Abstract
Based on in vitro studies, it is hypothesized that neurotrophic factor deprivation following deafferentation elicits an oxidative state change in the deafferented neuron and the formation of free radicals that then signal cell death pathways. This pathway to cell death was tested in vivo by assessing the efficacy of antioxidants (AOs) to prevent degeneration of deafferented CNVIII spiral ganglion cells (SGCs) in deafened guinea pigs. Following destruction of sensory cells, guinea pigs were treated immediately with Trolox (a water soluble vitamin E analogue) + ascorbic acid (vitamin C) administered either locally, directly in the inner ear, or systemically. Electrical auditory brainstem response (EABR) thresholds were recorded to assess nerve function and showed a large increase following deafness. In treated animals EABR thresholds decreased and surviving SGCs were increased significantly compared to untreated animals. These results indicate that a change in oxidative state following deafferentation plays a role in nerve cell death and antioxidant therapy may rescue SGCs from deafferentation-induced degeneration.
Keywords: vitamin E, vitamin C, Trolox, ascorbic acid, electrical auditory brainstem response, spiral ganglion cell, deafferentation
INTRODUCTION
The neurotrophic factor hypothesis (Mattson, 1998) suggests that with deafferentation of a neuron, neurotrophic factor deprivation leads to formation of reactive oxygen species (ROS) and a change in oxidant state (Dugan et al., 1997). The formation of free radicals acts directly to destroy phospholipids and as signaling molecules to upregulate cell death pathways leading to apoptosis or necrosis (Davies, 1996; Greenlund, et al., 1995; Deshmukh and Johnson, 1997; 1998; Van De Water et al., 2004). The aim of this study was to test the hypothesis that a change in oxidative state and the formation of free radicals play an important role in deafferentation-induced cell death in vivo.
The auditory nerve is an excellent model to study interventions to prevent deafferentation-induced cell death, because it can be readily deafferented by destruction of cochlear hair cells, cochlear fluids allow introduction of test articles and there is a direct clinical relevance. The cochlear implant has provided remarkable benefits for patients with profound hearing impairment, however, its benefits depend upon the presence of a remaining excitable spiral ganglion cell (SGC) population (Pfingst, 1990; Kileny et al., 1991; Skinner et al., 1995; Blamey et al., 1996; Nadol et al., 1989, Incesulu and Nadol, 1998; Miller et al., 2000). Loss of inner hair cells leads to a degeneration of denervated afferent CNVIII fibers and a loss of SGCs. The process begins with a rapid (days) degeneration of SGC peripheral processes followed by cell body degeneration and loss of the central axonal projection. In animal models the rate of degeneration can be rapid and profound, with > 50% loss of SGC in the ototoxically deafened guinea pig after 10 weeks (Yamagata et al., 2004) and 80% loss at 16 weeks post deafening (Jyung et al., 1989). In humans, the process is much slower, extending over years, depending on the etiology of the inner ear pathology (Bergstrom, 1975; Otte et al., 1978; Nadol, 1997; 2001).
Neurotrophic factors, including GDNF (glial cell line-derived neurotrophic factor), NT-3 (neurotrophin-3) and BDNF (brain derived neurotrophic factor), can prevent SGC degeneration following loss of hair cells when provided by direct intracochlear infusion (Ylikoski et al., 1998, Staecker et al., 1996, Ernfors et al., 1996, Miller et al., 1997; Shinohara et al., 2002; Yamagata et al., 2004) or via adenoviral vectors (Yagi et al., 2000), comparable to what has been seen in other models of deafferentation (e.g Mattson et al, 1998). Intracochlear or systemic administration of free-radical scavengers offers an alternative intervention. Hair cell death induced by intense noise, aminoglycosides or cisplatin reflects an increased production of ROS (Ohinata et al., 2000a; Ravi et al., 1995; Rybak et al., 1995; Clerici et al., 1996; Sha and Schacht, 1999) and intervention with antioxidants either before or shortly after the trauma can reduce hair cell death (Seidman et al., 1993; Quirk et al., 1994; Song and Schacht, 1996; Rybak et al., 1999; Conlon et al., 1999; Ohinata et al., 2000b; Teranishi et al., 2001, Yamashita et al., 2004). Gabaizadeh et al. (1997) found that neurotrophin deprivation of SGC leads to an early increase in intracellular level of ROS. The ROS induced lipid peroxidation product 8-isoprostane increases in the auditory nerve following noise exposure (Ohinata et al., 2000a) and can be prevented by antioxidants (Ohinata et al., 2000b). The goal of the present study was to determine if antioxidants in vivo can arrest degeneration of the auditory nerve following deafferentation, and for the benefit of the cochlear implant, maintain the electrical excitability of the deafferented auditory nerve.
