Abstract
Sensorineural hearing loss, which is often caused by degeneration of hair cells in the auditory epithelium, is permanent because lost hair cells are not replaced. Several conceptual approaches can be used to place new hair cells in the auditory epithelium. One possibility is to enhance proliferation of non-sensory cells that remain in the deaf ear and induce transdifferentiation of some of these cells into the hair cell phenotype. Several genes, including p27Kip1, have been shown to regulate proliferation and differentiation in the developing auditory epithelium. The role of p27Kip1 in the mature ear is not well characterized. We now show that p27Kip1 is present in the nuclei of non-sensory cells of the mature auditory epithelium. We determined that forced expression of Skp2 using a recombinant adenovirus vector, resulted in presence of BrdU-positive cells in the auditory epithelium. When SKP2 over-expression was combined with forced expression of Atoh1, ectopic hair cells were found in the auditory epithelium in greater numbers than were seen with Atoh1 alone. Skp2 over-expression alone did not result in ectopic hair cells. These findings suggest that the p27Kip1 protein remains in the mature auditory epithelium and therefore p27Kip1 can serve as a target for gene manipulation. The data also suggest that induced proliferation, by itself, does not generate new hair cells in the cochlea.
Keywords: Adenovirus, gene transfer, p27Kip1, Atoh1, Skp2, guinea pig, hair cell, supporting cell, BrdU
1. Introduction
The sensory epithelium of hearing in mammals consists of terminally differentiated epithelial cells: sensory hair cells and non-sensory supporting cells. These cells are quiescent in mammals. Therefore, hair cell degeneration is irreversible and leads to sensorineural hearing loss. One potential therapy for hearing loss is induction of hair cell regeneration in the organ of Corti, the sensory region of the auditory epithelium.
Recent data demonstrate that forced expression of genes encoding hair cell development can induce transdifferentiation of non-sensory cells into new hair cells in the developing (Woods et al., 2004; Zheng and Gao, 2000) and mature organ of Corti (Izumikawa et al., 2005; Kawamoto et al., 2003; Shou et al., 2003). As therapy, this procedure would be suboptimal because transdifferentiation of supporting cells into new hair cells does not involve mitosis in the tissue. Thus, formation of new hair cells would reduce the number of supporting cells and compromise the ability to restore normal cochlear structure and function. In birds, where hair cell regeneration leads to functional recovery (Dooling et al., 1997; Marean et al., 1995; Niemiec et al., 1994; Saunders et al., 1992), non-sensory cells divide after a lesion to the epithelium (Hashino and Salvi, 1993; Raphael, 1992; Stone and Cotanche, 1994). To induce proliferation in the mature organ of Corti as part of the reparative process, it may be necessary to manipulate expression of genes that regulate cell cycle.
Among the genes that regulate cell-cycle proteins in the developing inner ear are p27kip1 (Chen and Segil, 1999; Lowenheim et al., 1999), Ink4d (Chen et al., 2003) and Rb1 (Sage et al., 2005). Cell proliferation past the normal developmental cessation of mitosis has been shown in these transgenic mice. The ability to remove the inhibition of cell cycle in the mature inner ear, in a cell or organ specific manner, may potentially be used for developing clinical therapy for hair cell regeneration. One important step for inducing proliferation in the mature auditory epithelium is to identify and localize the cell cycle regulating molecules that are present in the tissue. This set of experiments was designed to determine whether p27Kip1 is present in the mature guinea pig auditory epithelium and to test outcome of blocking this protein with Skp2.
p27Kip1 is a cyclin-dependent kinase-2 (cdk-2) inhibitor (Sherr and Roberts, 1999). p27kip1 acts as a negative regulator of the G1–S transition of the cell cycle (Harper, 2001). Skp2 is an F-box protein and substrate of recognition component of Cullin 1 (CUL1) for SCF ubiquitin ligase (Nakayama et al., 2000). Skp2 induces the G1 to G0 transition of the cell cycle through ubiquitination of p27kip1 and cyclin E (Nakayama KI, 2001). As such, it may be used to antagonize the inhibition exerted on cell cycle by p27Kip1. During inner ear development in the mouse embryo, a down-regulation of Skp2 expression was noted to coincide with onset of p27Kip1 expression in the non-sensory cells of the auditory epithelium (Dong et al., 2003).
Removal of inhibition on cell cycle in the auditory epithelium may not necessarily lead to formation of new hair cells. In birds and other non-mammalian vertebrates, the process of hair cell regeneration occurs spontaneously, with or without mitosis (Cotanche, 1997; Stone and Rubel, 2000). In mice with dysfunctional p27Kip1 supernumerary hair cells are formed (Chen and Segil, 1999; Kanzaki et al., 2006; Lowenheim et al., 1999). The outcome of inducing cell proliferation in the mature auditory epithelium is unknown. If new cells do not take up the hair cell phenotype, it may be necessary to induce transdifferentiation with forced expression of genes such as Atoh1. Atoh1 (formerly Math1) is a basic helix-loop-helix (bHLH) transcription factor that is essential for generating hair cells in developing inner ear (Bermingham et al., 1999; Chen et al., 2002; Zine et al., 2001).
