Abstract
What is already known about this subject
Use of statins is growing worldwide and costs represent a burden to public budgets.
The introduction of simvastatin generics, generic substitution and price regulations have contributed to price reductions and resulted in overall cost reductions of statin use in Norway.
What this study adds
New reimbursement regulations for statins in Norway in June 2005, making simvastatin the drug of choice, had a great impact on physicians' prescribing of statins.
Nearly 40% of the atorvastatin users switched to simvastatin during the 13-month period after implementation of the new regulations.
Among the new users of statins the proportion receiving simvastatin increased from 48% in May 2005 to 92% in June 2006.
The new regulations have reduced costs of statins, even though the prevalence of statin use has increased.
Aims
To assess the changes in prescribing of statins in Norway after implementation of the new reimbursement regulations for statins in June 2005.
Methods
Data were retrieved from the Norwegian Prescription Database covering the total population in Norway (4.6 million). Outcome measures were the proportion of atorvastatin users switching to simvastatin and changes in the proportion of new statin users receiving simvastatin. Based on retail costs for all statin prescriptions dispensed in Norway, expenditure was measured in Norwegian currency.
Results
One-year prevalences of statin use increased from 6.3 to 6.8% for women and from 7.5 to 8.1% for men from the year before to the year after the new statin regulations. Of atorvastatin users (N = 131 222), 39% switched to simvastatin during the 13-month period after the implementation. The proportion of switching was higher in women (41%) than in men (36%). In May 2005, 48% of the new statin users received simvastatin. The proportion of new users receiving simvastatin increased rapidly after implementation of the new regulations to 68% in June 2005 and reached 92% in June 2006. Expenditure was reduced from €120 million to €95 million when comparing the year before with the year after the new statin regulations.
Conclusions
The new reimbursement policy for statins has had a great impact on physicians' prescribing of statins in Norway. Physicians in Norway acknowledge the importance of contributing to cost containment.
Keywords: adherence to regulations, drug costs, Norway, pharmacoepidemiology, prescription database, statins
Introduction
Many studies showing the benefits of statin use on cardiovascular morbidity and mortality have been published since the results of the first pivotal trial were published in 1994 [1]. The use of statins has been steadily increasing worldwide, but is higher in Norway compared with other European countries [2]. The costs of statin use are mainly covered by the Norwegian National Insurance Administration through the reimbursement scheme system [3]. Membership in this national insurance programme is mandatory for all Norwegian citizens.
Since 2003, introduction of simvastatin generics, generic substitution and price regulations have contributed to price reductions and reduced costs of statins in Norway [4–6]. However, the price regulations alone have had no influence on simvastatin's proportion of total sales measured in number of doses [6]. In June 2005, the Norwegian Medicines Agency introduced new reimbursement regulations for lipid-modifying agents to improve cost containment further. According to the new reimbursement regulations, all new users of statins should be prescribed simvastatin and present statin users switched to simvastatin at their first medical visit and within a 1-year transition period. Prescribing of other statins should be reserved for individuals only with solid medical reasons, including pharmacokinetic and/or interaction considerations. Physicians should clearly indicate the reasons for using other statins in the medical records. Norway is the first country to introduce such national restrictions on statin prescribing, and we are not aware of other studies presenting nationwide data.
The aim of this study was to assess the changes in prescribing of statins in Norway after the new regulations were introduced.
Methods
The Norwegian Prescription Database (NorPD) covers the entire population of Norway (4.6 million inhabitants). From January 2004 all Norwegian pharmacies have been obliged to send in data electronically on all prescriptions dispensed on a monthly basis to NorPD at the Norwegian Institute of Public Health. NorPD contains basic demographic information from all individuals having a prescription dispensed, reimbursed or not, at Norwegian pharmacies and includes details on items dispensed [7]. The variables used in the present study are: patient's unique encrypted identifying number, sex, age, date of dispensing and information on items dispensed [number of packages, tablet strength, Anatomical Therapeutic Chemical (ATC) code, retail cost]. All medicines in Norway are classified according to the ATC classification system [8, 9].
Study population
All individuals were included having a prescription of a statin (ATC group C10AA) dispensed in Norway from January 2004 to June 2006. Only prescriptions covered by the reimbursement regulations were included in the analysis, representing 99% of total statin costs. The following statins are marketed in Norway: simvastatin, atorvastatin, pravastatin, fluvastatin and lovastatin.
