Abstract
What is already known about this subject
Repeated cycles of electrical stimulation inhibit cutaneous vasoconstriction to noradrenaline, but the mechanism is unknown.
Investigating this is important because peripheral electrical stimulation is useful for pain modulation and appears to assist cutaneous wound healing.
What this study adds
Intermittent, brief electrical stimulation of the forearm over a 10-day period inhibited vasoconstriction and axon-reflex vasodilation to noradrenaline, but did not affect vasoconstriction to vasopressin or axon-reflex vasodilation to histamine.
Thus, electrical stimulation may evoke a specific reduction in responsiveness to noradrenaline.
Aim
To investigate whether desensitization to the vasomotor effects of noradrenaline is a specific effect of electrical stimulation.
Methods
Three sites on the forearm of 10 healthy volunteers were stimulated with 0.2 mA direct current for 2 min twice daily for 10 days. Noradrenaline and histamine were then displaced from ring-shaped iontophoresis chambers into two of the pretreated sites and two untreated sites on the contralateral forearm. Axon-reflex vasodilation was measured from the centre of the ring described by the iontophoresis chamber with a laser Doppler flowmeter. One or two days later, noradrenaline and vasopressin were introduced into pretreated and untreated sites by iontophoresis, and vasoconstriction at sites of administration was measured in the heated forearm.
Results
The pretreatment blocked vasoconstriction to noradrenaline [median increase in flow 1%, interquartile range (IR) −41 to 52%; median decrease at the untreated site 53%, IR. −70 to −10%; P < 0.05], but did not block vasoconstriction to vasopressin (median decrease 42% at the untreated site and 45% at the pretreated site). Axon-reflex vasodilation to noradrenaline was diminished at the pretreated site (median increase in flow 33%, IR 2–321%; untreated site 247%, IR 31–1087%; P < 0.05). However, axon-reflex vasodilation to histamine did not differ significantly between the pretreated site (median increase 1085%) and the untreated site (median increase 1345%).
Conclusions
The conditioning pretreatment appears to evoke a specific decrease in responsiveness to noradrenaline. Repeated cycles of electrical stimulation may downregulate neural and vascular responses to noradrenaline by repetitively activating cutaneous sympathetic nerve fibres.
Keywords: adrenergic vasoconstriction, axon reflex, desensitization, noradrenaline, sympathetic nervous system
Introduction
Local administration of noradrenaline by iontophoresis produces a dose-dependent decrease in skin blood flow in the heated human forearm [1]. Curiously, however, this adrenergic vasoconstrictor response is minimal in forearm skin conditioned by brief, occasional administration of direct anodal current over a 4–10-day period [2]. Investigating this effect might be of clinical interest, because peripheral electrical stimulation is useful for pain modulation and appears to assist cutaneous wound healing [3, 4].
At least three mechanisms could contribute to loss of adrenergic vasoconstriction following electrical stimulation of the skin. First, electrical stimulation induces the antidromic release of vasoactive neuropeptides such as calcitonin gene-related peptide and substance P from sensory nerve terminals [5]. Release of these neuropeptides from capsaicin-sensitive cutaneous nociceptors initiates a vasodilator response that spreads from the site of stimulation to encompass the terminal distribution of the stimulated neurone [6]. Persistent neurogenic vasodilation between cycles of electrical stimulation might counter adrenergic vasoconstriction. Second, prostaglandin synthesis under the contact electrodes used for iontophoresis contributes to current-induced vasodilation [7, 8]. This vasodilator response might also mask adrenergic vasoconstriction. Third, the electrical stimulus could provoke the local release of noradrenaline from sympathetic nerve terminals. Although this would initially oppose neurogenic vasodilation to the electrical current [9], repeated electrically evoked release of noradrenaline might downregulate cutaneous vascular adrenoceptors [10], thereby inhibiting adrenergic vasoconstriction.