After maturation of hair cells in developing mammals, the expression of Atoh1 is down-regulated (Zheng et al., 2000). However, over-expression of Atoh1 (or its homologs) in cultures of developing or mature rat cochleae results in the production of ectopic hair cells derived from non-sensory epithelial cells (Shou et al., 2003; Zheng and Gao, 2000). Over-expression of Atoh1 has also been shown to generate new hair cells in mature guinea pig cochleae in vivo (Izumikawa et al., 2005; Kawamoto et al., 2003). The goal of our experiments was to localize p27Kip1 in the mature auditory epithelium, to determine if forced expression of SKP2 can induce proliferation in the tissue and to assess the potential for generation of new ectopic hair cells by SKP2 alone versus SKP2 in combination with Atoh1 over-expression. We demonstrate that p27Kip1 is present in numerous types of non-sensory cells in the mature auditory epithelium and that over-expressing Skp2 can induce proliferation but no ectopic new hair cells are formed. Forced expression of SKP2 in combination with Atoh1 increases the number and alters the pattern of ectopic hair cell generation as compared with Atoh1 alone.
2. Materials and methods
Animal care and use were approved by institutional UCUCA committee and conformed to National Institutes of Health guidelines.
Adenovirus vectors
The vectors Ad.Atoh1 (5.2 × 1011 pfu/ml) and Ad.empty (5.1 × 1011 pfu/ml) were based on human adenovirus serotype 5 with E1, E3 and E4 regions deleted, as described previously (Brough et al., 1996). Ad.SKP2 (1.0 × 1012 pfu/ml) was constructed using the AdEasy system (He et al., 1998). Expression of the transgene insert in each of these vectors was driven by the human cytomegalovirus promoter. The recombinant adenoviruses were amplified and propagated as described previously (Gervais et al., 1998).
Animals and inoculation surgery
We used adult guinea pigs weighing 300–500 g at the beginning of the experiment. We inoculated 5 μl of the adenovirus vector or control solution into the 2nd turn scala media of the left ear, as previously described (Ishimoto et al., 2002). Briefly, animals were anesthetized with Rompun (i.m., xylazine, 10 mg/kg, Bayer, Shawnee Mission, KS) and Ketalar (i.m., ketamine HCl, 40 mg/kg, Parke Davis, Morris Plains, NJ). Chloramphenicol sodium succinate (i.m., 30 mg/kg) was administered as prophylaxis and 0.3 ml of 1% lidocaine HCl was injected subcutaneously in the post-auricular and neck areas, for local anesthesia. The animals were placed in a supine position on a thermo-regulated heated pad. Ventral skin was incised paramedially and the tympanic bulla was exposed. After opening the bony bulla, the lateral cochlea was revealed. A small perforation was made in the bone above the pigmented area of the stria vascularis using a fine surgical needle. A microcanula was inserted into the scala media through the perforation. The circumference of the inserted microcanula was sealed and covered with carboxylate cement (Durelon, 3M, St. Paul, MN).
To inoculate the fluid into the endolymph, we used a microcanula driven by an electromechanical infusion pump (Harvard Apparatus, Holliston, MA) operated at a rate of 1 μl/ml over 5 min. To inoculate Ad.SKP2 and Ad.Atoh1 combined, the two vector solutions were combined at a volume ration of 1:1 resulting in 50% reduction in the concentration of each vector. Once the inoculation was complete, a layer of carboxylate cement was placed over the inoculation site to minimize the leak from the fenestration after removing the canula. The incision was closed in two layers.
Scanning electron microscopy
SEM was performed in order to determine the distribution and the number of ectopic hair cells. We used 8 animals for the group receiving both Ad.SKP2 Ad.Atoh1, 5 animals for the Atoh1 alone group, 5 animals for the SKP2 alone group, 5 animals for the artificial endolymph group and 5 animals for the Ad.empty group. Animals were deeply anesthetized, exsanguinated and systemically perfused with glutaraldehyde (2% in phosphate buffer). Immediately following the perfusion, animals were decapitated, the temporal bones removed from the skull and the cochleae opened at the apical tip and the round and oval windows and immersed in fixative. Two hours later, the bony wall at was removed along with the lateral wall tissues (stria vascularis and spiral ligament) to reveal the surface of the sensory epithelium. In all samples, SEM evaluation was performed in areas including (from medial to lateral) the interdental cell region, the inner sulcus and the organ of Corti. Images were collected from all cochlear turns.
SEM analysis was performed for localizing and counting ectopic hair cells. To be counted, hair cells had to be localized to an ectopic site in the interdental cell area or the inner sulcus, and exhibit two or more stereocilia on the apical surface. The area immediately adjacent to the site of inoculation was excluded from the statistical analysis, because tissue in this area may have responded to the mechanical trauma (of the inoculation) as well as the presence of the transgenes.