Free radical scavengers, such as vitamin E and vitamin C, protect mammalian cells from oxidative damage in vivo (Chen and Tappel, 1995; Kontush et al., 2001). The antioxidative efficiency of α-tocopherol (vitamin E) can be increased by a co-supplementation with ascorbic acid, a co-antioxidant for the former (Stocker, 1994). In combination, vitamin E and vitamin C are more effective than either vitamin alone in preventing oxidative damages in vitro or in vivo (Sato et al., 1993, Chen and Tappel et al., 1995; Kontush et al., 2001). Moreover, these vitamins have no remarkable side effects. We therefore selected a combination of Trolox, a water-soluble vitamin E analogue, and ascorbic acid (vitamin C) to test the hypothesis that antioxidants can prevent auditory nerve degeneration and maintain electrical excitability of the nerve following deafness.
Materials and methods
This study was performed in two phases (Phase I and Phase II). In Phase I, AOs were administrated locally using osmotic mini-pumps, and in Phase II AOs were administrated systemically.
Subjects
A total of 44 male pigmented guinea pigs (250 ~ 400 g) with normal Preyer reflex were used in this study. The animals were anesthetized with xylazine (10 mg/kg, i.m.) and ketamine (40 mg/kg, i.m.) during the implant surgery and all recording sessions. All animal procedures were performed in accordance with ethical standards of Karolinska Institutet and consistent with national regulations for care and use of animals (approval no. N113/01).
Implant surgery
All animals underwent aseptic surgery to implant intracochlear stimulating electrodes and an epidural recording electrode as described previously (Shinohara et al., 2002). The left middle ear was exposed by a postauricular approach and a cochleostomy was performed on the basal turn to allow access to the scala tympani. A ball electrode was inserted through the round window membrane and placed approximately 1.5 mm into the scala tympani to elicit electrical auditory brainstem responses (EABRs). The ball electrode was constructed using 75 μm diameter, Pt-Ir 90%/10% wire, Teflon-insulated (Advent Research Materials Ltd., England) beyond the ball. The return electrode was a Teflon-insulated Pt-Ir wire, 125 μm in diameter (Advent Research Materials Ltd., England). In the Phase I study, the return electrode (stripped of insulation for 10 mm) was placed against the occipital bone beneath the dorsal neck musculature while in Phase II the return electrode (stripped of insulation for 5 mm) was placed in the left middle ear to enhance the first positive wave from stimulating artifacts. Prior to implantation, each stimulating electrode surface was activated to a stable minimal impedance value (2-3 kΩ) by applying a 2-volt triangular wave for 30 min in saline. Both electrodes were connected to a percutaneous socket (Scott Electronics, USA) and then secured with carboxylate dental cement. The epidural recording electrode was a stainless steel screw, implanted at the vertex. The intracochlear cannula was inserted 0.5mm through a carefully made fenestra in the otic capsule 2 mm ventral to the round window, sealed and secured, as described previously for the osmotic pump (Alzet model 2002, Alza Corp., USA) implantation (Shinohara et al., 2002). The bulla defect was covered with dental cement to fix the electrode and cannula to the temporal bone. All procedures were performed under aseptic conditions and doxycyclin (Nordic Drugs, Sweden) was administered after surgery, prophylactically.
