Homozygous CYP2D6*10 subjects appear to have higher exposures, but are not a clearly distinct group compared with other CYP2D6 extensive metabolizers.
Aims
To characterize atomoxetine pharmacokinetics, explore the effect of the homozygous CYP2D6*10 genotype on atomoxetine pharmacokinetics and evaluate the tolerability of atomoxetine, in healthy Chinese subjects.
Methods
Twenty-four subjects, all CYP2D6 extensive metabolizers (EM), were randomized to receive atomoxetine (40 mg qd for 3 days, then 80 mg qd for 7 days) or matching placebo (2 : 1 ratio) in a double-blind fashion. Atomoxetine serum concentrations were measured following single (40 mg) and multiple (80 mg) doses. Adverse events, clinical safety laboratory data and vital signs were assessed during the study.
Results
Atomoxetine was rapidly absorbed with median time to maximum serum concentrations of approximately 1.5 h after single and multiple doses. Atomoxetine concentrations appeared to decrease monoexponentially with a mean apparent terminal half-life (t1/2) of approximately 4 h. The apparent clearance, apparent volume of distribution and t1/2 following single and multiple doses were similar, suggesting linear pharmacokinetics with respect to time. Homozygous CYP2D6*10 subjects had 50% lower clearances compared with other EM subjects, resulting in twofold higher mean exposures. No clinically significant changes or abnormalities were noted in laboratory data and vital signs.
Conclusions
The pharmacokinetics of atomoxetine in healthy Chinese subjects appears comparable to other ethnic populations. Multiple dosing of 80 mg qd atomoxetine was well tolerated in this study.
Keywords: atomoxetine, Chinese, CYP2D6, CYP2D6*10, pharmacokinetics
Introduction
Atomoxetine hydrochloride [LY139603, benzenepropanamine, N-methyl-γ-(2-methylphenoxy), hydrochloride (–)], hereafter referred to as atomoxetine, is a selective norepinephrine uptake inhibitor with little or no affinity for other neuronal transporters or neurotransmitter receptor sites. Its effectiveness in the treatment of attention deficit hyperactivity disorder (ADHD) has been demonstrated in children, adolescents, and adults [1, 2]. It was approved in the USA in 2002 for the treatment of ADHD.
Atomoxetine is predominantly metabolized by cytochrome P450 2D6 (CYP2D6) [3, 4]. The majority of individuals are extensive metabolizers (EM) and possess normal metabolic capacity for CYP2D6 substrates. Some CYP2D6 gene mutations or deletions are associated with defective CYP2D6 metabolism. About 7% of Whites and < 1% of East Asians possess two nonfunctional alleles and are poor metabolizers (PM) [5]. CYP2D6 PM have significantly reduced activity in this pathway and thus exhibit a 10-fold higher area under the concentration–time curve (AUC), a fivefold higher peak plasma concentration and slower elimination (plasma half-life of about 24 h) of atomoxetine compared with EM [4]. One EM allele apparently specific to Asian populations is CYP2D6*10 and its prevalence in the Chinese population is approximately 50–70% (with about 24% homozygous CYP2D6*10), which is about 10-fold higher than that in Whites [6]. For some drugs, the decreased metabolic activity associated with the CYP2D6*10 allele results in slower clearance in vivo. Yin et al. have reported that the clearance of loratadine is 50% lower in homozygous CYP2D6*10 compared with the homozygous CYP2D6*1 in Chinese subjects [7]. In another study, Honda et al. have reported that the oral clearance of carvedilol is significantly lower in subjects with at least one CYP2D6*10 allele than with the heterozygous CYP2D6*1 in Japanese [8]. The high prevalence of CYP2D6*10 in the Chinese population may contribute to intersubject variability in exposure and response to atomoxetine.
The current study was the first atomoxetine study conducted in healthy adult subjects in China. The aims were to characterize pharmacokinetics of single and multiple doses of atomoxetine in healthy Chinese subjects and assess the tolerability of atomoxetine. In addition, the effect of the homozygous CYP2D6*10 genotype on atomoxetine pharmacokinetics was explored.