Immunohistochemistry
Whole mounts of the auditory sensory epithelium and surrounding tissues were used to localize p27Kip1, Atoh1 and SKP2. To localize p27Kip1 in the auditory epithelium, we stained normal guinea pig cochleae from 4 animals with a monoclonal anti-mouse antibody specific to p27Kip1 (Neomarkers, Fremont, CA), diluted 1:200. To localize Atoh1 and SKP2 after combined Ad.Atoh1 and Ad.SKP2 inoculation, we obtained cochleae from 4 animals, 4 days after the inoculation. Contralateral ears served as controls. We fixed cochleae in 4% paraformaldehyde in phosphate buffer, pH 7.4, removed the spiral ligament, stria vascularis and tectorial membrane and then permeabilized the tissue with 0.3% Triton X-100 in PBS with 1% goat serum for 10 min. Nonspecific binding of secondary antibodies was blocked with 5% BSA in PBS for 20 min. Tissues were reacted with primary antibody, rinsed and incubated with the secondary antibody. Specimens were mounted on glass slides using Crystal Mount (Biomeda, Foster City, CA). To perform double staining of Atoh1 and SKP2, we used a primary anti-Atoh1 monoclonal antibody (1:4 dilution, University of Iowa Hybridoma Core) and a rabbit polyclonal anti-SKP2 antibody (1:300 dilution, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies were TRITC-conjugated goat anti-mouse (diluted 1:200, Jackson ImmunoResearch, West Grove, PA) and FITC-conjugated goat anti-rabbit (diluted 1:300, Jackson ImmunoResearch). Samples were evaluated under a Leica DMRB epifluorescence microscope (Leica, Eaton, PA) using 40x and 100x oil objectives and a CCD-Cooled SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
BrdU study
This experiment was done to determine the uptake of BrdU by cells undergoing DNA synthesis. BrdU was given to 6 animals from the Ad.SKP2 group and 4 animals that received Ad.empty. Contralateral ears of these 10 animals served as additional controls. BrdU was administered in the drinking water from day 1 to day 14 after the inoculation of the viral vector(s). Two weeks after the surgery, all animals were euthanized and their ears and gut (positive control) were prepared for immuno-staining with anti-BrdU antibody. The cochleae were fixed with 4% paraformaldehyde in phosphate buffer, pH 7.4, permeabilized in 0.3% Triton X-100, incubated with 3% hydrogen peroxide for 30 min, immersed in 2N HCl for 30 min, and then incubated with mouse monoclonal antibody against BrdU (Sigma, Saint Louis, MO) for 30 min. A peroxidase-conjugated secondary anti-mouse antibody (ABC kit, Vector Laboratories, Burlingame, CA) was used, followed by DAB. After completion of immuno-staining, specimens were decalcified in 3% EDTA for 7 days, embedded in JB-4 resin (Electron Microscopy Sciences, Hatfield, PA) and sectioned (5 μm thickness). Every third section was collected, so that a given nucleus could only be counted once. Sections were mounted on glass slides and observed using light microscopy. The presence of BrdU-positive cells was assessed in 50 sections taken from serial sections of inoculated ears.
Data analysis
All analyses were performed using SPSS 13. Because of the small sample sizes, Fisher's exact test for the 2×2 contingency table comparing BrdU uptake between Ad.SKP2 treated animals and controls. A one-sided t-test was used to test the hypothesis that treatment with both Atoh1 and SKP2 produced more ectopic hair cells than treatment with Atoh1, alone.
3. RESULTS
p27Kip1 is expressed in the mature auditory epithelium in and around the organ of Corti
We used antibodies to localize to p27>Kip1 in whole-mounts of the normal mature auditory epithelium. The whole-mounts included tissues from the interdental cell layer (medially) to the outer sulcus (laterally). At a focal plane just above the basilar membrane, staining was detected in nuclei of Deiters and pillar cells within the sensory epithelium, and in Hensen cells (Fig. 1A). The staining intensity in Hensen cells was invariably higher compared to supporting cells in the sensory epithelium. At a slightly higher focal plane Hensen cell nuclei were strongly positive whereas hair cells were all negative (Fig. 1B). Other non-sensory cells that were p27Kip1 positive were inner sulcus cells and the interdental cells on the limbus (data not shown).
Figure 1.
