Abstract
Although the etiology of Parkinson’s disease (PD) is unknown, a common element of most theories is the involvement of oxidative stress, either as a cause or effect of the disease. There have been relatively few studies that have characterized oxidative stress in animal models of PD. In the present study a 6-hydroxydopamine (6-OHDA) rodent model of PD was used to investigate the in vivo production of oxidative stress after administration of the neurotoxin. 6-OHDA was injected into the striatum of young adult rats and the production of protein carbonyls and 4-hydroxynonenal (HNE) was measured at 1, 3, 7, and 14 days after administration. A significant increase in both markers was found in the striatum 1 day after neurotoxin administration, and this increase declined to basal levels by day 7. There was no significant increase found in the substantia nigra at any of the time points investigated. This same lesion paradigm produced dopamine depletions of 90–95% in the striatum and 63–80% in the substantia nigra by 14 to 28 days post 6-OHDA. Protein carbonyl and HNE levels were also measured in middle-aged and aged animals 1 day after striatal 6-OHDA. Both protein carbonyl and HNE levels were increased in the striatum of middle-aged and aged animals treated with 6-OHDA, but the increases were not as great as those observed in the young adult animals. Similar to the young animals, there were no increases in either marker in the substantia nigra of the middle-aged and aged animals. There was a trend for an age-dependent increase in basal amounts of oxidative stress markers when comparing the non-lesioned side of the brains of the three age groups. These results support that an early event in the course of dopamine depletion following intrastriatal 6-OHDA administration is the generation of oxidative stress.
Keywords: reactive oxygen species, protein carbonyls, 4-hydroxynonenal, striatum, substantia nigra, aging, Fischer-344 rat
Parkinson’s disease (PD) stems from the loss of dopamine (DA) caused by the degeneration of the dopaminergic neurons of the substantia nigra. The nature of this degeneration remains unclear, although current theories suggest that reactive oxygen species (ROS) are involved in some capacity early in the disease process (Jenner, 2003). One of the earliest changes in patients with PD and incidental Lewy body disease is the loss of glutathione (Sian et al., 1994, Owen et al., 1996). Post mortem tissue from PD patients has been shown to contain elevated levels of the oxidative stress products 4-hydroxynonenal (HNE) (Yoritaka et al., 1996), protein carbonyls (Alam et al., 1997a, Floor and Wetzel, 1998), 8-hydroxy-2-deoxyguanosine and 8-hydroxyguanine (Alam et al., 1997b, Zhang et al., 1999), and 3-nitrotyrosine (Good et al., 1998, Giasson et al., 2000).
The administration of 6-hydroxydopamine (6-OHDA) into the brain of the rat produces a well established model of PD (Kirik et al., 1998, Blum et al., 2001, Betarbet et al., 2002, Deumens et al., 2002). Many investigators have demonstrated that 6-OHDA induces oxidative stress (Kumar et al., 1995, Soto-Otero et al., 2000, Seth et al., 2002, Soto-Otero et al., 2002, Mazzio et al., 2004), which can lead to the induction of apoptosis and cellular loss (Choi et al., 1999, Marti et al., 2002, Seth et al., 2002, Liang et al., 2004). The effects of 6-OHDA are age-dependent as there is a greater effect seen in aged animals compared to young animals, particularly with lower doses of 6-OHDA (Marshall et al., 1983, Cass et al., 2002).
To identify oxidative stress that has occurred in vivo, it is more feasible to detect the products of oxidative damage as opposed to the causative agents, which exist only transiently. The measurement of protein carbonyls is one of the most widely accepted techniques to measure oxidative damage (Stadtman, 2002). HNE, the most abundant cytotoxic molecule generated under oxidative stress, is both a product of oxidative damage and a causative agent of cellular damage (Uchida, 2003). Both protein carbonyls and HNE are in abundance under oxidative conditions, and reliable methods of detection are available for each (Yatin et al., 1999, Poon et al., 2004, Theodore et al., 2006).
The purpose of the present study was to determine the time course of the development of oxidative stress that is generated in a striatal 6-OHDA lesion model of PD, and to ascertain if there are any age-related differences involving the generation of oxidative stress in this model. Establishing this time course in the rodent model will allow for the evaluation of experimental therapies on an early event in the neurodegenerative process. Young adult animals were lesioned with an intrastriatal injection of 6-OHDA. Protein carbonyls and HNE were assayed in both the striatum and substantia nigra in order to determine a time course of the generation of oxidative stress induced by 6-OHDA. Additionally, because 6-OHDA has been shown to have a greater effect on the DA systems of aged animals (Marshall et al., 1983, Cass et al., 2002), we examined the generation of protein carbonyls and HNE in middle-aged and aged animals to determine if there was an age-related effect in 6-OHDA-induced generation of oxidative stress.
EXPERIMENTAL PROCEDURES
Animals
Young adult (3–4 months old) (212g–336g), middle-aged (13–14 months old) (402g–498g) and aged (22–23 months old) (372g–524g) male Fischer-344 rats (Harlan Sprague Dawley, Indianapolis, IN) were used for all experiments. Animals were housed in groups of two under a 12-hour light/dark cycle with food and water freely available. All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the University of Kentucky.
6-OHDA lesions
The rats were anesthetized with isoflurane (2.0–2.5% as needed) and placed into a stereotaxic frame. The skull was exposed and 2 small burr holes were drilled in the skull above the right striatum (0.5 mm posterior to bregma, 4.2 mm right of midline; 0.5 mm anterior to bregma, 2.5 mm right of midline). The dura was cut and a SGE syringe with a 26 gauge blunt-tipped needle was slowly lowered to a depth of 5.0 mm below the surface of the brain. 2 μl of either vehicle (0.9% saline with 0.1% ascorbic acid, pH 5.5) or 10 μg of 6-OHDA (Sigma-Aldrich, St. Louis, MO) dissolved in vehicle was injected at a rate of 0.4 μl/minute for 5 minutes into each of the two sites. The needle was left in place for an additional 5 minutes following each injection and then slowly withdrawn. The burr holes were filled with Gelfoam and the incision closed with wound clips. The animals were placed in a heated recovery chamber until they recovered from the anesthetic, after which they were transferred back to their home cages.