The aim of the present study was to examine the effect of the conditioning pretreatment on vasoconstriction to noradrenaline and vasopressin and on axon-reflex vasodilation to noradrenaline and histamine in the skin of the human forearm. Selective attenuation of adrenergic responses would support the hypothesis that repetitive electrical stimulation promotes the desensitization of cutaneous adrenoceptors. Introduction of histamine into the skin of the forearm provokes a flare, mediated by H1 receptors, that spreads well beyond the site of stimulation [11]. A flare that is greater than the response to electrical stimulation alone, and that is inhibited by topically applied local anaesthetic agent, also develops after the iontophoresis of noradrenaline in the human forearm [12]. The mechanism of this vasodilator response is uncertain, but may involve direct stimulation of adrenergic receptors in the skin. If so, electrically provoked desensitization of these adrenergic receptors should attenuate the flare to noradrenaline, but should not affect the flare provoked by histamine. To investigate these hypotheses, vascular responses to vasopressin, histamine and noradrenaline were examined at pretreated and untreated sites in the human forearm.
Methods
Subjects
The sample consisted of 10 healthy volunteers (three men and seven women aged between 21 and 38 years) who were not taking prescription medication (apart from the oral contraceptive) for any medical condition. They each provided informed consent for the procedures, which were approved by the Murdoch University Human Research Ethics Committee.
Overview of the procedures
After 10 days of pretreatment at three sites on the forearm, subjects participated in two experiments at 1–2-day intervals. The pretreatment regime was maintained at the three experimental sites between the experiments. In each of the experiments described below, drugs were introduced into pretreated and untreated skin by iontophoresis, and effects of the drug on skin blood flow were evaluated. Different iontophoresis capsules were used for each drug solution to prevent any inadvertent mixing of the drugs.
Pretreatment
A perspex capsule with an internal chamber diameter of 20 mm was attached with an adhesive washer to a site on the dorsal aspect of the left or right forearm, and a 3 cm × 5 cm silver plate coated with conductive gel that acted as a cathode was attached to the upper arm. To ensure that the capsule adhered firmly and that good electrical contact was made, the site was shaved, if necessary, and cleaned with an isopropyl alcohol swab. Care was taken not to touch the skin with the razor while the hair was being removed. The chamber was filled with a conducting medium (0.9% saline) and the skin underneath the chamber was stimulated with direct anodal current of 0.2 mA for 2 min. The perimeter of the capsule was marked with indelible ink so that the capsule could be repositioned accurately when the electrical stimulation was repeated. The same procedure was carried out at two additional sites on the same forearm, separated from the other sites by at least 10 cm. Each site was stimulated twice per day for the next 10 days. This pretreatment sequence inhibits vasoconstrictor responses to noradrenaline administered by iontophoresis in the skin of the human forearm [2].
Experiment 1: axon-reflex vasodilation to noradrenaline and histamine
Solutions of 0.5 mm norepinephrine bitartrate (noradrenaline; Sigma, Sydney, Australia) and 0.5 mm histamine hydrochloride (Sigma) were prepared on the day of the experiment with distilled water from stock solutions at 10 mm. Fresh stock solutions were prepared fortnightly and stored in a refrigerator at 4°C. Two of the pretreated sites (selected at random) were cleaned with isopropyl alcohol and two additional sites on the contralateral forearm were shaved, if necessary, and cleaned. An iontophoresis capsule with a ring-shaped drug solution chamber (inner diameter 15 mm and outer diameter 19 mm) was attached to one of the prepared sites and filled with the noradrenaline or histamine solution. Adhesive tape was used to form a waterproof seal on each side of the drug solution chamber. During each iontophoresis, a weak electric current repelled positively charged noradrenaline or histamine molecules away from the anode into the underlying skin. For noradrenaline, a direct current of 350 µA was employed for 3 min, because the dose delivered by this current induces an axon reflex that is substantially greater than the axon reflex induced by the current itself [12]. A 2-min 100-µA direct current was used to introduce histamine into the skin because pilot studies have indicated that the dose delivered by this current induces substantial axon reflex vasodilation. The electrical circuit was completed with a cathode attached to the upper arm. The order of drug administration was counterbalanced across pretreated and untreated sites and was alternated between the left and right arms.
Before, during and after the iontophoreses, skin blood flow was detected with a wide surface area laser Doppler flow probe positioned in the centre of the ring described by the iontophoresis chamber, approximately 1 cm from the site of drug administration. Changes in skin blood flow to a depth of 1–2 mm were monitored with a Moor Instruments MBF3D laser Doppler flowmeter (Axminster, UK). In addition, changes in blood flow were detected with a second laser Doppler flow probe positioned 10–15 cm from the sites of iontophoresis. The signals were sampled at 5 Hz and later averaged using Moorsoft software for 2 min before the iontophoresis, and between 5 and 7 min after the iontophoresis when vasodilation peaked. Changes in skin blood flow after the iontophoresis were expressed as the difference in arbitrary units of flow and the percentage of levels recorded at the same site before the iontophoresis.