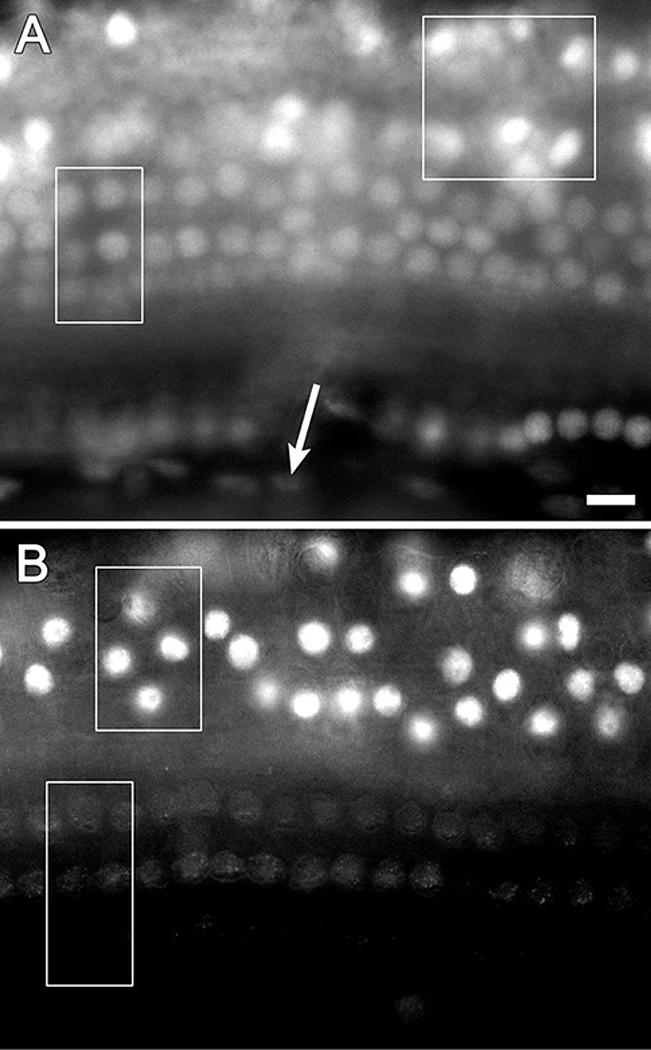
The normal distribution of p27Kip1 in mature guinea pig ears shown by immunofluorescence on whole-mounts of the auditory epithelium. A. At a focal plane immediately above the basilar membrane positive nuclei are found in Deiters cells (rectangle), Hensen cells (square) and inner pillar cells (round nuclei at bottom of image). Spindle shaped mesothelial cells located beneath the basilar membrane display background level staining (arrow). B. At a higher focal plane, p27Kip1 positive nuclei are found in Hensen cells (top rectangle) whereas nuclei of hair cells are at background staining level (bottom rectangle). Bar, 10 μm for A and B.
Over-expression of Skp2 induces proliferation in the auditory epithelium
In animals that received BrdU for 2 weeks following the Ad.SKP2 inoculation, and sacrificed 2 weeks later, several BrdU positive cells were found in the interdental cell area (Fig. 2) and the inner sulcus (data not shown). Some of the BrdU positive cells appeared in pairs. In the plastic sections we examined, cells within the organ of Corti did not exhibit BrdU staining. All 6 Ad.SKP2 inoculated animals that received BrdU had BrdU-positive cells, whereas none of the 4 control animals that received BrdU, but not Ad.SKP2, had positive cells. Fisher's exact test of the corresponding 2×2 contingency table indicates this difference in BrdU uptake is significant (p=0.005). The number of BrdU positive cells in each section was between 5 and 10, suggesting that with extrapolation the total number per ear could have reached many hundreds.
Figure 2.
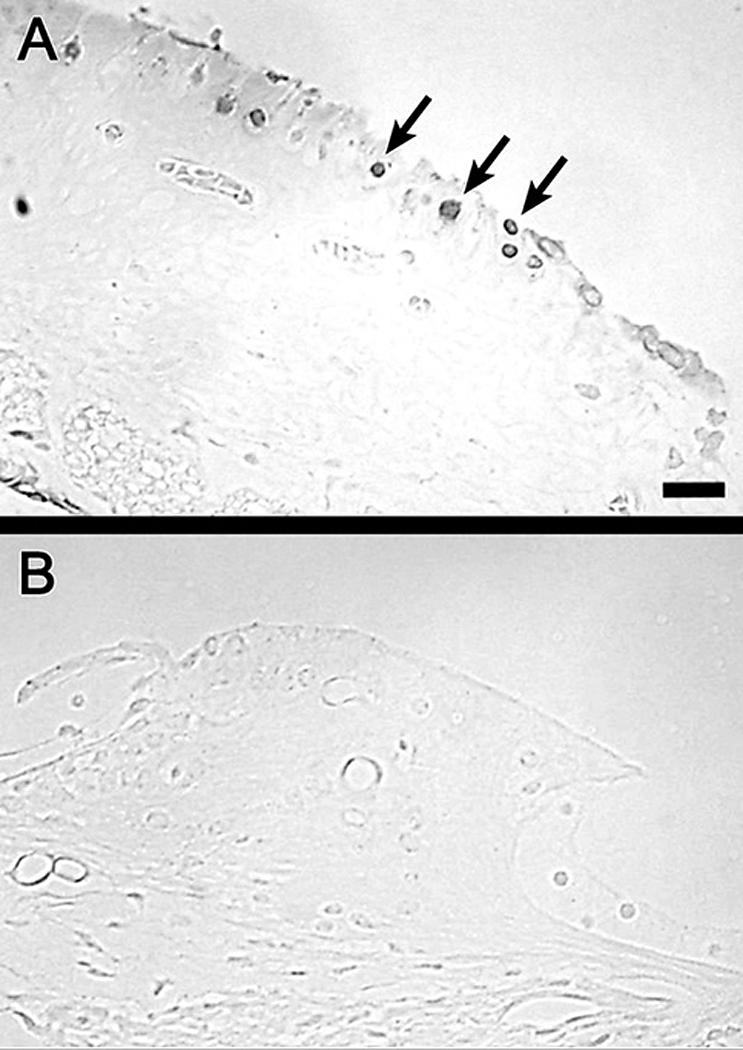
Plastic sections of the auditory epithelium of an Ad.SKP2 treated ear (A) and an Ad.empty control ear (B) stained with antibody to BrdU. A. Numerous BrdU-positive cells are found in the interdental cell region (arrows). B. No BrdU-positive cells are seen in a control ear. Bar, 25 μm for A and B.