Tissue collection for HPLC and Slot Blots
The animals were rendered unconscious with CO2, decapitated and the brains quickly removed and chilled in ice-cold saline. A coronal slice 2 mm thick was removed at the level of the striatum using a chilled brain mold (Rodent Brain Matrix; ASI Instruments, Warren, MI). The left and right striata were then dissected from the slice. A similar 2 mm coronal slice was made through the midbrain and the substantia nigra removed from both sides of the brain. All tissue samples were placed in pre-weighed vials, weighed and frozen on dry ice. The samples were stored at −80°C until analysis.
HPLC Analysis
Tissue samples were analyzed for DA using high pressure liquid chromatography (HPLC) with electrochemical detection as described previously (Cass et al., 2003). The retention times of standards were used to identify peaks, and the peak heights were used to determine amount of recovery of internal standard (dihydroxybenzylamine) and amounts of DA.
Quantification of markers for oxidative stress
Sample preparation for slot blots
Crude synaptosomes were made from the striatum. Tissue was homogenized with a Teflon pestle in cold 0.32 M sucrose buffer containing protease inhibitors (Complete, Mini; Roche Diagnostics, Indianapolis, IN) and centrifuged for 15 minutes at 1000 x g. The supernatant was transferred to a clean vial and centrifuged a second time for 15 minutes at 12,500 x g. The supernatant was discarded and the resulting crude synaptosomes were resuspended in 50 μL of buffer. The substantia nigra samples were homogenized in cold 0.32 M sucrose buffer containing protease inhibitors using a sonic dismembrator (Fisher Model 50 Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA). Protein concentrations of all samples were determined using the Pierce BCA method for protein quantification (Pierce Biotechnology, Rockford, IL).
Protein carbonyls
The methods for protein carbonyls and HNE by slot blots were as described previously (Poon et al., 2004) with slight modification. Crude synaptosomes (striatum) or whole tissue homogenate (substantia nigra) (15 μg protein/5 μl), 12% SDS, and 2,4-dinitrophenylhydrazine (DNP; OxyBlot Protein Oxidation Detection Kit; Chemicon International, Temecula, CA) were incubated at room temperature for 20 minutes, after which the reaction was stopped with a neutralizing solution. 250 ng of derivatized proteins were applied to a nitrocellulose membrane via vacuum filtration (Bio-Dot SF; BioRad, Hercules, CA). The membrane was blocked with 3% BSA for 1 hour then rinsed 3 times with Tris buffered saline with Tween 20 (TTBS) (Sigma-Aldrich, St. Louis, MO). The membrane was then incubated with rabbit anti-DNP (1:150) for 1 hour, rinsed 3 times with TTBS, followed by goat anti-rabbit conjugated to alkaline phosphatase (Sigma-Aldrich, St. Louis, MO) (1:15,000) for 1 hour followed by rinsing 3 times with TTBS. An alkaline phosphatase substrate (SigmaFast; Sigma-Aldrich, St. Louis, MO) was used to visualize the resulting bands. After drying, the developed membrane was scanned into Scion Image and the bands quantified by densitometry.
HNE
Crude synaptosomes or tissue homogenate (15 μg protein/5 μl) with 12% SDS was applied to a nitrocellulose membrane via vacuum filtration. The membrane was blocked with 3% BSA for 1 hour then rinsed 3 times with TTBS. The membrane was then incubated with rabbit anti-HNE (Alpha Diagnostic, San Antonio, TX) (1:10,000) for 2 hours, rinsed 3 times with TTBS, followed by goat anti-rabbit conjugated to alkaline phosphatase (Sigma-Aldrich, St. Louis, MO) (1:15,000) for 1 hour followed by rinsing 3 times with TTBS. An alkaline phosphatase substrate (SigmaFast; Sigma-Aldrich, St. Louis, MO) was used to visualize the resulting bands. After drying, the developed membrane was scanned into Scion Image, converted to a gray scale image, and the bands quantified by densitometry.
Data Analysis
Tissue levels of DA are expressed as ng/g wet weight of tissue, or as a ratio of the side ipsilateral to the lesion to the side contralateral to the lesion (I:C ratio). Data from the slot blots for both the protein carbonyl and HNE levels were expressed as a ratio of the side ipsilateral to the lesion to the side contralateral to the lesion (I:C ratio), or as a percentage of the results from young adult, naïve animals that had no surgical procedures. Results are expressed as mean ± SEM. All data were analyzed by one-, two-, or three-way ANOVA followed by a Newman-Keuls test for post hoc comparisons. P-values ≤ 0.05 were considered statistically significant.
