Abstract
What is already known about this subject
All inhaled corticosteroids are absorbed into the systemic circulation and hence have the potential to cause adverse systemic effects.
Plasma drug concentrations following inhalation of 1000 µg fluticasone are considerably lower in people with airflow obstruction than in healthy volunteers but this is not the case for budesonide.
What this study adds
This is the first study to determine whether changes in airflow obstruction within an individual affect the systemic absorption of inhaled fluticasone and budesonide;
Plasma concentrations of fluticasone and, to a lesser extent, those of budesonide were lower when the drugs were inhaled following induced bronchoconstriction;
The lower plasma concentrations of corticosteroids seen when the drugs were inhaled following induced bronchoconstriction is likely to reflect variations that will occur with fluctuations in airway caliber in asthma.
Aims
To determine whether and to what extent bronchoconstriction affects plasma concentrations of fluticasone and budesonide following inhalation.
Methods
Twenty people with mild asthma inhaled 1000 µg fluticasone (Accuhaler®) plus 800 µg budesonide (Turbohaler®) on two visits. On one occasion, prior to drug inhalation, FEV1 was decreased by at least 25% using inhaled methacholine. Plasma drug concentrations were measured for each drug over 5 h and area under the plasma concentration-time curve (AUC(0,5 h)) compared between visits.
Results
The mean difference in FEV1 prior to drug inhalation on the 2 days was 33%. AUC(0,5 h) values for fluticasone and budesonide were lower by a median of 60% (IQR 36–75) and 29% (IQR 2–44), respectively, when administered following bronchoconstriction; the reduction was greater for fluticasone than for budesonide, P = 0.007.
Conclusions
The lower plasma concentrations of fluticasone and, to a lesser extent, budesonide seen when the drugs were inhaled following induced bronchoconstriction, is likely to reflect variations that will occur with fluctuations in airway caliber in asthma.
Keywords: asthma, inhaled corticosteroid, pharmacology
Introduction
Adverse systemic effects from inhaled corticosteroids have been recognized increasingly over recent years and include adrenal suppression [1], reduced bone mineral density [2, 3] and an increase in fractures [4], cataracts [5] and bruising [6]. With about 5% of the population in more economically developed countries being prescribed an inhaled corticosteroid, often for many decades, an understanding of the factors that determine these adverse effects may help to improve their use.
All currently available inhaled corticosteroids are absorbed into the systemic circulation and hence have the potential to cause adverse systemic effects. However, the risk may differ between inhaled corticosteroids, due to their different physicochemical and pharmacokinetic properties [7], and there is evidence that these drug-related differences interact with patient factors such as airflow obstruction. Plasma drug concentrations following inhalation of 1000 µg fluticasone are considerably lower in people with airflow obstruction than in healthy subjects [8–10], but this is not the case for budesonide [9]. These differences between fluticasone and budesonide were attributed to differences in lipophilicity [9]. Whether changes in airflow obstruction within an individual affect systemic absorption in the same way is unknown, but of relevance in view of the fluctuations in airway caliber seen in asthma. The aim of this study was to compare the plasma concentrations of fluticasone and budesonide following inhalation of a single dose, with and without prior methacholine-induced bronchoconstriction.
Methods
Subjects
Twenty nonsmoking subjects with asthma aged between 18 and 70 years were recruited from our volunteer database. To be included subjects had to have a forced expiratory volume in one second (FEV1) of at least 80% predicted [11] and 1.5 l, a provocative dose of methacholine causing a 20% fall in FEV1 (PD20) below 8 µm, and stable asthma, defined as no change in asthma symptoms or treatment for 2 months. Subjects were excluded if they had significant comorbidity, were taking any medication known to alter the metabolism of corticosteroids, were pregnant or lactating, or had a greater than 20 pack year smoking history. The study was approved by Nottingham Research Ethics Committee and written informed consent was obtained from all subjects.
Protocol
This was a randomized open label cross-over study. Subjects were screened to assess suitability and to measure FEV1 and PD20 methacholine. Subjects familiarized themselves with the two dry powder inhalers (Accuhaler®, Glaxo-Wellcome and Turbohaler®, Astra-Zeneca) to ensure optimal inhaler technique during the study, and those taking fluticasone or budesonide were changed to an equivalent dose of beclomethasone dipropionate for 4 days before and until the completion of the study. Subjects were asked to avoid short and long acting β2-adrenoceptor agonists for 12 h, and to avoid exercise and caffeine on the morning of the study.