Gene Expression of Atoh1 and Skp2
The dual inoculation with Ad.Atoh1 and Ad.SKP2 was done by mixing equal amounts of each vector prior to inoculation. This leads to a dilution of each vector to half its original concentration and therefore to an overall reduction in the efficiency of gene expression. As such, the number of cells that are transduced by both vectors is limited. Cells expressing the Atoh1 and SKP2 transgenes are seen in the interdental cell region (Fig. 3A), in the organ of Corti, and in Hensen cell area (Fig. 3B). Staining for these transgenes was confined to the nucleus. The images were obtained at a focal plane immediately beneath the luminal surface. As expected, most transduced cells expressed only one transgene but dually transduced cells were found in all samples of the experimental group. Animals that received control inoculations did not show any positive staining with antibodies to these two proteins.
Figure 3.
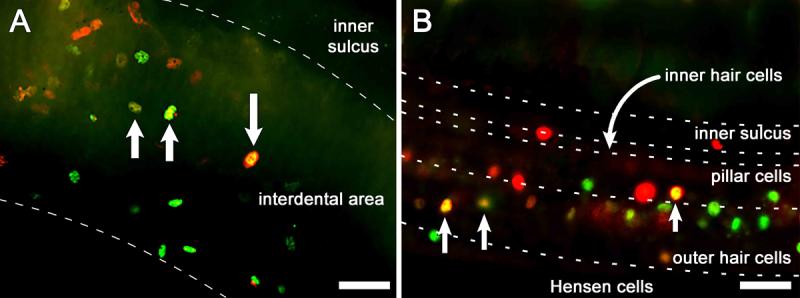
Epi-fluorescence images of whole-mounts of the organ of Corti double immuno-labeled for Atoh1 (green) and SKP2 (red) 4 days after Ad.Atoh1 and Ad.SKP2 inoculation. Images were obtained immediately beneath the luminal surface. A. In the interdental cell region, numerous cells are stained for Atoh1 or SKP2, and a small number of cells (yellow) express both proteins (arrows). B. In the organ of Corti and Hensen cell area, several cells (arrow) are yellow indicating dual expression of SKP2 and Atoh1 while others express either Atoh1 (green) or SKP2 (red). Dashed lines delimit regions within the tissue. Bar, 20 μm.
Ectopic hair cells
SEM analysis revealed numerous ectopic hair cells in ears inoculated simultaneously with Ad.Atoh1 and Ad.Skp2 (Fig. 4 A-C). Inoculation of Ad.Atoh1 alone also induced generation of ectopic hair cells (Fig. 4B), but their number was smaller than that seen in the combined Atoh1 and SKP2 group. The mean number of ectopic hair cells ± standard deviation in the 2nd turn of the 8 cochleae that received the combined (Atoh1 and SKP2) inoculation was 17.8 ± 17.4. In the group receiving the Ad.Atoh1 alone (5 animals) the mean number of ectopic hair cells was 1.40 ± 1.67. Thus the combined treatment produced more ectopic hair cells than did treatment with only Atoh1 (p=0.017).
Figure 4.
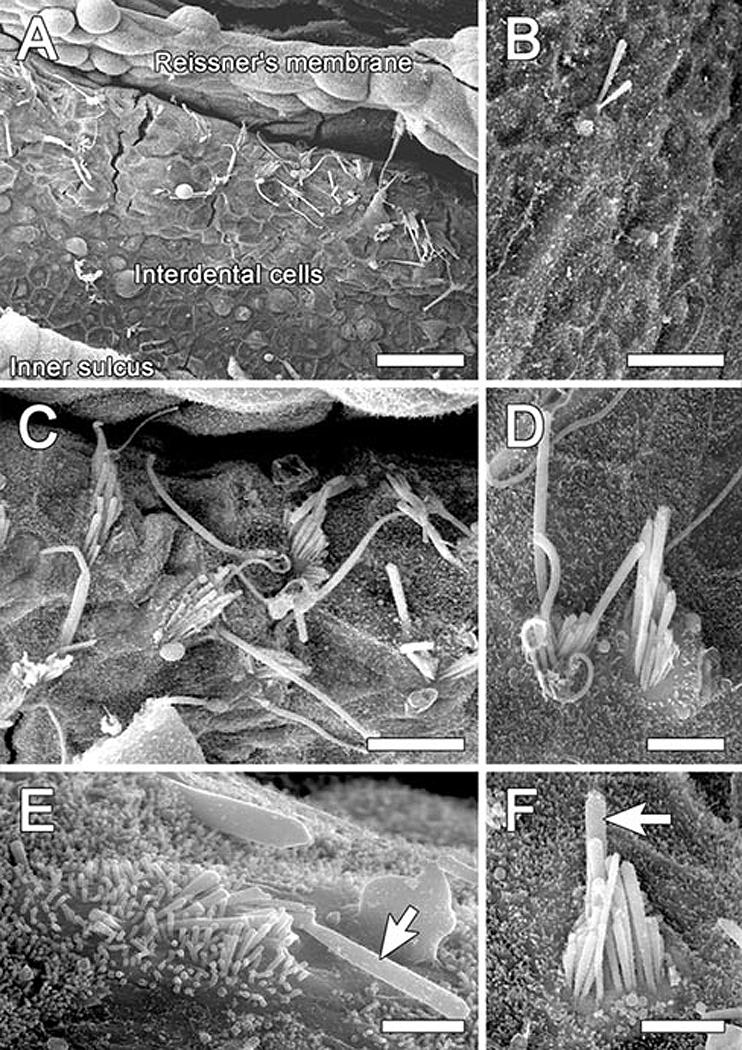
SEM images of interdental cell area (2nd turn) 2 months after inoculation of Ad.Atoh1 and Ad.SKP2 (A and C-F) or Ad.Atoh1 alone (B). A. Numerous ectopic stereocilia bundles on the limbus, reaching to the area where Reissner's membrane is inserted. B. A single ectopic bundle among interdental cells on the limbus. C. An enlarged area in (A) showing stereocilia bundles in an ectopic location on the limbus. Some bundles contain a graded array of stereocilia and others are rather disorganized. A kinocilium-like projection is seen on some of the bundles. D. Two ectopic hair cells appearing as a pair . E. Some ectopic hair cells appear like immature cells and exhibit a long and/or thick kinocilium-like protrusion (arrow). F. An ectopic hair cell with a staircase organization of stereocilia and a projection that appears like a kinocilium (arrow). Bars, 30 μm in A, 10μm in B, C, 5μm in D, 2 μm in E, and 3 μm in F.