Period (1 year) prevalence of statin use was calculated by identifying all individuals having at least one statin prescription dispensed during the following two periods: 1 year before the intervention (June 2004 to May 2005) and 1 year after the intervention (June 2005 to May 2006). Prevalence was calculated using gender-specific distribution of the population in Norway as of 1 January 2005 for the year before and 1 January 2006 for the year after. If an individual had more than one type of statin dispensed during a period, the latest statin dispensed was selected when calculating the distribution of the various statin users. Switching to simvastatin was studied among atorvastatin users only. The proportion of switching in the group of atorvastatin users was calculated for the 13-month period after implementation of the new statin regulations.
Individuals using many cardiovascular drugs may represent patients at increased cardiovascular risk. The proportion of atorvastatin users receiving other cardiovascular drugs (measured for separate subgroups in ATC group C, excluding C05, C06 and C10) was calculated for those who switched to simvastatin and compared with those who continued to use atorvastatin in the 13-month period after the new regulations.
New users were defined as individuals who had no statin dispensed during 2004 and who received a statin for the first time during the period January 2005 to June 2006.
Based on retail costs, total expenditures in Norwegian currency (NOK) were retrieved for the year period before (June 2004 to May 2005) and the year after (June 2005 to May 2006) implementation of the new regulations.
Results
Statin users before and after new regulations
The 1-year prevalence of statin use before the new regulations (June 2004 to May 2005) was 6.3% (N = 145 810) for women and 7.5% (N = 170 783) for men. One year after the new regulations (June 2005 to May 2006) the prevalences of statin use increased to 6.8% (N = 159 184) for women and 8.1% (N = 185 736) for men. Table 1 shows the proportion of simvastatin, atorvastatin and other statin users in the year before and after implementation of the new statin regulations.
Table 1.
Number of users of and expenditure (NOK) on statins in the year before (June 2004 to May 2005) and the year after (June 2005 to May 2006) the new reimbursement regulations. Norwegian Prescription Database
| Total (%) | Simvastatin (%) | Atorvastatin (%) | Other statins (%) | |
|---|---|---|---|---|
| Number of users | ||||
| June 2004 to May 2005 | 316 593 (100) | 123 033 (39) | 142 405 (45) | 51 155 (16) |
| June 2005 to May 2006 | 344 917 (100) | 210 960 (61) | 97 713 (28) | 36 244 (11) |
| Expenditure in million NOK | ||||
| June 2004 to May 2005 | 959 (100) | 186 (19) | 578 (60) | 195 (20) |
| June 2005 to May 2006 | 762 (100) | 187 (25) | 482 (63) | 92 (12) |
Expenditure on statin use
Total expenditure on statins was reduced from 959 million NOK (€120 million) in the year before to 762 million NOK (€95 million) in the year after the new regulations. Expenditure on simvastatin, atorvastatin and other statins is given in Table 1.
Switching from atorvastatin to simvastatin
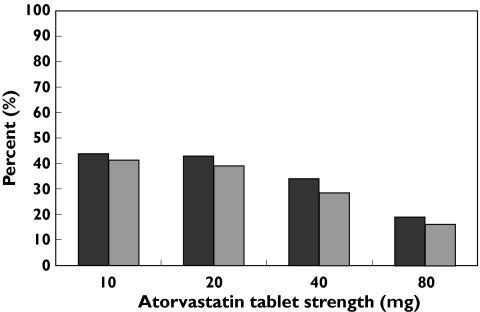
Overall, 142 405 individuals were users of atorvastatin during the 1 year before the new regulations (Table 1). Of these atorvastatin users, 8% had no statin dispensed (N = 10 840) or received other statins (pravastatin, lovastatin or fluvastatin, N = 343) in the 13 months after implementation of the new regulations. The remaining 92% (N = 131 222, 60 777 women and 70 445 men) of atorvastatin users had at least one atorvastatin or simvastatin prescription dispensed in the 13 months after implementation of the new regulations. Switching to simvastatin was studied among these atorvastatin users: 50 616 atorvastatin users (39%) switched to simvastatin, whereas 80 606 (61%) continued to use atorvastatin during the 13-month period after the new regulations. The proportion of switching was somewhat higher in women (41%) than in men (36%) and was highest in individuals previously using 10 mg or 20 mg atorvastatin (Figure 1). The switching proportions in patients using 40 mg and 80 mg atorvastatin were 31% and 17%, respectively. The proportion of atorvastatin users receiving other cardiovascular drugs was 72% in the group of users who switched to simvastatin, compared with 74% in the group who continued to use atorvastatin (Table 2).