Subjects attended for two study visits at the same time of day ± 1 h, 7 ± 3 days apart. A venous cannula was inserted and after 10 min rest, FEV1 was measured. One thousand µg fluticasone (Accuhaler®), given as two 500 µg doses, and 800 µg budesonide (Turbohaler®), given as two 400 µg doses, were then inhaled in random order. After each drug the subjects rinsed their mouths with water, which was discarded. Venous blood samples were drawn into heparinized tubes at intervals over the next 5 h (0, 5, 15, 30, 60, and 90 min, 2, 3, 4 and 5 h), centrifuged at 1500 rev min−1 for 10 min, and the resulting plasma was frozen at −70°C. The protocol for the two study visits was identical except that on one occasion prior to inhalation of the drugs, a methacholine challenge was carried out to induce a fall in FEV1 of at least 25% from baseline. The two studies were carried out in random order according to a computer generated code.
Power calculations showed that 20 patients would give 90% power to detect a 0.7 SD difference in the area under the plasma concentration time curve over 5 h, the primary outcome, between the two study visits.
Measurements
Spirometry
FEV1 and forced vital capacity (FVC) were measured with a dry bellows spirometer (Vitalograph, Buckingham, UK) as the higher of two successive readings within 100 ml of each other.
Methacholine inhalation challenge
Subjects inhaled three puffs of normal saline followed by doses of methacholine doubled from 0.048 µm to a maximum of 24.5 µm using a DeVilbiss nebulizer, and FEV1 was measured 1 min after each dose [12]. The test was stopped once FEV1 had fallen by 20% from the postsaline value during screening, for calculation of PD20 by interpolation, and once FEV1 had fallen by 25% in the study itself.
Drug analysis
Plasma concentrations of fluticasone propionate and budesonide were quantified with previously validated high performance liquid chromatography/tandem mass spectrometry methods [13, 14] using a Micromass Quattro LC-A triple quadrupole mass spectrometer (Beverley, MA) at the College of Pharmacy, Department of Pharmaceutics, University of Florida, USA. The lower limits of detection for the assays were 15 pg ml−1 for fluticasone propionate and 50 pg ml−1 for budesonide. The intra- and interassay coefficients of variation were below 13.6% for both fluticasone and budesonide at concentrations between 0.015 and 0.75 ng ml−1 for fluticasone and between 0.15 and 2.5 ng ml−1 for budesonide.
Data analysis
Plasma fluticasone and budesonide concentrations were plotted against time for each subject and the maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax) and area under the curve up to 5 h (AUC(0,5 h) were calculated using standard software (WinNonlin® Professional Version 3.1, Pharsight Corporation, Mountain View, CA). Cmax, tmax and AUC(0,5 h) values for the two study visits were compared for each drug using a paired t-test and 95% confidence intervals for the differences were calculated. To allow for the differences in concentrations of the two drugs the ratio of the AUC(0,5 h) values on the days with and without bronchoconstriction was calculated for each subject and for each drug and the between-drug values were analyzed by a Wilcoxon signed rank test. This analysis was repeated for Cmax values.
Results
All 20 subjects (mean age 48 years, 12 males) completed the study and their baseline characteristics are shown in Table 1. All were nonsmokers and only two had ever smoked.
Table 1.