Experiment 2: vasoconstriction to noradrenaline and vasopressin
Noradrenaline (0.5 mm) was introduced into one of the three pretreated sites (selected at random) from an iontophoresis capsule with a chamber diameter of 10 mm using a 50-µA direct current for 60 s. Noradrenaline was also introduced into an untreated site 3–5 cm away on the forearm at the same current intensity and duration. The dose of noradrenaline delivered by this current was expected to produce minor vasoconstriction at the site of administration [1]. Vasopressin was introduced into the second pretreated site and an untreated site nearby. The same stimulus parameters were used for noradrenaline and vasopressin, to ensure that any nonspecific effects of iontophoresis on skin blood flow were similar at both sites. The electric current was also passed through a conducting medium (0.9% saline) at the third pretreated site and another untreated site nearby in the same forearm, to delineate any nonspecific effect of iontophoresis. Laser Doppler flow probe holders were then attached over the sites of iontophoresis and over a reference site several centimetres away from the other sites with adhesive washers.
To ensure that normal endogenous constrictive influences on cutaneous vessels were minimal, the forearm was immersed in 42°C water for 10 min before vascular measures commenced. Skin blood flow increases for around 35 min in the heated forearm before reaching an upper limit [13]. However, only 10–15 min of heating was employed in the present study because laser Doppler measures of flow appear to saturate before skin blood flow peaks [13]. In forearm skin heated to 42°C for 5–10 min, vasoconstriction increases in direct proportion to the dose of noradrenaline administered by iontophoresis [1], indicating that the vasodilation induced by short periods of heating is great enough to overcome floor effects that would otherwise obscure responses to vasoconstrictive drugs. Blood flow was measured via a wide diameter laser Doppler flow probe for at least 30 s at each iontophoresis site in the immersed forearm until a stable recording was obtained. Blood flow was also measured at the reference site with the same flow probe before and after blood flow had been measured at the other sites. The iontophoreses were then repeated at each site, this time with a 50-µA direct current for 120 s. The dose of noradrenaline delivered by this current was expected to produce moderate vasoconstriction at the site of administration [1]. The arm was replaced in the 42°C water for 10 min, and blood flow was measured again at each site. Responses to noradrenaline and vasopressin were expressed as the difference in arbitrary units of flow and the percentage of levels recorded at the reference site.
Data analysis
Preliminary analyses indicated that many of the score distributions departed from a normal bell-shaped curve. Therefore, differences between pretreated and untreated sites were investigated with Wilcoxon's matched-pairs signed ranks test. The criterion of statistical significance was P < 0.05. As vasoconstriction to noradrenaline and vasopressin did not change significantly from the first to the second set of iontophoreses, responses to the second set of iontophoreses are presented in this study.
Results
Experiment 1: axon-reflex vasodilation to noradrenaline and histamine
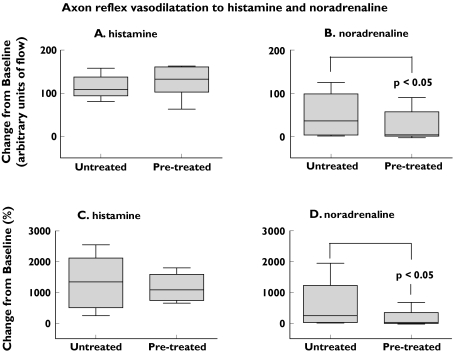
Skin blood flow did not differ significantly between the pretreated and untreated sites before the histamine iontophoreses [median flow at the untreated site 8.5 arbitrary units (AU), interquartile range (IR) 6.4–18.9; median flow at the pretreated site 10.5 AU, IR 8.1–17.4]. Blood flow at the central recording site approximately 1 cm from the site of drug administration increased substantially after the histamine iontophoresis, both in pretreated and untreated skin (difference between sites not significant) (Figure 1A,C).
Figure 1.