Experimental design
Phase I – Local treatment with antioxidants
Before implantation, the cannula was primed with 24 μl of 10% neomycin solution, and the pump was then filled with either 200 μl of artificial perilymph (AP) or a combination of 100 mM Trolox and 100 mM ascorbic acid. A small bubble was created in the cannula to reduce intermixing of the neomycin with the treatment/control solutions in the pump. This was done, based on many observations we have made under an operating microscope, with a variety of solutions, with dyes separated by such a small air bubble and found that this is most effective in restricting the mixing of the two solutions. AP was composed of 137mM NaCl, 2mM CaCl2, 5 mM KCl, 1mM MgCl2, 1 mM NaH2PO4, 12 mM NaHCO3, and 11 mM glucose. As the output of the pump is calibrated at 0.5 μl/h the neomycin was infused into the scala tympani for the first 2 days followed by either AP (untreated group, n=6) or a combination of 100 mM Trolox and 100 mM ascorbic acid (treated group, n=6). Intracochlear infusion of either AP or a combination of Trolox and ascorbic acid, was continued for a total of 26 days, respectively, by exchange of the osmotic pump on day 14 following implantation. Trolox (Fluka Chemie, Switzerland) was dissolved in artificial perilymph, with a few drops of 1N sodium bicarbonate (Sigma, USA) added to increase solubility. Following addition of ascorbic acid (Sigma-Aldrich Co Ltd., Germany), the final solution was adjusted to pH 7.2-7.4.
Phase II – Systemic treatment with antioxidants
Thirty-two guinea pigs were divided into 4 groups as follows: (1) control group, (2) untreated group, (3) treated group with high dose antioxidant, and (4) treated group with low dose antioxidant. The control group animals were normal hearing subjects, which were cannulated and received AP intracochlearly. The untreated and the two treated groups were deafened by intracochlear infusion of 10% neomycin for 2 days, using the osmotic pump. Before implantation, the cannula was primed with either 24 μl of AP (control group) or 24 μl of concentrated 10% neomycin solution (all others). For all subjects the pump was filled with AP. After 2 days the pump was removed and the cannula occluded.
Following deafening, the treated groups received daily intraperitoneal (IP) injections of Trolox and ascorbic acid for 4 weeks. The concentrations in the high dose group 10mg Trolox /kg/day and 200 mg ascorbic acid /kg/day. The treated group with low dose AOs received a drug concentration one-tenth that of the high dose group (i.e., Trolox 1mg/kg/day and ascorbic acid 20mg/kg/day). The control and untreated groups received daily IP injections of the same volume of saline for 4 weeks. Trolox (Fluka Chemie, Switzerland) was dissolved in 0.154 N NaOH and neutralized by 0.154 N HCl. The antioxidant solution was adjusted to pH 7.2-7.4 with 1N NaOH after the addition of ascorbic acid (Sigma-Aldrich Co Ltd., Germany). The antioxidant solution was made just before every injection.
Electrically-evoked auditory brainstem responses (EABRs)
The animals were anesthetized with xylazine (10 mg/kg, i.m.) and ketamine (40 mg/kg, i.m.), and placed in a sound-proof room. In Phase I, EABRs were recorded on day 3 and weekly for 4 weeks after deafening while in Phase II, EABRs were recorded on day 3 and weekly for 6 weeks. EABR recording procedures have been described previously (Shinohara et al., 2002). Thresholds were defined as the lowest stimulus level, which evoked at least a 0.4μV replicable waveform. Averages of 2048 responses, to 50-μs computer-controlled monophasic current pulses, presented at 50pps, with an alternating polarity, were amplified using preamplifier (HS4, Tucker-Davis Technologies Inc., USA) and recorded between the screw on the vertex (active) and a needle inserted at the left mastoid (reference) by an analogue to digital converter (Tucker-Davis Technologies Inc., USA). The ground was a needle electrode inserted to neck subcutaneously. Stimulus current was generated by a custom-built isolated constant current stimulator and monitored with an oscilloscope (PM3226, Philips, Germany). Intensity of stimulus current was changed from approximately 30 μA to 800 μA in 50 μA steps (in 10 μA steps near the threshold). High- and low-pass filters were set at 8 Hz and 15 kHz, respectively and a gain of 1000 was used. From these recordings, thresholds were evaluated in Phase I subjects and both thresholds and amplitudes of the first positive (P1) wave, at 0.l – 0.3 msec following stimulus onset, were measured in Phase II subjects. Amplitude assessment of P1 in Phase I subjects was not possible because of electrical artifact, reduced by the change in recording electrode placement in Phase II animals, thus P5, occurring 1.3 – 1.7 msec following stimulus onset, was assessed.
Figure 1 shows typical EABR waveforms of recorded in one of the deafened animals. P1 and P5 waves were clearly evident following, but separate from, the electrical stimulus artifacts (indicated by an asterisk). The threshold of this animal was 200 μA.