RESULTS
DA content of striatum and substantia nigra in young, middle-aged and aged rats
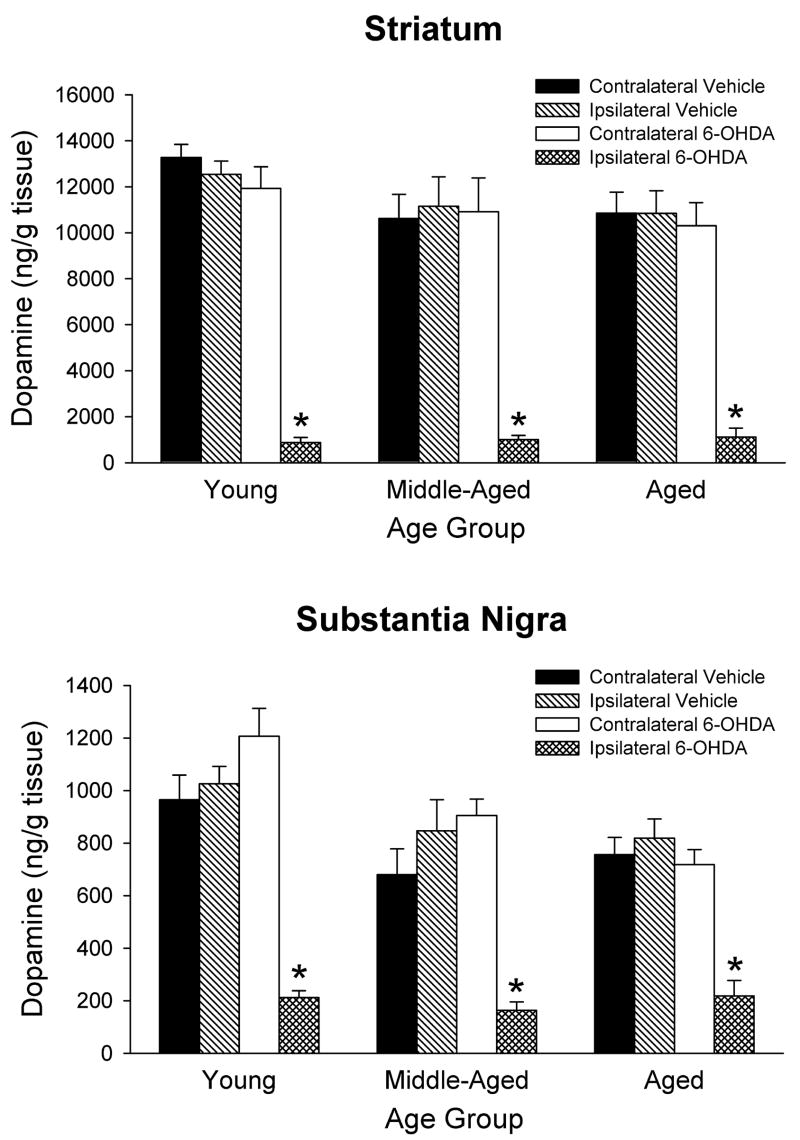
Tissue levels of DA in the striatum and substantia nigra were measured 3½ to 4 weeks after intrastriatal saline or 6-OHDA injections. The results for all three age groups are summarized in Figure 1. The data were statistically analyzed using 3-way ANOVAs with age and treatment as between factors, and side of brain as a within factor. The overall ANOVAs for both striatum and substantia nigra indicated main effects of treatment and side of brain (p < 0.0001 for all). In the animals that received 6-OHDA, there was a significant decrease in DA of 90–95% in the ipsilateral striatum compared to the contralateral striatum. In the substantia nigra the 6-OHDA treatment led to decreases in DA of 70–80%. The ANOVAs also indicated a main effect of age in the substantia nigra (p < 0.01) but not the striatum (p = 0.19). Newman-Keuls post hoc comparisons indicated overall DA levels in the substantia nigra were less in the middle-aged and aged groups compared to the young adults (p < 0.01 for both).
Figure 1.
Post-mortem levels of DA in rats treated with 6-OHDA or vehicle. Rats were injected with either saline vehicle or 20 μg of 6-OHDA in the right striatum. Striatal and nigral tissue were harvested 3½ to 4 weeks after treatment. Results displayed are mean ± SEM for 6–7 animals per group. * p<0.05 vs. contralateral side of same treatment and same age group, and vs. ipsilateral side of vehicle treatment of same age group (3-way ANOVA followed by Newman-Keuls post hoc comparisons).
Time course of oxidative stress markers in young adult rats
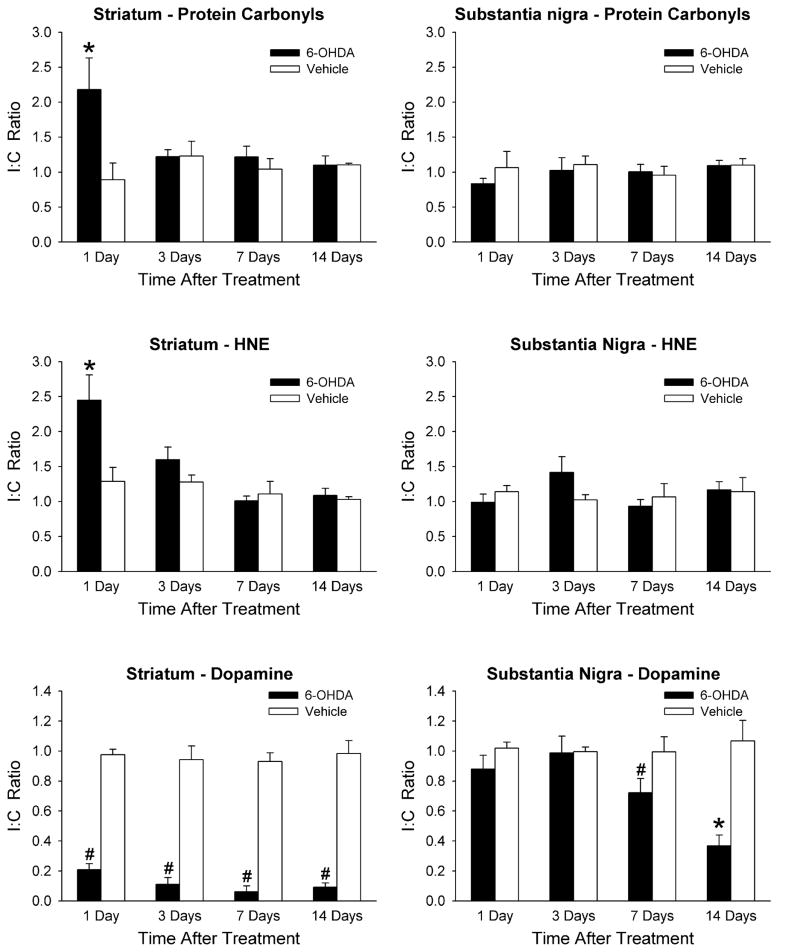
A slot-blotting technique was used for preparing membranes for Western blotting of protein carbonyls and HNE. This technique utilizes the ubiquitous nature of these markers. A representative example is shown in Figure 2. Ratios of the ipsilateral to contralateral sides for both the striatum and substantia nigra (I:C Ratio) were calculated for each animal. The data were statistically analyzed using 2-way ANOVAs with treatment and days after treatment as between factors. There was a statistically significant difference in the striatum between animals injected with 6-OHDA and those injected with vehicle 1 day after surgery for both protein carbonyl and HNE levels (Fig. 3). Protein carbonyl levels were increased by 144%, and HNE levels were increased by 90% in the 6-OHDA treated animals compared to the vehicle treated control animals. There were no differences found at any of the other time points examined within the striatum. There were also no differences in either protein carbonyl or HNE levels between the two treatments in the substantia nigra at any time point (Fig. 3).