Change in blood flow at central recording sites 1 cm distant from the site of administration of histamine or noradrenaline in pretreated and untreated skin. Increases in blood flow after the noradrenaline iontophoreses were greater in untreated than pretreated skin (P< 0.05). The box-and-whisker plots in Figures 1 and 2 represent the range of scores (whiskers), the 25th and 75th percentiles (the outline of the box represents the interquartile range), and the median (the line through the box)
Skin blood flow did not differ significantly between the pretreated and untreated sites before the noradrenaline iontophoreses (median flow at the untreated site 10.3 AU, IR 7.6–13.1; median flow at the pretreated site 11.9 AU, IR 8.2–18.9). Blood flow increased at the central recording site after the noradrenaline iontophoresis, more so at untreated than pretreated sites (for arbitrary units of flow, Wilcoxon's Z = 2.31, P < 0.05, Figure 1B; for percent change from baseline, Wilcoxon's Z = 2.19, P < 0.05, Figure 1D). Changes in blood flow at more distant sites in the forearm were minimal.
Experiment 2: vasoconstriction to noradrenaline and vasopressin
Median blood flow at the reference site in the heated skin changed from 100 AU to 102 AU during the 4–5-min period required for measuring flow at the various sites (Wilcoxon's Z = 0.0, not significant).
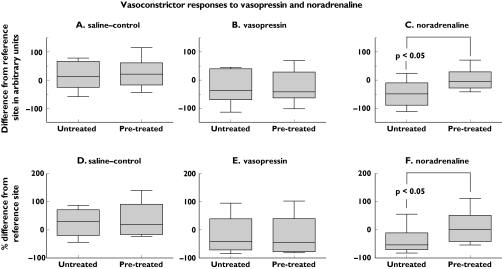
In the group as a whole, vasoconstriction to vasopressin was similar at the pretreated and untreated sites (Figure 2B,E). There was some individual variation in the intensity of vasoconstriction to vasopressin in untreated skin. Nevertheless, vasoconstriction to vasopressin did not differ significantly between untreated and pretreated sites in six subjects with a clear vasoconstrictor response to vasopressin [median response in untreated skin −55 AU (IR −82 to −35) compared with −37 AU (IR −75 to 28) in pretreated skin, Wilcoxon's Z = 0.73, not significant; in addition, the median response was 55% (IR 79–36%) below reference levels in untreated skin compared with 41% below reference levels (IR 80% below to 28% above reference levels) in pretreated skin, Wilcoxon's Z = 0.73, not significant].
Figure 2.
Vascular responses to vasopressin, noradrenaline and saline-control iontophoreses at pretreated and untreated sites in the forearm. Responses after the second set of iontophoreses are shown and are expressed in relation to blood flow at a reference site in the forearm. Decreases in blood flow after the noradrenaline iontophoreses were greater in untreated than pretreated skin (P< 0.05)
In contrast to vasopressin, adrenergic vasoconstriction developed at the untreated site in all but one subject and was significantly greater in untreated than pretreated skin (for arbitrary units of flow, Wilcoxon's Z = 2.31, P < 0.05, Figure 2C; for percent change from reference sites, Wilcoxon's Z = 2.31, P < 0.05, Figure 2E).
Discussion
Pretreatment of the skin of the forearm with brief cycles of electrical stimulation over a 10-day period produced two interesting effects. First, the pretreatment blocked vasoconstriction to noradrenaline, thus confirming previous findings [2], but did not block vasoconstriction to vasopressin. Second, the pretreatment reduced axon-reflex vasodilation to noradrenaline, whereas axon-reflex vasodilation to histamine remained largely unchanged.
Drummond and Lipnicki [2] reported that skin blood flow in the heated forearm was 58% lower than blood flow at reference sites after the iontophoresis of noradrenaline; however, flow at a site pretreated with brief cycles of electrical stimulation was only 7% lower than flow at reference sites. After similar procedures in the present experiment, the median decrease in blood flow was 53% below reference levels at the untreated site compared with an increase of 1% at the pretreated site. Thus, the conditioning pretreatment blocked adrenergic vasoconstriction in both studies. The vasoconstrictive response apparently peaked at the untreated site because doubling the dose produced no further vasoconstriction. Although adrenergic vasoconstriction was minimal at the pretreated site, vasoconstriction to vasopressin was unaffected by the pretreatment. Thus, the conditioning pretreatment appeared to evoke a specific decrease in the vascular response to noradrenaline.