Fig. 1.
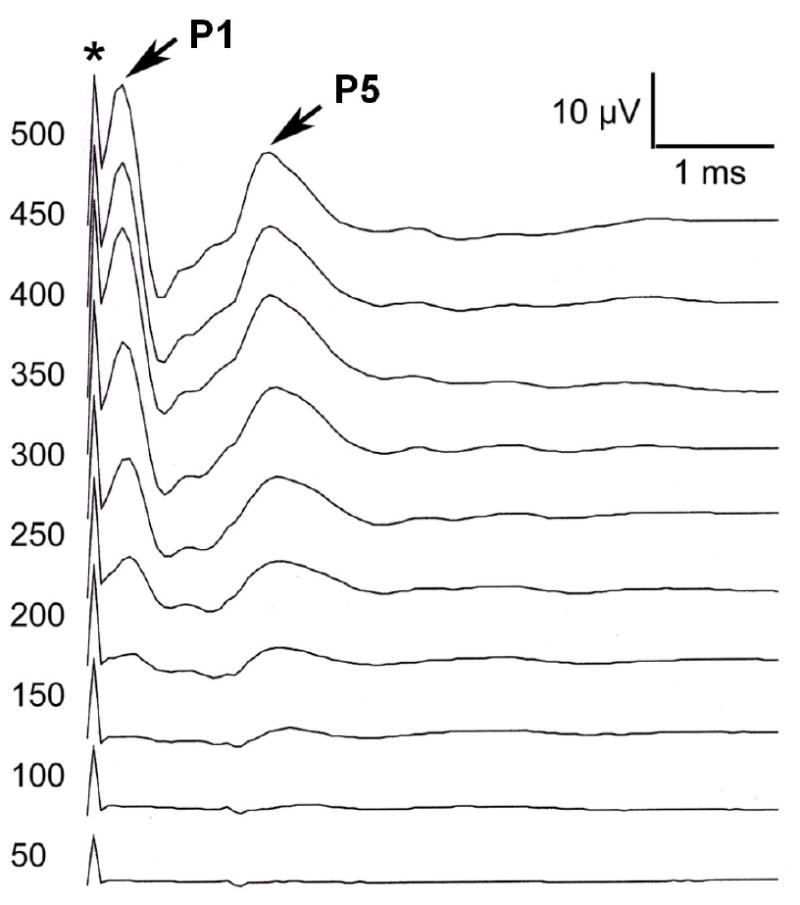
Electrically-evoked auditory brainstem responses (EABR) from a neomycin-deafened subject. The first positive (P1) wave after stimulus artifact (indicated by *) was clearly identified in each subject of this group; the later P5 wave is indicated.
Histology
To determine the survival of SGCs, the animals were sacrificed by cardiac perfusion (saline followed by 3% glutaraldehyde in 0.1M phosphate buffer) after final EABR measurement (at 4 weeks in Phase I study, at 6 weeks in Phase II). The bulla was then opened and the cochlea locally perfused with the same fixative, rinsed in buffer, decalcified with 0.1M EDTA, and embedded in paraffin. Six-μm sections were cut in a paramodiolar plane, and every fourth section was mounted on a glass slide and stained with toluidine blue. Six sections were randomly selected from the 10 most mid-modiolar sections for each animal and used for quantitative analysis of SGCs. The SGC counting was performed in a double blind manner as described in detail in Yamagata et al. (2004). All neurons meeting size and shape criteria to be considered type I SGCs within each profile of Rosenthal’s canal from base to apex of the cochlea were counted. The outline of the Rosenthal’s canal profile was then traced to generate a SGC density, expressed as the density of SGC for an area of 10,000 μm2.
Statistical analyses
Statistical assessment of differences in EABR thresholds and SGC density between the groups was performed using either one way ANOVA or one way repeated-measures ANOVA. All post-hoc tests were conducted using the Student Newman-Keuls Method.
