Abstract
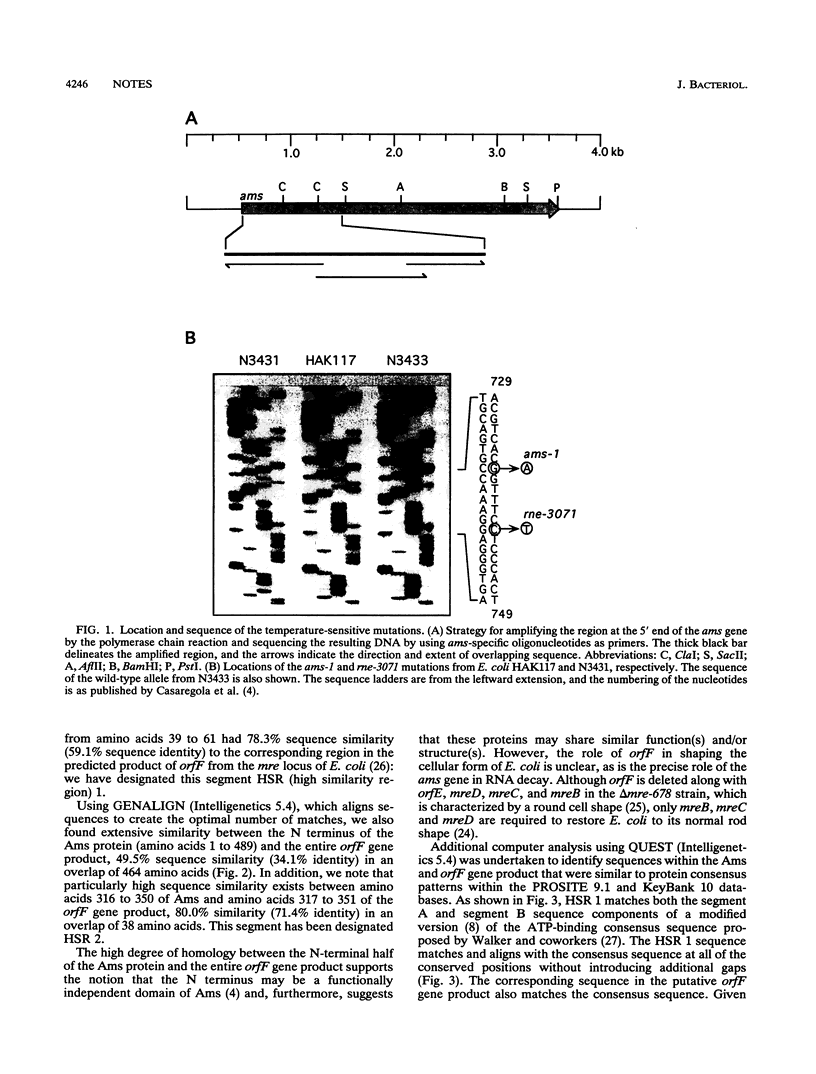
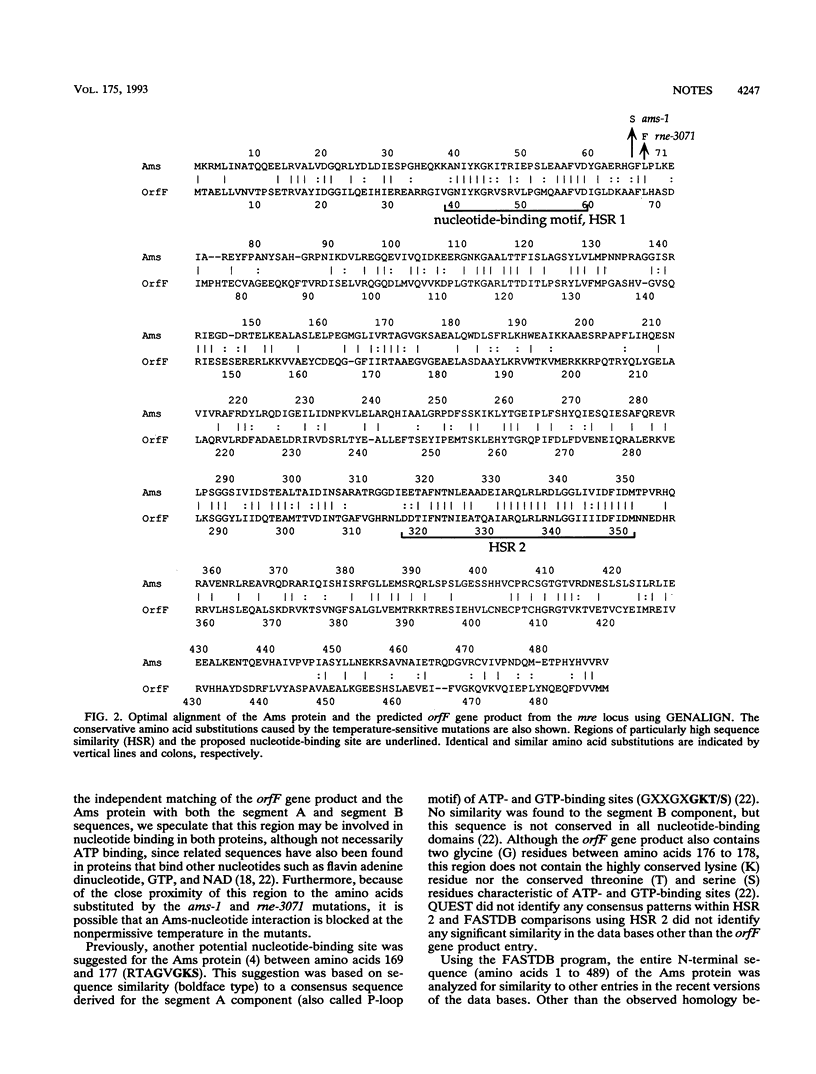
Two temperature-sensitive mutations, ams-1 and rne-3071, in the ams (altered mRNA stability) gene have been used extensively to investigate the processing and decay of RNA in Escherichia coli. We have sequenced these temperature-sensitive alleles and found that the mutations are separated by only 6 nucleotides and cause conservative amino acid substitutions next to a possible nucleotide-binding site within the N-terminal domain of the Ams protein. Computer analysis revealed that the region altered by the mutations has extensive sequence similarity to a predicted gene product from the mre (murein pathway cluster e) locus of E. coli, which has been implicated previously in determining bacterial cell shape.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Lassar A. B. A conditional lethal mutant of Escherichia coli which affects the processing of ribosomal RNA. J Biol Chem. 1978 Mar 10;253(5):1738–1742. [PubMed] [Google Scholar]
- Babitzke P., Kushner S. R. The Ams (altered mRNA stability) protein and ribonuclease E are encoded by the same structural gene of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):1–5. doi: 10.1073/pnas.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D. L., Dautricourt J. P., Maulik S., Relph J. Improved sensitivity of biological sequence database searches. Comput Appl Biosci. 1990 Jul;6(3):237–245. doi: 10.1093/bioinformatics/6.3.237. [DOI] [PubMed] [Google Scholar]
- Casaregola S., Norris V., Goldberg M., Holland I. B. Identification of a 180 kD protein in Escherichia coli related to a yeast heavy-chain myosin. Mol Microbiol. 1990 Mar;4(3):505–511. doi: 10.1111/j.1365-2958.1990.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Casarégola S., Jacq A., Laoudj D., McGurk G., Margarson S., Tempête M., Norris V., Holland I. B. Cloning and analysis of the entire Escherichia coli ams gene. ams is identical to hmp1 and encodes a 114 kDa protein that migrates as a 180 kDa protein. J Mol Biol. 1992 Nov 5;228(1):30–40. doi: 10.1016/0022-2836(92)90489-7. [DOI] [PubMed] [Google Scholar]