In the combined group, the highest number of ectopic hair cells was found near the site of inoculation. In this area, some of the ectopic hair cells existed in pairs (Fig. 4D). Some of the new hair cells exhibited a long and/or thick kinocilium-like projection in addition to the stereocilia (Fig. 4C and E). There were no ectopic hair cells in the cochleae of guinea pigs inoculated with the Ad.SKP2 vector alone. Ad.empty or artificial endolymph inoculations did not lead to formation of ectopic hair cells either (data not shown).
4. DISCUSSION
Our data show that p27Kip1 is expressed in non-sensory cells flanking the organ of Corti of the mature guinea pig. We demonstrate that forced expression of SKP2 leads to proliferation in non-sensory cells around the organ of Corti and that combined inoculation of Ad.SKP2 and Ad.Atoh1 enhances the number of newly generated ectopic hair cells as compared to over-expressing Atoh1 alone.
The presence of p27Kip1 in non-sensory cells of the membranous labyrinth has previously been documented in the developing mouse cochlea (Chen and Segil, 1999; Dong et al., 2003; Lowenheim et al., 1999). The present study extends the finding to the guinea pig model and provides whole-mount analysis that allows for the detection of the protein in large experimental fields. The data reveal that p27Kip1 expression is maintained into adulthood in supporting cells of the organ of Corti as well as in areas outside the organ of Corti. Interestingly, the staining intensity appeared weaker in the supporting cells of the sensory epithelium (Deiters and pillar cells) as compared to Hensen cells which flank the sensory epithelium. The reason for this difference is unclear.
In p27Kip1 null mice, proliferation of non-sensory cells continues in the organ of Corti, leading to generation of supplementary hair cells (Chen and Segil, 1999; Lowenheim et al., 1999). In the mature guinea pig ears examined in this study, forced expression of SKP2 did not cause a notable presence of BrdU-positive cells in the organ of Corti proper. BrdU-positive cells were localized in areas flanking the sensory epithelium. It is presently unclear why the localization of proliferation site differs between the two models.
The number of ectopic cells generated by Atoh1 over-expression alone is limited (Izumikawa et al., 2005; Kawamoto et al., 2003). The ability of SKP2 to increase the number of ectopic cells generated by Atoh1 suggests that therapies for cell cycle enhancement and transdifferentiation can be combined. It also indicates that mature non-sensory cells that undergo cell division can respond to the developmental signals provided by Atoh1. The data suggest that in areas adjacent to the organ of Corti the expression of SKP2 leads to proliferation of the non-sensory cells, which by itself is not sufficient for generating new hair cells. These findings are in agreement with the outcome of disrupted p27Kip1 in mice, where the number of hair cells is increased only in the organ of Corti and not in areas flanking the sensory epithelium (Chen and Segil, 1999; Kanzaki et al., 2006; Lowenheim et al., 1999), despite the fact that cell division continues in flanking areas (Chen and Segil, 1999). Our overall interpretation of these data is that p27Kip1 blocks proliferation in non-sensory cells in and around the organ of Corti, and that in the absence of this block, new cells that are generated in the organ of Corti proper can become new hair cells without further intervention. In ectopic areas, however, it is necessary to force expression of hair cells genes such as Atoh1 to generate new hair cells.
The area immediately adjacent to the site of inoculation was excluded from the statistical analysis, because tissue in this area responded to the mechanical trauma (related to the inoculation) as well as the presence of the transgenes. Therefore the numbers presented here may represent an underestimate of the total number of newly generated hair cells. The area of the organ of Corti was also excluded from the counting, because the procedure was performed on non-deafened guinea pigs. Many hair cells degenerate in response to the procedure of endolymphatic inoculation, and regenerated hair cells could have taken their place. However, we were unable to distinguish between original hair cells and possibly regenerated hair cells within the organ of Corti and therefore did not count hair cells in this area.