Figure 1.
Proportion (%) of atorvastatin users who switched to simvastatin in the period June 2005 to June 2006, related to dose (tablet strength) of atorvastatin. Norwegian Prescription Database. Women ( ); men (
); men ( )
)
Table 2.
Proportion (%) of atorvastatin users receiving at least one cardiovascular drug (ATC group C) in the 13 months after implementation of new regulations in June 2005. Comparison of atorvastatin users who switched to simvastatin with users who continued on atorvastatin. Norwegian Prescription Database
| ATC groups | Atorvastatin users, switched†(N = 50 616) | Atorvastatin users, not switched† (N = 80 606) | |
|---|---|---|---|
| C* | Cardiovascular system | 72% | 74% |
| C01 | Cardiac therapy | 15% | 18% |
| C02 | Antihypertensives | 2% | 2% |
| C03 | Diuretics | 18% | 19% |
| C07 | β-Blocking agents | 40% | 45% |
| C08 | Calcium channel blockers | 23% | 22% |
| C09 | Agents acting on the renin–angiotensin system | 47% | 46% |
Excluding C04, C05 and C10.
The range of the 95% confidence interval is <1% for all the Anatomical Therapeutic Chemical (ATC) groups.
New users of statins
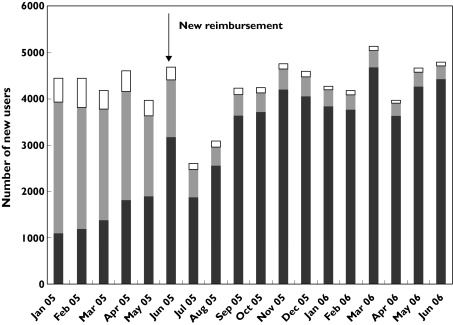
During the 13-month period after implementation of the new policy, 55 145 individuals were new users of statins (26 109 women and 29 036 men; Figure 2). Among new statin users, the atorvastatin proportion decreased from 64% in January 2005 to 44% in May 2005, whereas the simvastatin proportion increased from 25 to 48%. The proportion of new users receiving simvastatin increased rapidly after implementation of the new regulations to 68% in June 2005 and reached 92% in June 2006. Small gender differences in the proportion of simvastatin prescribed to new users were observed. The total number of new atorvastatin users gradually decreased after implementation of the new statin regulations (Figure 2). Among new atorvastatin users, the proportion receiving 80 mg increased from 9% (total number of new atorvastatin users, N = 1734) in May 2005 to 25% (total number of new atorvastatin users, N = 287) in May 2006.
Figure 2.
Monthly number of new statin users in Norway from January 2005 to June 2006. Norwegian Prescription Database. Other statins (□); Atorvastatin ( ); Simvastatin (
); Simvastatin ( )
)
Discussion
The new reimbursement policy for statins has had a great impact on physicians' prescribing of statins in Norway. Among atorvastatin users, 39% switched to simvastatin during the 13 months after implementation of the new regulations. In June 2006, 92% of new statin users received simvastatin.
Even though the 1-year prevalence of statin use increased from 6.3 to 6.8% in women and 7.5 to 8.1% in men, the expenditure on statins was reduced by nearly 200 million NOK (€25 million) from the year before the new regulations compared with the year after. The estimated reduced costs are attributed both to price reductions and an increased proportion of simvastatin users.
Many European countries discuss how switching to the cheaper generics of simvastatin could save money for the public budgets [10], but very few have yet implemented a switching policy as part of their national regulations or recommendations. Already in December 2004, the Norwegian health authorities distributed the drafted new regulations to interested parties. The increased proportion of simvastatin prescribed to new users observed in the period January to May 2005 reflects that physicians adjusted their prescribing before the new regulations were implemented.