Baseline characteristics of the subjects studied
| Subject | Gender | Age (years) | Height (cm) | Weight (kg) | FEV1 (l) | FEV1 (% predicted) | Usual inhaled corticosteroid |
|---|---|---|---|---|---|---|---|
| 1 | M | 55 | 192 | 78 | 3.8 | 91 | Beclometasone diproprionate |
| 2 | M | 50 | 173 | 78 | 2.8 | 80 | None |
| 3 | F | 50 | 160 | 86 | 2.55 | 103 | Beclometasone diproprionate |
| 4 | M | 63 | 174 | 77 | 2.8 | 89 | Beclometasone diproprionate |
| 5 | F | 67 | 155 | 68 | 1.55 | 84 | None |
| 6 | M | 23 | 170 | 79 | 4 | 96 | None |
| 7 | F | 39 | 161 | 63 | 3.5 | 126 | Beclometasone diproprionate |
| 8 | M | 38 | 189 | 92 | 3.8 | 84 | None |
| 9 | M | 41 | 182 | 96 | 3.8 | 92 | Budesonide |
| 10 | M | 41 | 176 | 87 | 3.8 | 98 | None |
| 11 | M | 52 | 172 | 88 | 3.05 | 90 | None |
| 12 | F | 62 | 166 | 105 | 1.95 | 81 | Beclometasone diproprionate |
| 13 | M | 66 | 173 | 78 | 2.4 | 80 | Beclometasone diproprionate |
| 14 | M | 48 | 166 | 79 | 2.75 | 84 | Fluticasone |
| 15 | M | 49 | 164 | 68 | 2.8 | 89 | Budesonide |
| 16 | M | 42 | 179 | 67 | 3.55 | 89 | Beclometasone diproprionate |
| 17 | F | 65 | 161 | 64 | 2 | 93 | None |
| 18 | F | 27 | 157 | 57 | 2.8 | 96 | Fluticasone |
| 19 | F | 34 | 161 | 71 | 2.9 | 100 | Fluticasone |
| 20 | F | 44 | 166 | 60 | 2.35 | 82 | Fluticasone |
| Mean (SD) | 48 (13) | 167 (10) | 77 (13) | 2.9 (0.7) | 91 (11) |
Subjects had a geometric mean PD20 methacholine of 1.2 µm (range 0.06–6.1). Mean (SD) FEV1 was 2.9 (0.7) l (91% predicted) on screening and 2.8 (0.7) and 2.9 (0.6) l on arrival on the study days. All subjects had a fall in FEV1 of at least 25% (range 25–47%) following methacholine, giving an FEV1 of 1.9 (0.5) l (60% predicted) prior to drug inhalation, and a mean difference in FEV1 prior to drug inhalation on the 2 days of 0.9 l (range 0.5–1.35) or 33% (range 20–45%).
Data for budesonide plasma concentrations were lost for two subjects due to a computer problem during drug analysis. Therefore, complete data were available for 20 subjects for fluticasone and 18 subjects for budesonide.
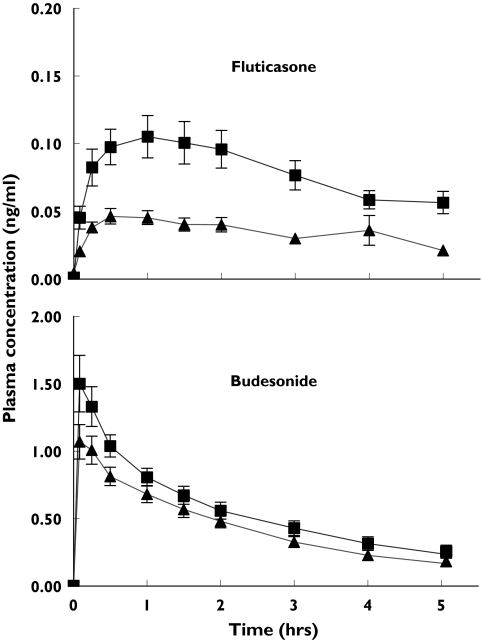
The shape of the fluticasone and budesonide plasma concentration-time curves showed marked differences on the study day without bronchoconstriction. Peak plasma concentrations of budesonide were 14 times higher than those of fluticasone and occurred considerably earlier (Figure 1). Mean (SD) time to maximum concentration (tmax) values were 0.21 (0.24) h and 1.21 (0.91) h for budesonide and fluticasone, respectively. The shape of the plasma drug concentration-time curves for each drug was similar on the 2 study days, although plasma concentrations were lower following methacholine-induced bronchoconstriction (Figure 1 and Table 2).
Figure 1.
Mean (SEM) plasma drug concentrations following inhalation of 1000 mg fluticasone via an Accuhaler® (n = 20) and 800 μg budesonide via a Turbohaler® (n = 18) in subjects with asthma with (▴) and without (▪) prior methacholine-induced bronchoconstriction (note y axes have different scales)
Table 2.