The increase in ectopic hair cell number and the presence of paired hair cells following the combined treatment with SKP2 and Atoh1 indicate that new hair cells may be generated via mitotic division followed by transdifferentiation. This finding has important implications for future clinical use of hair cell regeneration therapy. The fact that post-mitotic cells can attain the hair cell phenotype is encouraging in that these cells are less likely to continue dividing and form a tumor. It is also important to observe that non-sensory cells retain their responsiveness to Atoh1 after division. This is not trivial, because these cells de-differentiate morphologically in order to divide. However, the data corroborate finding in vertebrates other than mammals, including avian species, where mitotic transdifferentiation is the main spontaneous route of hair cell regeneration.
Ectopic hair cells were not found following forced expression of SKP2 alone, despite the presence of BrdU positive cells. This suggests that proliferation by itself is insufficient for inducing generation of new hair cells in the mature ear. This finding appears to contrast with the situation in the developing ear of mice with loss of function of p27Kip1 or Rb1, where the cell cycle regulation is disrupted and the phenotypic outcome is excessive number of hair cells (Chen and Segil, 1999; Lowenheim et al., 1999; Sage et al., 2005). It is possible that once an animal is mature, the addition of supernumerary hair cells due to the defect in cell cycle regulation is reduced, as seen in mature mice deficient for p27Kip1 (Kanzaki et al., 2006).
In this study, the forced expression of SKP2 and Atoh1 was accomplished by two different viral vectors, one for each gene. For several reasons, this approach leads to a small number of cells that are transduced by both vectors. First, mixing Ad.Atoh1 and Ad.SKP2 prior to inoculation dilutes the concentration of each vector by 50%, thereby lowering the overall efficiency of transgene expression. Second, the chance for a cell to be transduced by both vectors is further reduced. The timing of vector delivery may also matter for maximizing the effect on proliferation and transdifferentiation. In these experiments, the vectors were applied simultaneously, which may have further compromised production of a large number of new hair cells. The efficiency of generating new hair cells would likely be enhanced by utilization of a virus vector encoding both Skp2 and Atoh1, which would increase the rate of cells simultaneously expressing both transgenes, and/or by optimized timing for sequential use of the vectors.
The presence of newly generated cochlear hair cells does not necessarily imply that hearing improves toward normal hearing. It is possible that tinnitus may also result from the new hair cells, and the presence of ectopic hair cells further complicates the physiological outcome. Interestingly, mice with deficient p27Kip1 expression have very poor hearing (Chen and Segil, 1999; Kanzaki et al., 2006; Lowenheim et al., 1999). It is currently unclear whether the functional quality of hair cells the arise due to p27Kip1 deficiencies is lacking in some way, or if other problems in these mice, in the ear and elsewhere, contribute to the deficiency. The next step in assessing the feasibility of the combined SKP2 / Atoh1 treatment would be histological and physiological assessment of such therapy in deafened mature mammals.
Manipulation of cell cycle regulation for therapeutic purposes is usually aimed at treating cancer, by stabilizing and enhancing p27Kip1 expression (Sumimoto et al., 2005; Supriatno et al., 2005). In contrast, our study looked at the effects of antagonizing p27Kip1 by over-expressing SKP2. This strategy is relevant to cases where adding new cells may contribute to the therapeutic goals. Promoting cell cycle with SKP2 has also been attempted in cultured primary hepatocytes and in hepatocytes in vivo and resulted in mitosis in hepatocytes that were otherwise quiescent (Nelsen et al., 2001). Although this therapy is attractive, it would be important to improve the control of gene expression to ascertain that proliferation remains limited in place and time so as not to promote tumor formation.
In addition to p27Kip1, enhanced proliferation in the post-mitotic organ of Corti has been shown with disruption of cell cycle regulatory gene Rb1 (Sage et al., 2005). It is presently unclear if the two genes act on the same signaling cascade in regulating cell cycle arrest in non-sensory cells of the auditory epithelium. Based on studies in other tissue models, it is possible that Rb1 represses Skp2 resulting in stabilizing p27Kip1, leading to arrest of the cell cycle (Ji and Zhu, 2005). Better understanding of the specific role of each gene in regulating proliferation in the auditory epithelium will help design robust yet well regulated means for increasing the number of cells in the tissue. This is important, because therapy for hair cell regeneration based on transdifferentiation of non-sensory cells requires that a large enough number of supporting cells remain in the deaf auditory epithelium.
The results we present do not provide evidence for a direct causative relationship between SKP2 and p27Kip1. It is possible that proliferation due to SKP2 forced expression is accomplished by another signaling cascade, not directly involving p27Kip1. Further work is necessary for elucidating the molecular signaling initiated by SKP2 in non-sensory cochlear cells resulting in a proliferative response in these cells.