Iontophoresis of noradrenaline triggered vasodilation in nearby skin, both in the present and a previous study [12]. Topical application of local anaesthetic agent blocks the flare to noradrenaline, consistent with neural mediation of this response [12]. Houghton et al. [14] have recently reported that low-dose noradrenaline infusion via intradermal microdialysis fibres facilitates axon-reflex vasodilation to gradual local heating of the skin; conversely, adrenergic blockade inhibits axon-reflex vasodilation to direct heating. However, in the absence of iontophoretic currents or local heating, intradermal administration of noradrenaline through microdialysis fibres does not induce axon-reflex vasodilation [15]. One potential explanation for these findings is that an axon reflex to direct heat or iontophoretic currents disrupts protective barriers that normally shield adrenoceptors from adrenergic agents in the extracellular fluid [16, 17]. Excitation of the unshielded adrenoceptors might then facilitate the axon reflex initiated by heating the skin or by iontophoretic currents.
The conditioning pretreatment inhibited the flare to noradrenaline, consistent with desensitization of adrenergic receptors on the afferent limb of the axon reflex. In contrast to noradrenaline, responses to histamine in pretreated skin were similar to (although less variable than) responses in untreated skin, at least when expressed as percent change from baseline. This decrease in variability may have been due to depletion of neuropeptide stores in sensory nerve fibres or downregulation of vascular responses to these neuropeptides. The alternative possibility, that the pretreatment disrupted drug delivery into the skin, seems unlikely because the vasoconstrictor response to vasopressin was unaffected by the pretreatment. In the short term, passage of an electric current through a saline solution enhances vasoconstriction to adrenergic agents administered subsequently by iontophoresis [18], possibly because an axon reflex initiated by the iontophoretic current assists the entry of pharmacological agents into the skin.
Taken together, the findings suggest that repeated cycles of electrical stimulation downregulate neural and vascular responses to noradrenaline. Periarterial nerve stimulation triggers the release of noradrenaline from adrenergic nerves and calcitonin gene-related peptide from capsaicin-sensitive sensory nerves [19, 20]. Exposure to a high concentration of adrenergic agonists desensitizes vascular smooth muscle to these agents by decreasing the number of postjunctional α-adrenergic binding sites [10]. In the present study, desensitization may have spilt over to adrenergic receptors that directly or indirectly excite the sensory afferents that mediate axon reflexes. Evidence from several sources suggests that α-adrenoceptors are normally present in the cell bodies of primary afferent neurones. For example, messenger RNA for α1-adrenoceptors was detected in the superficial dorsal horn and dorsal root ganglia of rats [21]. In addition, noradrenaline and the α1-adrenoceptor agonist phenylephrine increased the excitability of cultured dorsal root ganglion neurones [22, 23]. Further investigation is required to determine whether noradrenaline binds directly to neural adrenoceptors or stimulates sensory nerves indirectly by releasing nociceptive mediators such as prostaglandins or nitric oxide [24, 25].
Noradrenaline increases sensitivity to heat, particularly in inflamed skin [16, 26]. Conversely, the conditioning pretreatment inhibits thermal hyperalgesia induced by the topical application of capsaicin, and reduces adrenergic hyperalgesia in heat-sensitized skin [2]. Thus, the conditioning pretreatment could potentially be beneficial for pain control in patients with painful neuropathies associated with heightened adrenergic sensitivity [27, 28].
Electrical stimulation may also accelerate the healing of chronic superficial wounds, in part by increasing blood flow and tissue oxygenation around the site of injury [4]. A reduction in adrenergic vasoconstrictor tone following brief cycles of electrical stimulation could facilitate the healing of ischaemic wounds. The persistence of axon-reflex vasodilation to histamine, despite a reduction in adrenergic sensitivity, is particularly encouraging, because in the presence of an adequate blood supply neurogenic inflammation may assist normal healing processes [29].
Vasodilator responses to histamine and noradrenaline were extremely variable, possibly because responses were expressed in relation to low levels of blood flow at baseline. In future studies, standardizing responses against an estimate of maximal flow at each site (induced by sustained local heating or sodium nitroprusside administration) [30–32] might reduce variability and permit more precise analysis of treatment effects. Delineation of dose–response relationships would also help to clarify treatment effects.