Mean (SD) values for maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax) and area under the curve (AUC(0,5 h)) for fluticasone and budesonide when inhaled with and without prior methacholine-induced bronchoconstriction
| Without bronchoconstriction | With bronchoconstriction | Difference (95% CI) | P value | |
|---|---|---|---|---|
| Fluticasone | ||||
| Cmax (ng ml−1) | 0.12 (0.07) | 0.06 (0.04) | 0.06 (0.03, 0.08) | <0.001 |
| tmax (h) | 1.21 (0.91) | 0.78 (0.62) | 0.43 (−0.01, 0.87) | 0.05 |
| AUC(0,5 h) (ng ml−1 h) | 0.40 (0.23) | 0.16 (0.10) | 0.23 (0.14, 0.33) | <0.001 |
| Budesonide | ||||
| Cmax (ng ml−1) | 1.67 (0.82) | 1.16 (0.48) | 0.51 (0.16, 0.85) | 0.007 |
| tmax (h) | 0.21 (0.24) | 0.23 (0.24) | −0.02 (−0.15, 0.12) | |
| AUC(0,5 h) (ng ml−1 h) | 2.87 (1.19) | 2.28 (0.90) | 0.6 (0.02, 1.17) | 0.04 |
Mean Cmax values for both fluticasone and budesonide were lower when the drugs were inhaled following bronchoconstriction (Table 2). Cmax values were a median 44% lower (interquartile range (IQR) 21–60) for fluticasone and 33% lower (IQR −7–51) for budesonide following bronchoconstriction but the difference between fluticasone and budesonide did not differ significantly (P= 0.18).
Mean AUC(0,5 h) values, the primary endpoints, for both fluticasone and budesonide were lower when the drugs were inhaled following bronchoconstriction (Table 2). AUC(0,5 h) values were a median 60% lower (IQR 36–75) for fluticasone and 29% lower (IQR 2–44) for budesonide following bronchoconstriction. These values for fluticasone and budesonide differed significantly (P= 0.007).
Discussion
This is the first study to explore the effect of change in airflow obstruction within an individual on plasma concentrations of fluticasone and budesonide following their inhalation. There was an average 33% difference in FEV1 prior to drug inhalation on the two study visits as a result of methacholine challenge. Plasma concentrations of both fluticasone and budesonide were lower when FEV1 was decreased but the magnitude of this effect was greater for fluticasone.
The greater effect of bronchoconstriction on plasma concentrations of fluticasone compared with budesonide is similar to the findings in patients with and without airflow obstruction [8–10], and is probably due to differences in lipophilicity [16]. Bronchoconstriction will cause a greater proportion of both drugs to be deposited in more central airways, where mucociliary clearance is better able to remove them [15]. This would be expected to affect fluticasone more than budesonide because the former, being more lipophilic, dissolves more slowly in airway lining fluid and hence is available to be cleared for longer [16]. Furthermore, little of the fluticasone that is removed by mucociliary clearance will reach the systemic circulation due to its low oral bioavailability (<1% compared with about 10% for budesonide) [16].
Peak plasma drug concentrations occurred earlier and were considerably higher following inhalation of budesonide than fluticasone, which is in keeping with previous data [9]. These differences are likely to reflect high pulmonary deposition of budesonide from the Turbohaler® [17], its higher oral bioavailability [16], greater water solubility [16], and lower volume of distribution [7].
We gave fluticasone and budesonide at the same time to ensure that they were studied under identical conditions. This approach was validated by Agertoft & Pederson who found similar plasma fluticasone and budesonide concentrations following inhalation, whether the drugs were given separately or together [18]. Drug concentrations were measured over 5 h since our previous study showed that the major differences in plasma concentrations between subjects with and without airflow obstruction occurred during this time [9]. We did not determine pharmacokinetic parameters other than Cmax and tmax, since these data are already available [7], and a longer sampling period would have been required. We believe our findings are due to decreased rather than delayed drug absorption, in view of the shape of the plasma concentration-time curves, and the observation that the changes within subjects in the present study are very similar to the differences seen over 8 and 12 h between subjects with and without airflow obstruction [8–10].
By using a methacholine challenge we were able to study the effect of changes in lung function within an individual on plasma concentrations of fluticasone and budesonide under controlled conditions. This is likely to be a reasonable model to study changes in airflow that occur as asthma control deteriorates. A 33% reduction in FEV1 in our study caused a 60% reduction in AUC(0,5 h) for fluticasone, suggesting a 60% reduction in systemic exposure. Equally, as FEV1 increases with treatment, systemic exposure would be expected to increase in a similar way. Therefore, the risk of adverse systemic effects from inhaled fluticasone is likely to vary considerably in relation to lung function within an individual, whereas that from budesonide is unlikely to be affected to the same extent. These findings re-enforce the importance of reviewing the need for higher doses of inhaled corticosteroids and particularly fluticasone, as lung function improves and in patients with relatively normal lung function.