Methods
Study design
This was a double-blind, randomized, single period study. It was approved by the Ethical Review Board of Peking University First Hospital, Beijing, China, and was conducted in accordance with the Declaration of Helsinki.
Twenty-four healthy Chinese subjects (16 men, eight nonpregnant women; age range 20–39 years, weight range 53–72 kg and body mass index of 22.4 ± 1.6 kg m−2, mean ± SD) were enrolled. This sample size was considered sufficient to achieve the primary objective of characterizing the pharmacokinetics of atomoxetine in Chinese subjects. They were genotyped for the CYP2D6*1, *2, *3, *4, *5, *6, *7, *8, *9,*10, *11, *14A, *14B, *15, *17, *19, *20, *25, *26, *29, *30, *31, *35, *36, *40 and *41 alleles using a validated, polymerase chain reaction-based method (Roche AmpliCip CYP450; MDS Pharma Services, Beijing, China). If subjects had two nonfunctional alleles in any combination of *3, *4, *5, *6, *7, *8, *11, *14A, *15, *19, *20 and *40 alleles, a PM genotype was assigned; otherwise, an EM genotype was assigned. In this study, all subjects were identified as EM, with eight subjects identified as homozygous CYP2D6*10, 13 subjects as heterozygous CYP2D6*10 and three as homozygous CYP2D6*1. Although this study allowed for enrolment of CYP2D6 PM subjects, none was identified for inclusion.
Subjects were all in good health as determined from their medical history, physical examination, electrocardiogram, chest X-ray, vital signs (blood pressure, pulse rate and temperature) and safety laboratory tests (blood chemistry, haematology and urine analysis). All subjects were required not to take any medication that might interfere with the pharmacokinetics of atomoxetine. Written informed consent was obtained from each subject before participating in the study.
Subjects were randomly assigned to receive either atomoxetine or placebo in a 2 : 1 ratio. Atomoxetine 40-mg capsules and matching placebo capsules were supplied by Eli Lilly and Co. (Indianapolis, IN, USA). Each subject received atomoxetine, 40 mg once daily (qd) or placebo for 3 days (days 1, 2 and 3), followed by atomoxetine or placebo, 80 mg qd for 7 days (days 4–10). This was in accordance with the current recommended clinical dosage and administration regime, where atomoxetine is initiated as a total daily dose of approximately 0.5 mg kg−1 and increased after a minimum of 3 days to a target total daily dose of approximately 1.2 mg kg−1 administered either as qd or twice daily (b.i.d.) [9]. All subjects fasted for at least 8 h prior to, and for at least 60 min after dosing.
Blood samples were collected for the determination of serum atomoxetine concentrations as follows: at predose, 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, 16 and 24 h postdose, on days 1 and 10; at predose on days 7, 8 and 9 (trough samples). Safety laboratory tests and vital signs were measured, and adverse events were collected during the study.
Analytical methods
Human serum samples were analysed for LY404363 (free base of atomoxetine hydrochloride) using a validated liquid chromatography/atmospheric pressure chemical ionization/mass spectrometry/mass spectrometry (LC/APCI/MS/MS) method previously described [4] (Taylor Technology, Inc., Princeton, NJ, USA). The validated range for the assay was 2.50–2000.00 ng ml−1. The precision and accuracy of the assay expressed as the intrarun percentage relative standard deviation (%RSD) and percentage relative error (%RE) were between 1.72 and 4.65% and between −5.20 and 7.48%, respectively.
Pharmacokinetic analysis
Noncompartmental pharmacokinetic parameters for atomoxetine were derived in WinNonlin Professional Edition, Version 3.1 (Pharsight Corp., Mountain View, CA, USA) using actual sampling times. Pharmacokinetic parameters determined after single dose on day 1 and multiple doses on day 10 included maximum serum concentration (Cmax and Cmax,ss), time to maximum serum concentration (tmax and tmax,ss), apparent clearance (CL/F and CLss/F) and terminal half-life (t1/2). Areas under the serum concentration–time curves from the time of dosing extrapolated to infinity (AUC0–∞) and during one dosing interval at steady state (AUCτ,ss) were also calculated. Additional parameters determined at steady state included the average steady-state concentration (Cav,ss), the accumulation ratio (RA), defined as the ratio of dose-normalized AUCτ,ss/AUCτ,day1, and the linearity index (LI), defined as the ratio of dose-normalized AUCτ,ss/AUC0–∞,day1.