- Chauhan A. K., Apirion D. The rne gene is the structural gene for the processing endoribonuclease RNase E of Escherichia coli. Mol Gen Genet. 1991 Aug;228(1-2):49–54. doi: 10.1007/BF00282446. [DOI] [PubMed] [Google Scholar]
- Chauhan A. K., Miczak A., Taraseviciene L., Apirion D. Sequencing and expression of the rne gene of Escherichia coli. Nucleic Acids Res. 1991 Jan 11;19(1):125–129. doi: 10.1093/nar/19.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. T., Goff S. A., Webster T., Smith T., Goldberg A. L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988 Aug 25;263(24):11718–11728. [PubMed] [Google Scholar]
- Claverie-Martin F., Diaz-Torres M. R., Yancey S. D., Kushner S. R. Analysis of the altered mRNA stability (ams) gene from Escherichia coli. Nucleotide sequence, transcriptional analysis, and homology of its product to MRP3, a mitochondrial ribosomal protein from Neurospora crassa. J Biol Chem. 1991 Feb 15;266(5):2843–2851. [PubMed] [Google Scholar]
- Grandea A. G., 3rd, Tuyen L. K., Asikin N., Davis T. B., Philipp M., Cohen C., McReynolds L. A. A lambda gt11 cDNA recombinant that encodes Dirofilaria immitis paramyosin. Mol Biochem Parasitol. 1989 Jun 1;35(1):31–41. doi: 10.1016/0166-6851(89)90139-4. [DOI] [PubMed] [Google Scholar]
- Gurevitz M., Apirion D. Processing of bacteriophage T4 tRNAs: a precursor of species 1 RNA. FEBS Lett. 1983 Aug 8;159(1-2):180–184. doi: 10.1016/0014-5793(83)80442-6. [DOI] [PubMed] [Google Scholar]
- Kagawa H., Gengyo K., McLachlan A. D., Brenner S., Karn J. Paramyosin gene (unc-15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence and models for thick filament structure. J Mol Biol. 1989 May 20;207(2):311–333. doi: 10.1016/0022-2836(89)90257-x. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Ono M., Endo H., Hori K., Nakamura K., Hirota Y., Ohnishi Y. Gene affecting longevity of messenger RNA: a mutant of Escherichia coli with altered mRNA stability. Mol Gen Genet. 1977 Sep 9;154(3):279–285. doi: 10.1007/BF00571283. [DOI] [PubMed] [Google Scholar]