Finally, the sample was small and consisted of healthy young adults, some of whom were taking oral contraceptives. Effects of age, gender, cardiovascular disease, inflammation, peripheral neuropathy and drug treatments on electrically evoked release of noradrenaline in the skin, and on desensitization of responses to noradrenaline, require further study.
Acknowledgments
This project was supported by the Medical Research Fund of Western Australia. The author gratefully acknowledges the research assistance of Miss Etsuko Uno and Mr Darren Hocking.
References
- 1.Lipnicki DM, Drummond PD. Facilitating laser Doppler measurements of cutaneous adrenergic vasoconstriction: a comparison of methods. Clin Auton Res. 2001;11:93–8. doi: 10.1007/BF02322052. [DOI] [PubMed] [Google Scholar]
- 2.Drummond PD, Lipnicki DM. Repeated local administration of noradrenaline or saline inhibits thermal hyperalgesia in pain-sensitized human skin. Br J Clin Pharmacol. 2001;52:289–95. doi: 10.1046/j.0306-5251.2001.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojingwa JC, Isseroff RR. Electrical stimulation of wound healing. J Invest Dermatol. 2003;121:1–12. doi: 10.1046/j.1523-1747.2003.12454.x. [DOI] [PubMed] [Google Scholar]
- 4.Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds. 2005;4:23–44. doi: 10.1177/1534734605275733. [DOI] [PubMed] [Google Scholar]
- 5.Sauerstein K, Klede M, Hilliges M, Schmelz M. Electrically evoked neuropeptide release and neurogenic inflammation differ between rat and human skin. J Physiol. 2000;529:803–10. doi: 10.1111/j.1469-7793.2000.00803.x. Part 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magerl W, Szolcsanyi J, Westerman RA, Handwerker HO. Laser Doppler measurements of skin vasodilation elicited by percutaneous electrical stimulation of nociceptors in humans. Neurosci Lett. 1987;82:349–54. doi: 10.1016/0304-3940(87)90281-3. [DOI] [PubMed] [Google Scholar]
- 7.Tartas M, Bouye P, Koitka A, Jaquinandi V, Tan L, Saumet JL, Abraham P. Cathodal current-induced vasodilation to single application and the amplified response to repeated application in humans rely on aspirin-sensitive mechanisms. J Appl Physiol. 2005;99:1538–44. doi: 10.1152/japplphysiol.00258.2005. [DOI] [PubMed] [Google Scholar]
- 8.Tartas M, Bouye P, Koitka A, Durand S, Gallois Y, Saumet JL, Abraham P. Early vasodilator response to anodal current application in human is not impaired by cyclooxygenase-2 blockade. Am J Physiol Heart Circ Physiol. 2005;288:H1668–73. doi: 10.1152/ajpheart.00415.2004. [DOI] [PubMed] [Google Scholar]
- 9.Hornyak ME, Naver HK, Rydenhag B, Wallin BG. Sympathetic activity influences the vascular axon reflex in the skin. Acta Physiol Scand. 1990;139:77–84. doi: 10.1111/j.1748-1716.1990.tb08899.x. [DOI] [PubMed] [Google Scholar]
- 10.Colucci WS, Gimbrone MA, Jr, Alexander RW. Regulation of the postsynaptic alpha-adrenergic receptor in rat mesenteric artery. Effects of chemical sympathectomy and epinephrine treatment. Circ Res. 1981;48:104–11. doi: 10.1161/01.res.48.1.104. [DOI] [PubMed] [Google Scholar]
- 11.Clough GF, Bennett AR, Church MK. Effects of H1 antagonists on the cutaneous vascular response to histamine and bradykinin: a study using scanning laser Doppler imaging. Br J Dermatol. 1998;138:806–14. doi: 10.1046/j.1365-2133.1998.02217.x. [DOI] [PubMed] [Google Scholar]
- 12.Drummond PD, Lipnicki DM. Noradrenaline provokes axon reflex hyperaemia in the skin of the human forearm. J Auton Nerv Syst. 1999;77:39–44. doi: 10.1016/s0165-1838(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 13.Savage MV, Brengelmann GL. Reproducibility of the vascular response to heating in human skin. J Appl Physiol. 1994;76:1759–63. doi: 10.1152/jappl.1994.76.4.1759. [DOI] [PubMed] [Google Scholar]