Figure 2.

Representative example of a slot blot of crude synaptosomes from the striatum stained for protein carbonyls. Animals S1-S3 were injected with 20 μg 6-OHDA and animals S4-S6 were injected with vehicle in right striatum. All six animals were allowed to recover for 1 day. Controls were supplied from the antibody manufacturer.
Figure 3.
Time course of oxidative stress markers and DA levels in striatum and substantia nigra. Animals received intrastriatal injections of 6-OHDA or saline vehicle and were allowed to recover for 1, 3, 7, or 14 days. Protein carbonyls (top graphs), HNE content (middle graphs), and DA content (bottom graphs) were measured in the striatum and substantia nigra and a ratio of the ipsilateral to contralateral sides (I:C Ratio) was calculated for each animal for each structure. Results are mean ± SEM for 6–8 animals per group for protein carbonyls and HNE, and 5 animals per group for DA content. * p < 0.05 vs. vehicle treatment on same day, and vs. 6-OHDA treatment on all other days; # p < 0.05 vs. vehicle treatment on same day (2-way ANOVA followed by Newman-Keuls post hoc comparisons).
Time course for changes in tissue DA levels in young adult rats
DA levels in the striatum and substantia nigra were determined at 1, 3, 7 and 14 days post-lesion in groups of animals separate from those used for measuring protein carbonyl and HNE levels. I:C ratios were calculated for DA for each animal for both the striatum and substantia nigra, and the data were statistically analyzed using 2-way ANOVAs with treatment and days after treatment as between factors. In the 6-OHDA treated animals, striatal DA levels were decreased by 79% at 1 day after the lesion (Fig. 3). A similar level of DA loss was observed for the other time points and by 14 days DA levels in the striatum had decreased by 91% compared to the vehicle treated control rats. Substantia nigra DA levels in the 6-OHDA treated rats were not significantly decreased at 1 or 3 days post-lesion, but were decreased by 28% at 7 days, and 63% at 14 days, post-lesion compared to the vehicle injected rats.
Oxidative stress markers in middle-aged and aged animals
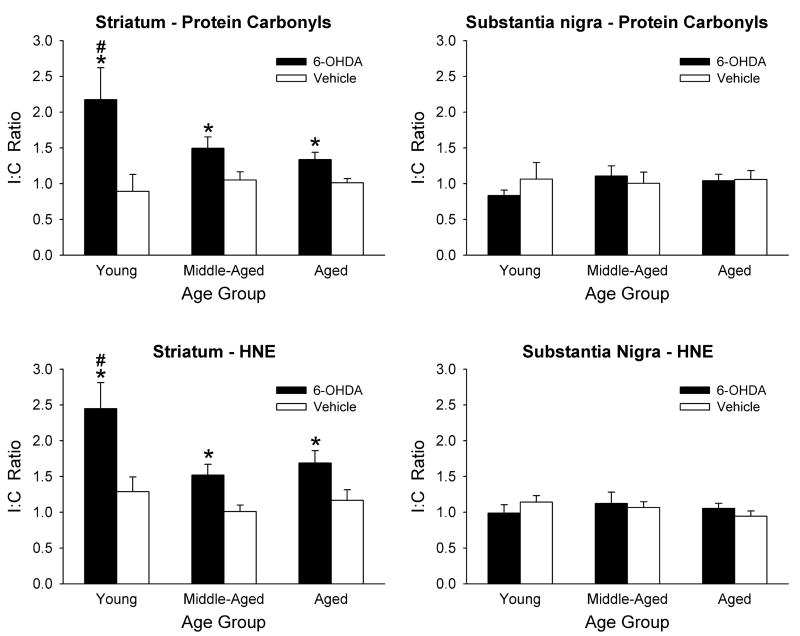
Protein carbonyl and HNE levels were analyzed in young, middle-aged, and aged rats 24 hours after 6-OHDA or vehicle injections, the time point determined in the above experiments that revealed significant 6-OHDA-induced elevations in the two markers of oxidative damage. The data were statistically analyzed using 2-way ANOVAs with treatment and age as between factors. A significant difference in the I:C ratios of both protein carbonyls and HNE was found in the striata of all three age groups (Fig. 4). However, similar to the time course experiments above, there were no significant differences found in the substantia nigra. In addition, the I:C ratios for both protein carbonyl and HNE levels in the young animals treated with 6-OHDA were significantly greater than the corresponding ratios for the middle-aged and aged animals (Fig. 4).