- Lanar D. E., Pearce E. J., James S. L., Sher A. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986 Oct 31;234(4776):593–596. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- Mackie G. A. Specific endonucleolytic cleavage of the mRNA for ribosomal protein S20 of Escherichia coli requires the product of the ams gene in vivo and in vitro. J Bacteriol. 1991 Apr;173(8):2488–2497. doi: 10.1128/jb.173.8.2488-2497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Gomi T., Mueckler M. M., Fujioka M., Backlund P. S., Jr, Aksamit R. R., Unson C. G., Cantoni G. L. Amino acid sequence of S-adenosyl-L-homocysteine hydrolase from rat liver as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1987 Feb;84(3):719–723. doi: 10.1073/pnas.84.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez C. G., Myers J. C., Shows T. B., Leinwand L. A. Human nonmuscle myosin heavy chain mRNA: generation of diversity through alternative polyadenylylation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1164–1168. doi: 10.1073/pnas.87.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Srivastava R. K., Miczak A., Apirion D. Maturation of precursor 10Sa RNA in Escherichia coli is a two-step process: the first reaction is catalyzed by RNase III in presence of Mn2+. Biochimie. 1990 Nov;72(11):791–802. doi: 10.1016/0300-9084(90)90188-m. [DOI] [PubMed] [Google Scholar]
- Wachi M., Doi M., Okada Y., Matsuhashi M. New mre genes mreC and mreD, responsible for formation of the rod shape of Escherichia coli cells. J Bacteriol. 1989 Dec;171(12):6511–6516. doi: 10.1128/jb.171.12.6511-6516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi M., Doi M., Tamaki S., Park W., Nakajima-Iijima S., Matsuhashi M. Mutant isolation and molecular cloning of mre genes, which determine cell shape, sensitivity to mecillinam, and amount of penicillin-binding proteins in Escherichia coli. J Bacteriol. 1987 Nov;169(11):4935–4940. doi: 10.1128/jb.169.11.4935-4940.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi M., Doi M., Ueda T., Ueki M., Tsuritani K., Nagai K., Matsuhashi M. Sequence of the downstream flanking region of the shape-determining genes mreBCD of Escherichia coli. Gene. 1991 Sep 30;106(1):135–136. doi: 10.1016/0378-1119(91)90578-y. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