Statistical analysis
All pharmacokinetic parameters were summarized using descriptive statistics. Geometric means and coefficient of variation percentage (CV%) were calculated for all parameters except for tmax, tmax,ss and t1/2. The parameters tmax and tmax,ss were summarized with median and range, whereas t1/2 was expressed as geometric mean (range).
The effect of the CYP2D6*10 allele on atomoxetine metabolism was explored by comparison of pharmacokinetic parameters (AUC, Cmax, CL/F and t1/2) between homozygous CYP2D6*10 subjects and other EM subjects using Student's t-test for independent samples. The parameters were log-transformed prior to statistical analysis. Ratio of geometric means and 90% confidence interval (CI) were obtained. All statistical analyses were performed using SAS® for Windows, Version 8.2 (SAS Inc., Cary, NC, USA).
Results
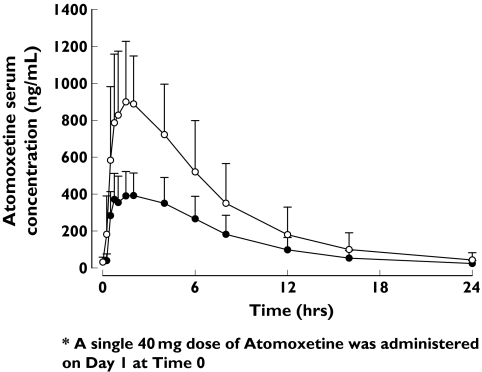
Following a single dose of 40 mg and multiple qd doses of 80 mg, atomoxetine was rapidly absorbed with a median tmax or tmax,ss of 1.25 and 1.50 h, respectively (Table 1). Atomoxetine concentrations appeared to decrease monoexponentially with a mean t1/2 of approximately 4 h, for both the single- (4.15 h) and multiple-dose regimens (4.31 h) (Figure 1). Consistent with the relatively short t1/2, the mean (CV%) accumulation ratio, RA, was 1.01 (14.9). Subjects had attained steady state by day 10, with average trough atomoxetine concentrations of 39.7, 42.1, 40.4 and 31.5 ng ml−1 on days 7, 8, 9 and 10, respectively. The steady-state parameters of CLss/F and t1/2 parallel the results shown after single-dose administration, suggesting linear pharmacokinetics with respect to time. The mean (CV%) linearity index was 0.982 (15.3).
Table 1.
a.Summary of geometric means (CV%) of pharmacokinetic parameters on day 1 after a single dose of 40 mg atomoxetine and on day 10 after multiple doses of 80 mg qd atomoxetine
| Arithmetic mean (SD) | Geometric mean (CV%) | |||
|---|---|---|---|---|
| Parameters | (N = 16)Single dose (40 mg) | (N = 16)Multiple dose (80 mg) | Single dose(40 mg) | Multipledose (80 mg) |
| Cmax or Cmax,ss (ng ml−1) | 449 (144) | 1020 (334) | 427 (34.0) | 965 (36.2) |
| Cav,ss (ng ml−1) | – | 297 (143) | – | 260 (60.5) |
| tmax* or tmax,ss* (h) | 1.25 (0.50–4.00) | 1.50 (0.50–4.00) | – | – |
| AUC0–∞ or AUCt,ss (h ng−1 ml−1) | 3630 (1730) | 7120 (3430) | 3170 (62.4) | 6240 (60.5) |
| CL/F or CLss/F (l h−1 kg−1) | 0.241 (0.151) | 0.242 (0.142) | 0.204 (62.7) | 0.208 (61.4) |
| t1/2† (h) | 4.15 (2.44–5.94) | 4.31 (2.22–6.39) | – | – |
| LI | – | 0.993 (0.158) | – | 0.982 (15.3) |
| RA | – | 1.02 (0.159) | – | 1.01 (14.9) |
Figure 1.