Results
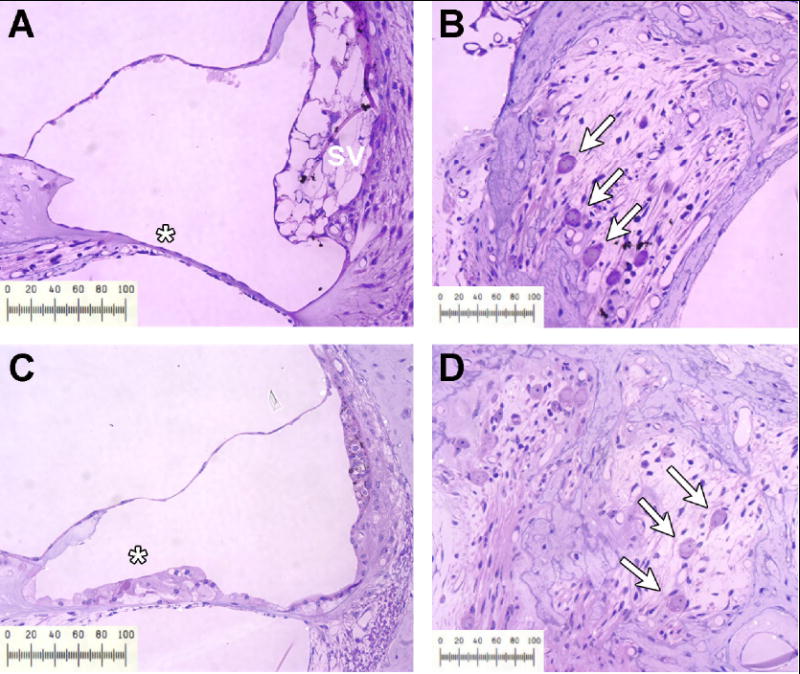
The present experimental approach is based on the assumption that sensory hair cells and spiral ganglion neurons are destroyed throughout most of the cochlea as a result of the deafening procedure. As seen in Figure 2, the organ of Corti was completely destroyed four weeks after neomycin infusion, especially in the base of the cochlea (2A), leaving only an epithelial scar. In the most apical turn (third turn; Figure 2C), many of the supporting cells and occasionally a hair cell survived the neomycin treatment. However, the density of SGC in the base (2B), as well as in the more apical modiolar region (2D) was dramatically reduced.
Fig. 2.
Light micrographs illustrating the organ of Corti region (A and C) and spiral ganglion region (B and D) in an untreated animal 6 weeks following neomycin-deafening. In the first turn (A) the organ of Corti is completely eliminated (*); whereas the apical third turn (C) demonstrates a few supporting cells remaining (*). Occasionally the stria vascularis (SV) in the lower turn of deafened animals showed marked edema as seen in panel A. Rosenthal’s canal demonstrates a dramatic loss of SGC in both the basal (B) and apical (D) regions of the modiolus. Scale bar, 100 μm.
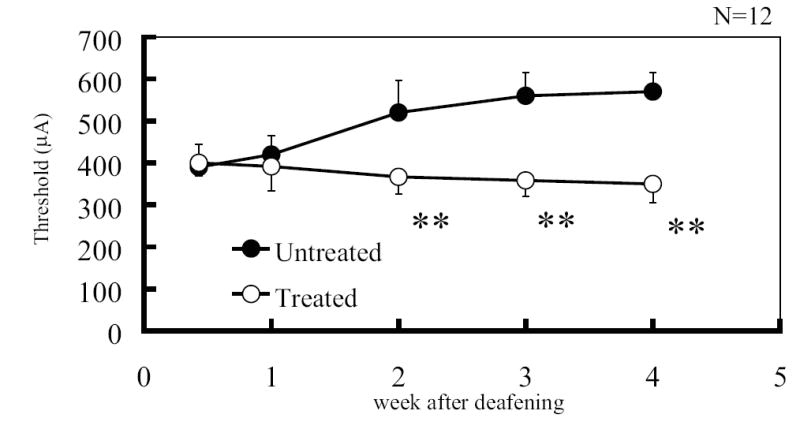
Phase I – Local administration of antioxidants
The mean and standard deviation of EABR thresholds of both untreated and treated groups (n = 6 each) are shown in Figure 3. Thresholds of the untreated group demonstrated a gradual and continuing elevation following deafness, which by 4 weeks was 570 μA. In contrast, the thresholds of those groups treated with AOs showed a clear decrease following deafness over 4 weeks, reaching a final stable level of 350 μA. There was a statistically significant difference between the treated groups and untreated groups at weeks 2, 3 and 4 (t-Test, p<0.01).