Acknowledgments
We thank the 20 volunteers who took part in the study, Sarah Pacey (Senior Pharmacist at Nottingham City Hospital) for supplying the inhalers and randomization schedule and the Scadding Morriston Davies Joint Fellowship for helping to fund the project.
Conflicts of interest: The department of respiratory medicine has previously received financial support for clinical research from AstraZeneca and GlaxoSmithKline.
References
- 1.Todd GRG, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child. 2002;87:457–61. doi: 10.1136/adc.87.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong CA, Walsh LJ, Smith CJP, Wisniewski AF, Lewis SA, Hubbard R, Cawte S, Green DJ, Pringle M, Tattersfield AE. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000;355:1399–403. doi: 10.1016/S0140-6736(00)02138-3. [DOI] [PubMed] [Google Scholar]
- 3.Israel E, Banerjee TR, Fitzmaurice GM, Kotlov TV, LaHive K, LeBoff MS. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med. 2001;345:941–7. doi: 10.1056/NEJMoa002304. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard RB, Smith CJP, Smeeth L, Harrison TW, Tattersfield AE. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166:1563–6. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 5.Smeeth L, Boulis M, Hubbard R, Fletcher AE. A population based case-control study of cataract and inhaled corticosteroids. Br J Ophthalmol. 2003;87:1247–51. doi: 10.1136/bjo.87.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, Ohlsson SV. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 7.Winkler J, Hochhaus G, Derendorf H. How the lung handles drugs pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1:356–63. doi: 10.1513/pats.200403-025MS. [DOI] [PubMed] [Google Scholar]
- 8.Brutsche MH, Brutsche IC, Munavvar M, Langley SJ, Masterson CM, Daley-Yates PT, Brown R, Custovic A, Woodcock A. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomised crossover study. Lancet. 2000;356:556–61. doi: 10.1016/S0140-6736(00)02581-2. [DOI] [PubMed] [Google Scholar]
- 9.Harrison TW, Tattersfield AE. Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects. Thorax. 2003;58:258–60. doi: 10.1136/thorax.58.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SD, Whale C, Houghton N, Daley-Yates P, Kirby SM, Woodcock AA. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2003;55:375–81. doi: 10.1046/j.1365-2125.2003.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standardised lung function testing. Bull Europ Physiopath Resp. 1983;19(Suppl 5):1–95. [PubMed] [Google Scholar]
- 12.Yan K, Salome C, Woolcock AJ. Rapid method for measurement of bronchial responsiveness. Thorax. 1983;38:760–5. doi: 10.1136/thx.38.10.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimova H, Wang Y, Pommery S, Moellmann H, Hochhaus G. SPE/RIA vs LC/MS for measurement of low levels of budesonide in plasma. Biomed Chromatogr. 2003;17:14–20. doi: 10.1002/bmc.204. [DOI] [PubMed] [Google Scholar]
- 14.Krishnaswami S, Mollmann H, Derendorf H, Hochhaus G. A sensitive LC-MS/MS method for the quantification of fluticasone propionate in human plasma. J Pharmaceut Biomed Anal. 2000;22:123–9. doi: 10.1016/s0731-7085(99)00246-0. [DOI] [PubMed] [Google Scholar]
- 15.Saari SM, Vidgren MT, Koskinen MO, Turjanmaa VM, Waldrep JC, Nieminen MM. Regional lung deposition and clearance of 99mTc-labeled beclomethasone-DLPC liposomes in mild and severe asthma. Chest. 1998;113:1573–9. doi: 10.1378/chest.113.6.1573. [DOI] [PubMed] [Google Scholar]
- 16.Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol. 1996;97:169–76. doi: 10.1016/s0091-6749(96)80217-x. [DOI] [PubMed] [Google Scholar]
- 17.Thorsson L, Edsbacker S, Conrandson TB. Lung deposition of budesonide from Turbohaler is twice that from a pressurized metered-dose inhaler (p-MDI) Eur Respir J. 1994;7:1839–44. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 18.Agertoft L, Pedersen S. Lung deposition and systemic availability of fluticasone Diskus and budesonide Turbuhaler in children. Am J Respir Crit Care Med. 2003;168:779–82. doi: 10.1164/rccm.200302-200OC. [DOI] [PubMed] [Google Scholar]