Figure 4.
Effects of aging on oxidative stress markers from 6-OHDA treated animals. Young, middle-aged and aged rats were injected with either 6-OHDA or saline vehicle and allowed to recover for 1 day. Protein carbonyls (top graphs) and HNE content (bottom graphs) were measured in the striatum and substantia nigra and a ratio of the ipsilateral to contralateral sides (I:C Ratio) was calculated for each animal for each structure. Results are mean ± SEM for 6–8 animals per group. * p < 0.05 vs. vehicle group of same age group; # p < 0.05 vs. both middle-aged and aged 6-OHDA groups (2-way ANOVA followed by Newman-Keuls post hoc comparisons).
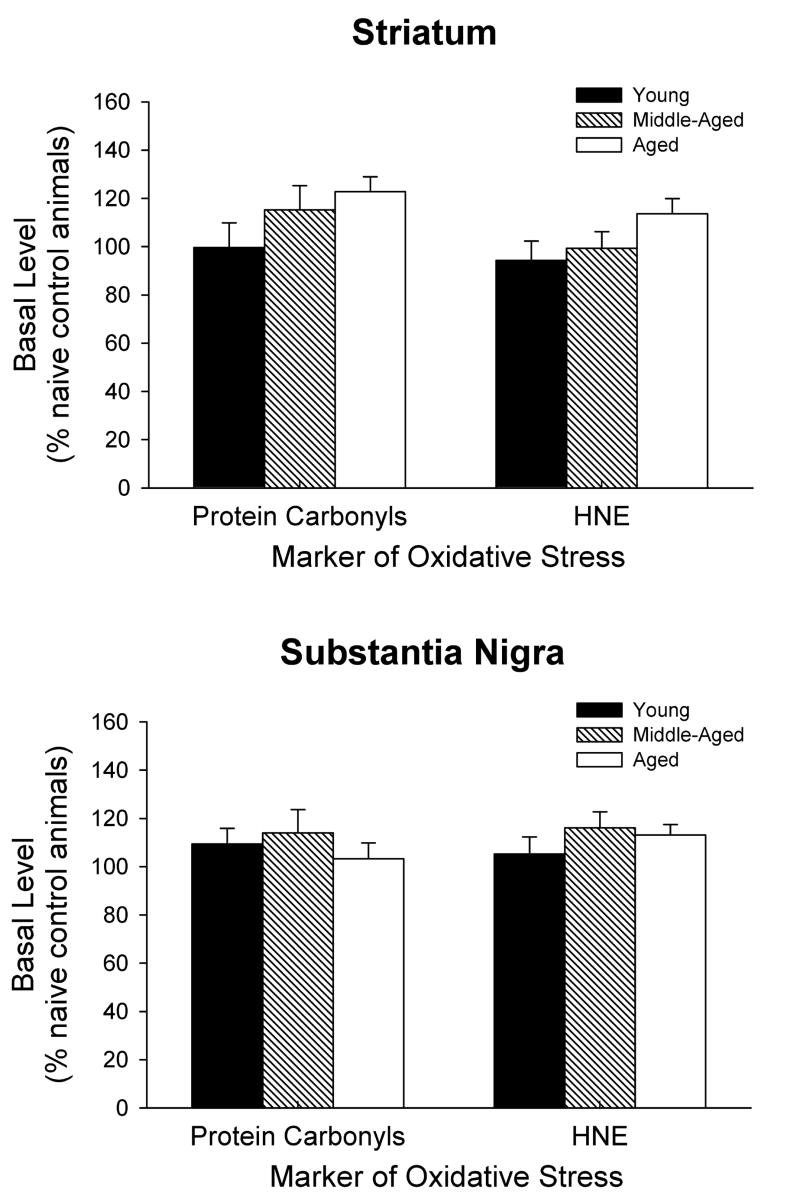
It was also of interest to determine if there is was an age-dependent difference in the amount of basal levels of oxidative stress within the striatum and substantia nigra. Data from the non-treated, contralateral sides of the brains were expressed as a percentage of the level in naïve, young adult control animals. There was no difference in the results between the contralateral sides of the vehicle and 6-OHDA treated animals within each age group so the results were pooled together. One-way ANOVAs indicated there was no statistically significant difference between the three age groups in the striatum or substantia nigra for either protein carbonyl or HNE levels (Fig. 5). However, there was a trend for increasing basal levels of protein carbonyls and HNE with age in the striatum (t-tests between the young and aged groups were significant at the p < 0.05 level for both protein carbonyls and HNE).
Figure 5.
Basal levels of protein carbonyls and HNE in the striatum and substantia nigra of young, middle aged, and aged rats. The data are expressed as a percentage of the level in naïve, young adult control animals, and are the pooled results from the contralateral sides of the vehicle and 6-OHDA treated animals within each age group. Results are mean ± SEM for 12–16 animals per group.
DISCUSSION
The present results demonstrate that in vivo indices of oxidative stress are markedly increased in the striatum within 24 hours following an intrastriatal lesion with 6-OHDA. Other investigators have shown that in vitro exposure to 6-OHDA increases oxidative stress and may lead to apoptosis (Kumar et al., 1995, Park et al., 2002, Soto-Otero et al., 2002, Holtz and O’Malley, 2003, Salinas et al., 2003, Ugarte et al., 2003, Zhuo et al., 2003, Datla et al., 2004, Elkon et al., 2004, Mazzio et al., 2004), and some elements of apoptosis and degeneration occur shortly after in vivo administration of 6-OHDA (Jeon et al., 1995, Joo et al., 1998, Zuch et al., 2000, Kramer and Mytilineou, 2004, Mladenovic et al., 2004). However, little has been reported on in vivo markers of oxidative stress after 6-OHDA administration.