Arithmetic mean (+SD) serum concentration–time profiles of atomoxetine on day 1 following a single dose of 40 mg (N = 16) ( ) and on day 10 following multiple doses of 80 mg qd (N = 16) (○)
) and on day 10 following multiple doses of 80 mg qd (N = 16) (○)
b.Comparison of *10/*10 subjects vs. other extensive metabolizer (EM) subjects for AUC, Cmax, t1/2 and CL/F
| Parameters | Dose | Geometric mean* 10/* 10(n = 7) | Geometric mean,other EMs(n = 9) | Ratio (90% CI) | P-value |
|---|---|---|---|---|---|
| AUC0–∞ (h ng−1 ml−1) | 40 mg single | 4962 | 2242 | 2.21 (1.53, 3.21) | 0.002 |
| Cmax (ng ml−1) | 530 | 360 | 1.47 (1.15, 1.88) | 0.014 | |
| CL/F (l h−1 kg−1) | 0.13 | 0.29 | 0.45 (0.31, 0.65) | 0.002 | |
| t1/2 (h) | 5.44 | 3.36 | 1.62 (1.34, 1.96) | 0.001 | |
| AUCt,ss (h ng−1 ml−1) | 80 mg multiple | 9693 | 4427 | 2.19 (1.53, 3.13) | 0.002 |
| Cmax,ss (ng ml−1) | 1199 | 815 | 1.47 (1.13, 1.92) | 0.023 | |
| SDED/F (l h−1 kg−1) | 0.13 | 0.29 | 0.45 (0.32, 0.65) | 0.002 | |
| t1/2 (h) | 5.17 | 3.74 | 1.38 (1.08, 1.76) | 0.034 |
Median (range).
Geomean (range).
Cmax and Cmax,ss, Maximum serum concentration; Cav,ss, average steady-state concentration; tmax and tmax,ss, time to maximum serum concentration; AUC0–∞and AUCτ,ss, areas under the serum concentration–time curves, from the time of dosing extrapolated to infinity and during one dosing interval at steady state, respectively; CL/F and CLss/F, apparent clearance; t1/2, terminal half-life; LI, linearity index defined as the ratio of dose-normalized AUCτ,ss/AUC0–∞,day1; RA, accumulation ratio of dose-normalized AUCτ,ss/ AUCτ,day1.)
The comparison of AUC, Cmax, CL/F and t1/2 between homozygous CYP2D6*10 (n = 7) and other EM subjects (n = 9, with seven heterozygous CYP2D6*10 and two homozygous CYP2D6*1) following single and multiple doses of atomoxetine is summarized in Table 1b. Mean clearance was approximately 50% lower in homozygous CYP2D6*10 subjects, resulting in higher AUC and Cmax and longer t1/2 compared with other EM subjects in this study.
The most common adverse events reported were dizziness, nausea and upper abdominal pain. All adverse events were of mild severity and had resolved by the time of study completion. No obvious difference was observed with respect to the frequency, severity and type of adverse events reported by homozygous CYP2D6*10 and other EM subjects. No clinically significant changes or abnormalities were seen in laboratory data and vital signs.
Discussion
The pharmacokinetics of atomoxetine was characterized in healthy Chinese EM subjects. Variability among subjects as assessed by geometric CV% was generally similar between single- and multiple-dose treatments for all parameters.
Previous studies have shown that for atomoxetine, the dose-normalized Cmax,ss and clearance for CYP2D6 PMs are approximately five times higher and 10 times lower than for EMs, respectively [4]. In a combined analysis of clinical studies of subjects taking at least 1.2 mg kg−1 day−1 of atomoxetine, a comparison of 1290 EMs with 67 PMs showed a slightly higher increase in heart rate and slightly more weight loss in PMs, but there was little difference in discontinuations or reporting rates of adverse events [9]. Therefore, these pharmacokinetic differences have not necessitated dose adjustment required based on genotype [10].