Some patients with established coronary heart disease (CHD) or at high risk of developing CHD may benefit from initiating statin therapy with a high dose of atorvastatin [11–13]. While the overall number of new atorvastatin users gradually decreased after the new regulations (Figure 2), the proportion of the new atorvastatin users receiving 80 mg increased. This trend may reflect a tendency towards reserving atorvastatin for new patients considered to need aggressive treatment.
Switching to simvastatin was studied only in the population of previous users of atorvastatin. However, the number of other statin users has also decreased after the new regulations, indicating that switching to simvastatin has also occurred among these users (Table 1). Individuals using 20 mg atorvastatin should be switched to 40 mg simvastatin according to recommendations given by the Norwegian Medicines Agency [5]. Our study has shown that switching decreases with increasing doses of atorvastatin used, reflecting that physicians consider switching to be less appropriate in the higher dose range. The maximum approved recommended dose for simvastatin is 80 mg, and it is therefore not possible to double the simvastatin dose when switching from a dose of 80 mg atorvastatin. Nevertheless, 17% of patients using 80 mg atorvastatin were switched to simvastatin. Clinical considerations related to achievement of lipid treatment goals or tolerance issues could be reasons for switching from 80 mg atorvastatin to simvastatin, rather than the new reimbursement regulations.
A slightly lower proportion received other cardiovascular drugs in the group of atorvastatin users who switched to simvastatin, compared with those who continued on atorvastatin (Table 2). However, it is difficult to judge whether these differences reflect a true difference in overall cardiovascular risk in these two groups.
The strength of this study is that it provides detailed information about all dispensed prescriptions of statins to individuals in the total population in Norway. The NorPD contains information that makes it possible to follow each individual over time in order to study changes in pharmacological treatment. This approach eliminates the possibility of selection and recall bias. Several other countries have healthcare databases containing information on drug prescriptions. However, many of these are based on insurance plans which cover only parts of the population, which may introduce selection bias.
The lack of information about baseline clinical characteristics (e.g. lipid levels, patient cardiovascular risk) as well as clinical outcome measures in the population of statin users is a limitation of our study. The follow-up period after the new statin regulations was too short to assess changes in treatment outcomes. However, it would be important to follow the population of statin users in the future to ensure that the new statin regulations have no negative impact on cardiovascular morbidity or mortality. Compliance with and persistence of statin treatment related to switching of statins have not been studied.
The success of a new reimbursement policy depends on collaboration with the physicians. Our study indicates that physicians acknowledge their responsibility to contribute to cost containment in Norway.
Acknowledgments
Competing interests: None declared.
References
- 1.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 2.Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol. 2005;60:543–51. doi: 10.1111/j.1365-2125.2005.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hågå A, Sverre JM. Pricing and reimbursement of pharmaceuticals in Norway. Eur J Health Econom. 2002;3:215–20. doi: 10.1007/s10198-002-0135-4. [DOI] [PubMed] [Google Scholar]
- 4. [2007 April]. Evaluering av Trinnprismodellen (In English: Assessment of the Graded Pricing System). Norwegian Medicines Agency 2006. Available at URL: http://www.legemiddelverket.no/upload/25534/Evaluering%20av%20Trinnprissystemet%20-%20versjon%202.pdf.
- 5. [2007 April]. Refusjon: Statiner—nye vilkår for refusjon (In English: Statins—New Reimbursement Regulations). Norwegian Medicines Agency 2005. Available at URL: http://www.legemiddelverket.no/templates/InterPage_21566.aspx.
- 6.Rønning M, editor. Legemiddelforbruket i Norge 2001–2005. Oslo: Norwegian Institute of Public Health; 2006. [Google Scholar]
- 7.Furu K, Strøm H, Rønning M, Skurtveit S, Engeland A, Tverdal A. The Norwegian Prescription Database (NorPD)—a new register for pharmacoepidemiologic research covering a whole nation. Pharmacoepidemiol Drug Safety. 2005;14:S49. [Google Scholar]
- 8.ATC Classification Index with DDDs 2006. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2005. [Google Scholar]
- 9.Guidelines for ATC Classification and DDD Assignment 2006. 9. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2005. [Google Scholar]
- 10.Moon JC, Bogle RG. Switching statins. BMJ. 2006;332:1344–5. doi: 10.1136/bmj.332.7554.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holmel, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 12.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 13.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]