Our findings that indices of oxidative stress in the striatum are elevated within 24 hours after administration of 6-OHDA, and return to near basal levels by 7 days, are similar to other models of brain lesions where oxidative stress occurs shortly after insult. In a mouse model of traumatic brain injury, 3-nitrotyrosine and HNE were both elevated within one hour after injury. Silver staining for degenerating cell bodies and processes in the same region was not elevated until 48 hours after injury (Hall et al., 2004). In a model of HIV induced dementia, Tat was injected into the striatum of rats that were evaluated for oxidative damage and neurodegeneration at various time points (Aksenov et al., 2003). Protein carbonyls were detected within 2 hours of injection of Tat and returned to basal levels by 24 hours, but neurodegeneration, characterized by FluroJade staining, was not detected until 24 hours after Tat injection. In another study, lysed autologous red blood cells were injected into the striatum of rodents to simulate an intracerebral hemorrhage (Wu et al., 2002). At 24 hours post-surgery, protein carbonyls were significantly elevated and superoxide dismutase levels were significantly decreased. Animals were not positive for TUNEL staining until 48 hours after surgery. A study looking at the production of hydroxyl radicals following a striatal 6-OHDA lesion produced results that also correlate with our findings. Henze et al. (2005) found reactive oxygen and nitrogen species are formed in the striatum 25 minutes following striatal 6-OHDA lesioning and these levels tended to return to control levels by 7 days. The increase and subsequent falloff of reactive species correlates with our findings of increased secondary indicators of oxidative stress. These results demonstrate the transient nature of oxidative stress markers, and suggest that oxidative stress is involved in the early stages of neuronal loss. These studies, along with our present experiments, suggest that in widely different models of neuronal injury, a common component is the early presence of oxidative stress.
The current results indicate that the extent of oxidative stress initially induced by 6-OHDA does not increase as the age of the animal increases. Rather, we found that while 6-OHDA led to increases in both oxidative stress markers in all three age groups, the increase was actually greater in the young animals compared to the middle-aged and aged animals. This was unexpected as we anticipated that the magnitude of any increase observed would be the same as, or higher, in the older groups compared to the youngest group. Others have suggested that there is a maturation process that occurs in antioxidant efficiency in the rodent brain, in that middle-aged and aged animals are better equipped to detoxify free radicals than young animals (Zhang et al., 1993, Zhang et al., 1994). A reduced ability in younger animals to detoxify free radicals could help explain the present results of a greater increase in oxidative stress markers following 6-OHDA administration in the young animals compared to both the middle-aged and aged animals.
Our present results indicated a trend for an increase in the basal levels of oxidative stress markers in aged animals compared to young adults. However, there are conflicting studies in the literature concerning age-dependent increases of oxidative stress within the brain. Some studies have reported age-related increases in oxidative stress (Aksenova et al., 1998, Calabrese et al., 2004), and some studies have reported no change with aging (Cao and Cutler, 1995, Cini and Moretti, 1995, Goto et al., 1999, Davies et al., 2001). In a review on oxidative stress and aging, Stadtman notes how differing results may have to do with animal breeding conditions or that the animals may have adapted over time to become more resistant to oxidative stress (Stadtman, 2002). The use of different methodologies, strains and species of animals, and indicators of oxidative stress could all contribute to the lack of agreement concerning age-related changes in oxidative stress.
The time points that we utilized for this study were determined based upon previous work that was done in the characterization of the 6-OHDA rodent model and other models of neuronal injury. After injecting 6-OHDA into the striatum, the majority of cell loss within the substantia nigra occurs between 1 and 2 weeks post lesion (Sauer and Oertel, 1994). In a study looking at acute traumatic brain injury, Hall noted an increased intensity of HNE and 3-nitrotyrosine staining 1 hr after injury, peaking at 48 hrs, and persisting through 96 hrs (Hall et al., 2004). Silver staining for neurodegeneration was not detected until 24 hours, peaked at 72 hours, and persisted through 120 hours. Similar results were seen from a time course study investigating oxidative stress and neurodegeneration using the paraquat model of PD (McCormack et al., 2005). These studies suggest that markers of oxidative stress are increased before neuronal degeneration is detected. The time points used in the present study encompass the start of the neurodegenerative process proposed by others (Sauer and Oertel, 1994).
The time course for changes in striatal DA levels indicates that intrastriatal administration of 6-OHDA leads to a rapid and substantial loss of DA content in the striatum. Thus, at 1 day post-lesion, protein carbonyl and HNE levels are significantly elevated in the striatum, while DA content is substantially reduced. The loss of striatal DA levels 1 day after 6-OHDA administration is consistent with toxicity to dopaminergic terminals, as free radical damage to, or destruction of, DA terminals would be expected to disrupt normal physiological possesses such as DA synthesis and storage. In contrast, nigral DA levels were normal at 1 and 3 days following intrastriatal injection of 6-OHDA, but were decreased at 7 days and further decreased at 14 days. This time course is consistent with previous studies that have documented loss of nigral DA cells following an intrastriatal lesion with 6-OHDA (Sauer and Oertel, 1994; Kramer and Mytilineou, 2004).