In conclusion, we determined that p27Kip1 is expressed in the mature auditory epithelium in the organ of Corti and in adjacent regions. Co-expression of Ad.Atoh1 and Ad.SKP2 enhances the number of ectopic hair cells compared to Atoh1 over-expression alone. Forced expression of SKP2 alone induces proliferation but does not enhance generation of new hair cells. These findings demonstrate that targeted enhancement of proliferation is by itself insufficient for inducing hair cell regeneration, but when combined with forced expression of Atoh1, regeneration in the mature auditory epithelium can be enhanced. The data suggest that therapies can be designed to induce proliferation in the auditory epithelium by removing the inhibition on cell cycle.
Suggested Cover Image.
SEM micrograph of an ectopic hair cell residing on the spiral limbus following inoculation of adenoviral vectors expressing Atoh1 and SKP2 into the cochlear endolymph of guinea pigs.
Acknowledgements
We thank Lisa Beyer for technical assistance and Don Swederski for help with statistics and manuscript preparation. We thank GenVec for providing the Ad.Atoh1 and control vectors. The work is supported by the Williams Professorship, a gift from Berte and Alan Hirschfield, and by NIH/NIDCD Grants DC-01634, DC-05401, DC-03685 and DC05188.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4.PG - 6497-501. J. Virol. 1996;70 doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat. Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Hair cell regeneration in the avian cochlea. Annals Otol. Rhinol. Laryngol.- Suppl. 1997;168:9–15. [PubMed] [Google Scholar]
- Dong Y, Nakagawa T, Endo T, Kim TS, Iguchi F, Yamamoto N, Naito Y, Ito J. Role of the F-box protein Skp2 in cell proliferation in the developing auditory system in mice. Neuroreport. 2003;14:759–761. doi: 10.1097/00001756-200304150-00020. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Ryals BM, Manabe K. Recovery of hearing and vocal behavior after hair-cell regeneration. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14206–14210. doi: 10.1073/pnas.94.25.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais JL, Seth P, Zhang H. Cleavage of CDK inhibitor p21(Cip1/Waf1) by caspases is an early event during DNA damage-induced apoptosis. J. Biol. Chem. 1998;273:19207–19212. doi: 10.1074/jbc.273.30.19207. [DOI] [PubMed] [Google Scholar]
- Harper J. Protein destruction: adapting roles for Cks proteins. Curr. Biol. 2001;11:R431–435. doi: 10.1016/s0960-9822(01)00253-6. [DOI] [PubMed] [Google Scholar]
- Hashino E, Salvi RJ. Changing spatial patterns of DNA replication in the noise-damaged chick cochlea. J. Cell Sci. 1993;105:23–31. doi: 10.1242/jcs.105.1.23. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear. Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Ji P, Zhu L. Using kinetic studies to uncover new Rb functions in inhibiting cell cycle progression. Cell Cycle. 2005;4:373–375. doi: 10.4161/cc.4.3.1535. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Beyer LA, Swiderski DL, Izumikawa M, Stover T, Kawamoto K, Raphael Y. p27(Kip1) deficiency causes organ of Corti pathology and hearing loss. Hear. Res. 2006;214:28–36. doi: 10.1016/j.heares.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of Corti. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marean GC, Cunningham D, Burt JM, Beecher MD, Rubel EW. Regenerated hair cells in the European starling: are they more resistant to kanamycin ototoxicity than original hair cells? Hear. Res. 1995;82:267–276. doi: 10.1016/0378-5955(94)00183-q. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kitagawa M, Hatakeyama S. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama KI HS, Nakayama K. Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem. Biophys. Res. Commun. 2001;282:853–860. doi: 10.1006/bbrc.2001.4627. [DOI] [PubMed] [Google Scholar]
- Nelsen CJ, Hansen LK, Rickheim DG, Chen C, Stanley MW, Krek W, Albrecht JH. Induction of hepatocyte proliferation and liver hyperplasia by the targeted expression of cyclin E and skp2. Oncogene. 2001;20:1825–1831. doi: 10.1038/sj.onc.1204248. [DOI] [PubMed] [Google Scholar]
- Niemiec AJ, Raphael Y, Moody DB. Return of auditory function following structural regeneration after acoustic trauma: behavioral measures from quail. Hear. Res. 1994;79:1–16. doi: 10.1016/0378-5955(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J. Neurocytol. 1992;21:663–671. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Adler HJ, Pugliano FA. The structural and functional aspects of hair cell regeneration in the chick as a result of exposure to intense sound. Exp. Neurol. 1992;115:13–17. doi: 10.1016/0014-4886(92)90213-a. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell. Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J. Comp. Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Cellular studies of auditory hair cell regeneration in birds. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11714–11721. doi: 10.1073/pnas.97.22.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H, Yamagata S, Shimizu A, Miyoshi H, Mizuguchi H, Hayakawa T, Miyagishi M, Taira K, Kawakami Y. Gene therapy for human small-cell lung carcinoma by inactivation of Skp-2 with virally mediated RNA interference. Gene Ther. 2005;12:95–100. doi: 10.1038/sj.gt.3302391. [DOI] [PubMed] [Google Scholar]
- Supriatno, Harada K, Yoshida H, Sato M. Basic investigation on the development of molecular targeting therapy against cyclin-dependent kinase inhibitor p27Kip1 in head and neck cancer cells. Int. J. Oncol. 2005;27:627–635. [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat. Neurosci. 2004 doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]