The atomoxetine pharmacokinetic data from healthy EM Chinese subjects in this study are similar to data obtained in White subjects [4]. The pharmacokinetic differences (Cmax, AUC and CL/F) between homozygous CYP2D6*10 and other EMs observed in this study are also comparable to those observed in previous studies (data on file at Lilly). In this study, the proportion of Chinese subjects homozygous for CYP2D6*10 allowed for a comparison between homozygous CYP2D6*10 and other EM (which included heterozygous CYP2D6*10 and homozygous CYP2D6*1; homozygous CYP2D6*1 was not analysed separately owing to its low frequency). Although the homozygous CYP2D6*10 subjects had higher mean exposures than other EM subjects in this study, their data fall within the range of clearance values previously observed for EMsin other studies and they are thus not considered a clearly distinct group [9].
There were no clinically significant observations relating to safety or tolerability in this study. Whilst the number of homozygous CYP2D6*10 subjects was too small to support definitive conclusions, higher average drug exposures in this group did not appear to result in differences in safety or tolerability. No obvious difference was observed with respect to the frequency, severity or type of adverse events reported by homozygous CYP2D6*10 subjects from other EMs.
In conclusion, atomoxetine pharmacokinetics observed in healthy Chinese subjects appears comparable to other ethnic populations. The higher mean exposure in homozygous CYP2D6*10 subjects compared with other EM subjects observed in this study is not expected to be clinically significant. However, extrapolation of these data to the relevant patient group and to CYP2D6 PMs should be made with caution. Oral administration of 80 mg atomoxetine was well tolerated by healthy Chinese subjects in this study.
Acknowledgments
Competing interests: This study was funded by Eli Lilly and Company., Indianapolis, IN, USA. Authors of Eli Lilly and Company and Lilly-NUS Centre for Clinical Pharmacology are employees of Eli Lilly and Company, and hold stock options on own stock in Eli Lilly and Company.
We thank the following persons for their assistance in these experiments: Qiu Qing Ang, Yu Cheng Liu, Yuwang Liu and Peihong Sun.
References
- 1.Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ, Kelsey D, Wernicke J, Dietrich A, Milton D. Atomoxetine in adults with attention-deficit/hyperactivity disorder: two randomised, placebo-controlled studies. Biol Psychiatry. 2003;53:112–20. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- 2.Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomised, placebo-controlled study. Am J Psychiatry. 2002;159:1896–901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 3.Ring BJ, Gillespie JS, Eckstein JA, Wrighton SA. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30:319–23. doi: 10.1124/dmd.30.3.319. [DOI] [PubMed] [Google Scholar]
- 4.Sauer JM, Ponsler GD, Mattiuz EL, Long AJ, Witcher JW, Thomasson HR, Desante KA. Disposition and metabolic fate of atomoxetine hydrochloride. The role of CYP2D6 in human disposition and metabolism. Drug Metab Dispos. 2003;31:98–107. doi: 10.1124/dmd.31.1.98. [DOI] [PubMed] [Google Scholar]
- 5.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism and Biochemistry. New York: Plenum Press; 1995. pp. 473–535. [Google Scholar]
- 6.Ji L, Pan S, Wu J, Marti-Jaun J, Hersberger M. Genetic polymorphisms of CYP2D6 in Chinese mainland. Chin Med J (Engl) 2002;115:1780–4. [PubMed] [Google Scholar]
- 7.Yin OQP, Shi XJ, Tomlinson B, Chow MS. Effect of CYP2D6*10 allele on the pharmacokinetics of loratadine in Chinese subjects. Drug Metab Dispos. 2005;33:1283–7. doi: 10.1124/dmd.105.005025. [DOI] [PubMed] [Google Scholar]
- 8.Honda M, Nozawa T, Igarashi N, Inoue H, Arakawa R, Ogura Y, Okabe H, Taguchi M, Hashimoto Y. Effect of CYP2D6*10 on the pharmacokinetics of R- and S-carvedilol in healthy Japanese volunteers. Biol Pharm Bull. 2005;28:1476–9. doi: 10.1248/bpb.28.1476. [DOI] [PubMed] [Google Scholar]
- 9.Sauer JM, Ring BJ, Witcher JW. Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet. 2005;44:571–90. doi: 10.2165/00003088-200544060-00002. [DOI] [PubMed] [Google Scholar]
- 10.Strattera®, PV5311 AMP. Indianapolis, IN: Eli Lilly; 2002. [Google Scholar]