Several investigators have shown that there is a loss of tyrosine hydroxylase positive staining in the striatum within 7 days following intrastriatal 6-OHDA (Sauer and Oertel, 1994; Stromberg et al., 2005). Munoz et al. (2005) showed a loss of tyrosine hydroxylase positive staining in the striatum at 36 hours following 6-OHDA that was accompanied by an increase in glial cell markers. Mladenovic et al. (2004), showed that the maximal amount of apoptotic striatal neurons occurs at 24 hours and then declines by 7 days after intrastriatal 6-OHDA. These studies complement our finding of an increase in markers of oxidative stress, and a decrease in DA content, in the striatum 24 hours following intrastriatal injection of 6-OHDA. Taken together, the above studies and the present results suggest that loss of dopaminergic markers begins shortly after 6-OHDA administration and may be initiated by excessive oxidative stress.
Although our study used a lesion model that resulted in a 70–80% decrease of DA in the substantia nigra, we did not find evidence indicating an increased amount of oxidative stress in the substantia nigra following intrastriatal 6-OHDA administration. In contrast, markers of oxidative stress are increased in the substantia nigra following intranigral administration of 6-OHDA (Inden et al., 2005; Smith and Cass, unpublished observations). However, concentrations of proteins involved with programmed cell death are altered in the substantia nigra after a striatal 6-OHDA lesion (Marti et al., 2002, Kramer and Mytilineou, 2004, Mladenovic et al., 2004). Mattson has speculated that ROS initiate signal transduction pathways, such as that of apoptosis, which commence in the neuronal terminals and terminate in the cell bodies (Mattson et al., 1998, Mattson and Duan, 1999). Thus, apoptotic events initiated in striatal DA terminals may be leading to retrograde degeneration or damage to DA cell bodies in the substantia nigra. The relatively slow loss of nigral DA neurons following intrastriatal 6-OHDA administration, together with the rapid clearance of oxidized cellular elements that may be forming in the substantia nigra during degenerative processes, may explain the lack of significant increases in nigral levels of protein carbonyls and HNE in the present study.
In conclusion, our results support that oxidative stress plays a role in the damage produced by intrastriatal injection of 6-OHDA, and that indices of oxidative stress could potentially be important markers for evaluating therapeutic strategies and their effects on 6-OHDA-induced dopaminergic neurotoxicity. Whether it is the mitigating source of degeneration, or the result of an earlier event, oxidative stress occurs very early in the disease process. In addition, our results provide further support that agents that reduce oxidative stress may prove to be of therapeutic benefit against the ongoing loss of DA neurons and motor function that occurs in PD.
Acknowledgments
We thank Laura Peters, Brian Thompson, Dr. H. Fei Poon, and Dr. Ed Hall for technical assistance and for discussions concerning the experiments. This study was supported in part by United States Public Health Service Grants AG17963 and AG00242.
List of Abbreviations
- 6-OHDA
6-hydroxydopamine
- ANOVA
analysis of variance
- DA
dopamine
- DNP
dinitrophenylhydrazine
- HNE
4-hydroxynonenal
- HPLC
high pressure liquid chromatography
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- TTBS
Tris buffered saline with Tween 20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Carney JM, Butterfield DA. Protein oxidation and enzyme activity decline in old brown Norway rats are reduced by dietary restriction. Mech Ageing Dev. 1998;100:157–168. doi: 10.1016/s0047-6374(97)00133-4. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalized increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997a;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997b;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Cao G, Cutler RG. Protein oxidation and aging. I. Difficulties in measuring reactive protein carbonyls in tissues using 2,4-dinitrophenylhydrazine. Arch Biochem Biophys. 1995;320:106–114. doi: 10.1006/abbi.1995.1347. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Bailey SL. Enhanced effects of 6-hydroxydopamine on evoked overflow of striatal dopamine in aged rats. Brain Res. 2002;938:29–37. doi: 10.1016/s0006-8993(02)02481-2. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Choi WS, Yoon SY, Oh TH, Choi EJ, O’Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- Datla KP, Bennett RD, Zbarsky V, Ke B, Liang YF, Higa T, Bahorun T, Aruoma OI, Dexter DT. The antioxidant drink effective microorganism-X (EM-X) pre-treatment attenuates the loss of nigrostriatal dopaminergic neurons in 6-hydroxydopamine-lesion rat model of Parkinson’s disease. J Pharm Pharmacol. 2004;56:649–654. doi: 10.1211/0022357023222. [DOI] [PubMed] [Google Scholar]
- Davies SM, Poljak A, Duncan MW, Smythe GA, Murphy MP. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med. 2001;31:181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Elkon H, Melamed E, Offen D. Oxidative stress, induced by 6-hydroxydopamine, reduces proteasome activities in PC12 cells: implications for the pathogenesis of Parkinson’s disease. J Mol Neurosci. 2004;24:387–400. doi: 10.1385/JMN:24:3:387. [DOI] [PubMed] [Google Scholar]
- Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson’s disease. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Goto S, Nakamura A, Radak Z, Nakamoto H, Takahashi R, Yasuda K, Sakurai Y, Ishii N. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech Ageing Dev. 1999;107:245–253. doi: 10.1016/s0047-6374(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Henze C, Earl C, Sautter J, Schmidt N, Themann C, Hartmann A, Oertel WH. Reactive oxidative and nitrogen species in the nigrostriatal system following striatal 6-hydroxydopamine lesion in rats. Brain Res. 2005;1052:97–104. doi: 10.1016/j.brainres.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Holtz WA, O’Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- Inden M, Kitamura Y, Kondo J, Hayashi K, Yanagida T, Takata K, Tsuchiya D, Yanagisawa D, Nishimura K, Taniguchi T, Shimohama S, Sugimoto H, Akaike A. Serofendic acid prevents 6-hydroxydopamine-induced nigral neurodegeneration and drug-induced rotational asymmetry in hemi-parkinsonian rats. J Neurochem. 2005;95:950–961. doi: 10.1111/j.1471-4159.2005.03413.x. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36–38. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Joo WS, Jin BK, Park CW, Maeng SH, Kim YS. Melatonin increases striatal dopaminergic function in 6-OHDA-lesioned rats. Neuroreport. 1998;9:4123–4126. doi: 10.1097/00001756-199812210-00022. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of Behavioral and Neurodegenerative Changes Following Partial Lesions of the Nigrostriatal Dopamine System Induced by Intrastriatal 6-Hydroxydopamine in the Rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kramer BC, Mytilineou C. Alterations in the cellular distribution of bcl-2, bcl-x and bax in the adult rat substantia nigra following striatal 6-hydroxydopamine lesions. J Neurocytol. 2004;33:213–223. doi: 10.1023/b:neur.0000030696.62829.ec. [DOI] [PubMed] [Google Scholar]
- Kumar R, Agarwal AK, Seth PK. Free radical-generated neurotoxicity of 6-hydroxydopamine. J Neurochem. 1995;64:1703–1707. doi: 10.1046/j.1471-4159.1995.64041703.x. [DOI] [PubMed] [Google Scholar]
- Liang Q, Liou AK, Ding Y, Cao G, Xiao X, Perez RG, Chen J. 6-Hydroxydopamine induces dopaminergic cell degeneration via a caspase-9-mediated apoptotic pathway that is attenuated by caspase-9dn expression. J Neurosci Res. 2004;77:747–761. doi: 10.1002/jnr.20198. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Drew MC, Neve KA. Recovery of function after mesotelencephalic dopaminergic injury in senescence. Brain Res. 1983;259:249–260. doi: 10.1016/0006-8993(83)91255-6. [DOI] [PubMed] [Google Scholar]
- Marti MJ, Saura J, Burke RE, Jackson-Lewis V, Jimenez A, Bonastre M, Tolosa E. Striatal 6-hydroxydopamine induces apoptosis of nigral neurons in the adult rat. Brain Res. 2002;958:185–191. doi: 10.1016/s0006-8993(02)03694-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- Mazzio EA, Reams RR, Soliman KF. The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res. 2004;1004:29–44. doi: 10.1016/j.brainres.2003.12.034. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Mladenovic A, Perovic M, Raicevic N, Kanazir S, Rakic L, Ruzdijic S. 6-Hydroxydopamine increases the level of TNFalpha and bax mRNA in the striatum and induces apoptosis of dopaminergic neurons in hemiparkinsonian rats. Brain Res. 2004;996:237–245. doi: 10.1016/j.brainres.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Munoz AM, Rey P, Parga J, Guerra MJ, Labandeira-Garcia JL. Glial overexpression of heme oxygenase-1: a histochemical marker for early stages of striatal damage. J Chem Neuroanat. 2005;29:113–126. doi: 10.1016/j.jchemneu.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Owen AD, Schapira AH, Jenner P, Marsden CD. Oxidative stress and Parkinson’s disease. Ann N Y Acad Sci. 1996;786:217–223. doi: 10.1111/j.1749-6632.1996.tb39064.x. [DOI] [PubMed] [Google Scholar]
- Park JW, Youn YC, Kwon OS, Jang YY, Han ES, Lee CS. Protective effect of serotonin on 6-hydroxydopamine- and dopamine-induced oxidative damage of brain mitochondria and synaptosomes and PC12 cells. Neurochem Int. 2002;40:223–233. doi: 10.1016/s0197-0186(01)00072-9. [DOI] [PubMed] [Google Scholar]
- Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, Butterfield DA. Antisense directed at the Abeta region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res. 2004;1018:86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Seth K, Agrawal AK, Aziz MH, Ahmad A, Shukla Y, Mathur N, Seth PK. Induced expression of early response genes/oxidative injury in rat pheochromocytoma (PC12) cell line by 6-hydroxydopamine: implication for Parkinson’s disease. Neurosci Lett. 2002;330:89–93. doi: 10.1016/s0304-3940(02)00714-0. [DOI] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol. 2002;64:125–135. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Munoz-Patino AM, Labandeira-Garcia JL. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson’s disease. J Neurochem. 2000;74:1605–1612. doi: 10.1046/j.1471-4159.2000.0741605.x. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Importance of individuality in oxidative stress and aging. Free Radic Biol Med. 2002;33:597–604. doi: 10.1016/s0891-5849(02)00904-8. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Gemma C, Vila J, Bickford PC. Blueberry- and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp Neurol. 2005;196:298–307. doi: 10.1016/j.expneurol.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006;137:925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. J Neurosci Res. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953:45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Varadarajan S, Link CD, Butterfield DA. In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid beta-peptide (1–42) Neurobiol Aging. 1999;20:325–330. doi: 10.1016/s0197-4580(99)00056-1. discussion 339–342. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Andrus PK, Hall ED. Age-related regional changes in hydroxyl radical stress and antioxidants in gerbil brain. J Neurochem. 1993;61:1640–1647. doi: 10.1111/j.1471-4159.1993.tb09798.x. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Andrus PK, Hall ED. Age-related phospholipid hydroperoxide levels in gerbil brain measured by HPLC-chemiluminescence and their relation to hydroxyl radical stress. Brain Res. 1994;639:275–282. doi: 10.1016/0006-8993(94)91741-8. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Yu F, Xu de H, Sun L, Liu X. Baculovirus p35 gene greatly enhances PC12 cell’s resistance against oxidative stress. J Neurol Sci. 2003;216:135–141. doi: 10.1016/j.jns.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Zuch CL, Nordstroem VK, Briedrick LA, Hoernig GR, Granholm AC, Bickford PC. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J Comp Neurol. 2000;427:440–454. doi: 10.1002/1096-9861(20001120)427:3<440::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]