Fig. 3.
Mean electrically evoked auditory brainstem response (EABR) thresholds in untreated and AO (100mM Trolox and 100mM ascorbic acid) locally-treated groups at each week after deafening (n = 6 each group). The vertical bars indicate one standard deviation. EABR thresholds in the AO-treated group decreased 2 weeks after deafening compared to those of untreated group. An asterisk indicates a significant difference (** p<0.01), at weeks 2, 3, and 4.
SGC survival in untreated and AO-treated groups are shown in Figure 4. The number of subjects in each group are 5, one from each group having been lost in histological preparation. Across the whole cochlea, mean SGC survival density for the untreated group was 2.1 per 10,000 μm2, whereas for the AO treated group SGC density was 3.2 per 10,000 μm2. There was a statistically significant enhancement in SGC survival for the treated group after deafening compared with the untreated group (p<0.05).
Fig. 4.
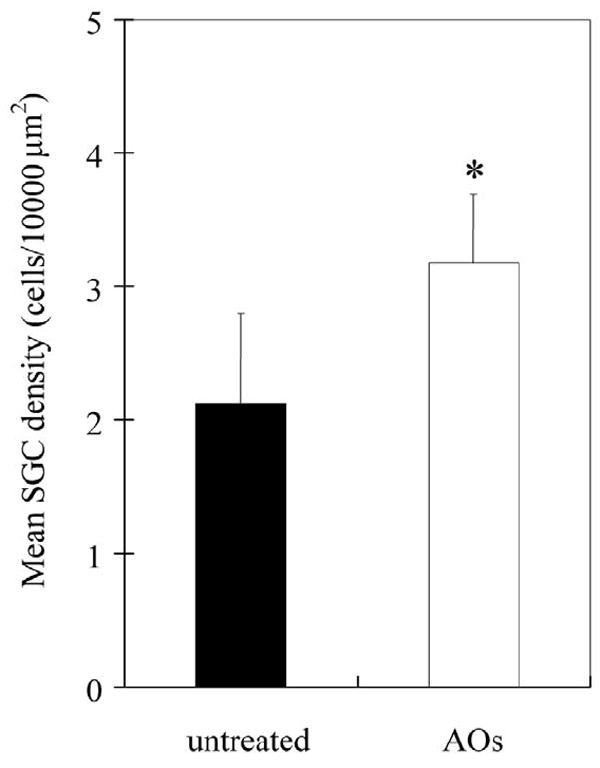
Mean spiral ganglion cell (SGC) density in untreated and AO (100mM Trolox and 100mM ascorbic acid) locally-treated groups (n=5 each). The vertical bars indicate one standard deviation. SGC density in AO-treated group is significantly higher than that of untreated group (* p<0.05).
Phase II – Systemic administration of antioxidants
Input/output functions of P1 amplitude in each group are shown in Figure 5. The data represent mean values for 8 animals each. In the control group (Figure 5A, normal hearing, no treatment), P1 amplitudes were stable throughout the experiment (p=0.9956). In the untreated group (Figure 5B, deafened), intracochlear neomycin was followed by a marked initial decrease (after 3 days) and then a continuing gradual significant decrease of P1 amplitude growth functions through the observation period (p<0.05). In the deafened groups treated systemically with high and low dose AOs (Figures 5C and 5D, respectively) significant decreases of P1 amplitude, compared to normal hearing animals, were also observed but of smaller magnitude to those of the deafened group receiving no AO treatment (Figure 5B vs. 5C and 5D, p<0.05). This difference was most obvious in the maximum output of the system to high levels of stimulation at 800μA between the treated and untreated groups. It is noteworthy, that P1 amplitudes were well-maintained in the treated groups following an initial decrease, even 5 and 6 weeks after deafening, well after the termination of AO treatment. The high dose AO-treated group stabilized after the initial decrease at 3 days; the low dose AO-treated animals stabilized after 1 week.
Fig. 5.