- 14.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–20. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahn S, Leis S, Schick C, Schmelz M, Birklein F. No alpha-adrenoreceptor-induced C-fiber activation in healthy human skin. J Appl Physiol. 2004;96:1380–4. doi: 10.1152/japplphysiol.00990.2003. [DOI] [PubMed] [Google Scholar]
- 16.Drummond PD. Enhancement of thermal hyperalgesia by alpha-adrenoceptors in capsaicin-treated skin. J Auton Nerv Syst. 1998;69:96–102. doi: 10.1016/s0165-1838(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 17.Drummond PD. The effect of sympathetic activity on thermal hyperalgesia in capsaicin-treated skin during body cooling and warming. Eur J Pain. 2001;5:59–67. doi: 10.1053/eujp.2001.0224. [DOI] [PubMed] [Google Scholar]
- 18.Drummond PD. Prior iontophoresis of saline enhances vasoconstriction to phenylephrine and clonidine in the skin of the human forearm. Br J Clin Pharmacol. 2002;54:45–50. doi: 10.1046/j.1365-2125.2002.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maynard KI, Saville VL, Burnstock G. Sensory-motor neuromodulation of sympathetic vasoconstriction in the rabbit central ear artery. Eur J Pharmacol. 1990;187:171–82. doi: 10.1016/0014-2999(90)90004-p. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki H, Nuki C, Saito A, Takasaki K. Role of calcitonin gene-related peptide-containing nerves in the vascular adrenergic neurotransmission. J Pharmacol Exp Ther. 1990;252:403–9. [PubMed] [Google Scholar]
- 21.Nicholson R, Dixon AK, Spanswick D, Lee K. Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci Lett. 2005;380:316–21. doi: 10.1016/j.neulet.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 22.Kasai M, Mizumura K. Increase in spontaneous action potentials and sensitivity in response to norepinephrine in dorsal root ganglion neurons of adjuvant inflamed rats. Neurosci Res. 2001;39:109–13. doi: 10.1016/s0168-0102(00)00201-7. [DOI] [PubMed] [Google Scholar]
- 23.Pluteanu F, Ristoiu V, Flonta ML, Reid G. Alpha (1)-adrenoceptor-mediated depolarization and beta-mediated hyperpolarization in cultured rat dorsal root ganglion neurones. Neurosci Lett. 2002;329:277–80. doi: 10.1016/s0304-3940(02)00665-1. [DOI] [PubMed] [Google Scholar]
- 24.Khasar SG, Green PG, Chou B, Levine JD. Peripheral nociceptive effects of alpha 2-adrenergic receptor agonists in the rat. Neuroscience. 1995;66:427–32. doi: 10.1016/0306-4522(94)00562-j. [DOI] [PubMed] [Google Scholar]
- 25.Hu ZW, Miller JW, Hoffman BB. Induction of enhanced release of endothelium-derived relaxing factor after prolonged exposure to alpha-adrenergic agonists: role in desensitization of smooth muscle contraction. J Cardiovasc Pharmacol. 1994;23:337–43. [PubMed] [Google Scholar]
- 26.Fuchs PN, Meyer RA, Raja SN. Heat, but not mechanical hyperalgesia, following adrenergic injections in normal human skin. Pain. 2001;90:15–23. doi: 10.1016/s0304-3959(00)00381-x. [DOI] [PubMed] [Google Scholar]
- 27.Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–8. doi: 10.1016/S0304-3959(00)00327-4. [DOI] [PubMed] [Google Scholar]
- 28.Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case–control study. Lancet. 2002;359:1655–60. doi: 10.1016/S0140-6736(02)08589-6. [DOI] [PubMed] [Google Scholar]
- 29.Khalil Z, Helme R. Sensory peptides as neuromodulators of wound healing in aged rats. J Gerontol A Biol Sci Med Sci. 1996;51:B354–61. doi: 10.1093/gerona/51a.5.b354. [DOI] [PubMed] [Google Scholar]
- 30.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol. 1984;57:191–6. doi: 10.1152/jappl.1984.57.1.191. [DOI] [PubMed] [Google Scholar]
- 31.Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–9. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 32.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]