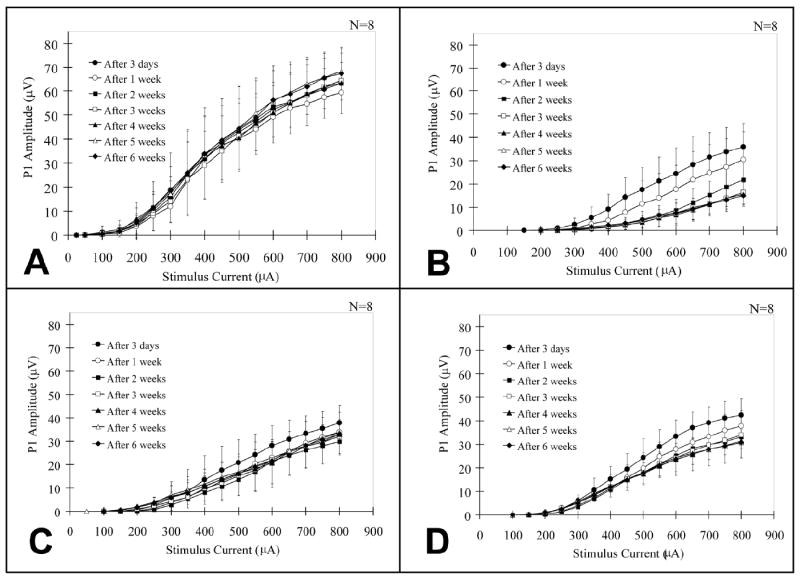
Input-output functions of P1 amplitude in all Phase II (systemic administration) groups at 3 days and weeks 1-6 after the onset of deafness. The vertical bars indicate one standard deviation. (A) P1 amplitudes in control group are stable throughout the experiment. (B) P1 amplitudes in untreated group decreased rapidly and continually for 3 weeks after deafening. (C) Decreases of P1 amplitude in the high dose (10mg/kg/day Trolox and 200mg/kg/day ascorbic acid) AO-treated group are smaller than those of the untreated group. (D) Decreases of P1 amplitude in the low dose (1mg/kg/day Trolox and 20mg/kg/day ascorbic acid) AO-treated group are smaller than those of the untreated group.
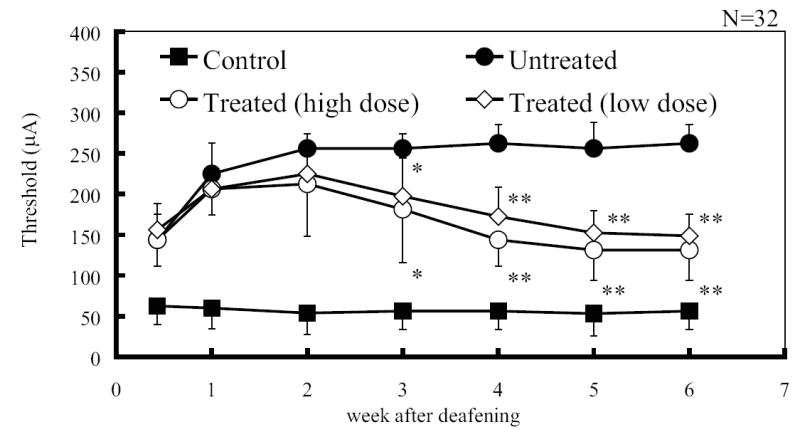
Figure 6 shows mean EABR thresholds and standard deviations in each group. Mean EABR thresholds of the control group were stable (53.1~62.5μA) throughout the experiment, as well as P1 amplitudes. In both treated groups (high and low dose AOs) and the untreated group (i.e., all deafened groups), EABR thresholds increased immediately with deafening (compare control group EABR thresholds with those of all deafened groups at the first measurement, day 3 following deafening). The EABR thresholds then continued to increase through the first 2 weeks following deafening, compared to the control group. The EABR thresholds of the two deafened-treated groups began to decrease 3 weeks following initiation of treatment, and this decrease in threshold continued through week 4 and was maintained at a level of approximately 140 μA after the four weeks of treatment to week 6. The mean threshold of the treated group with high dose antioxidant, at 6 weeks following deafness, was 131.3μA and that of the low dose treatment group was 148.8μA. On the other hand, the threshold of the untreated group increased to a plateau at 3 weeks following deafness of 262.5μA and remained stable to 6 weeks. There were statistically significant differences between each of treated groups and the untreated group on weeks 3, 4, 5 and 6 (One way ANOVA on Ranks, p<0.05). However, there is no significant difference between the high and low dose treated groups.
Fig. 6.
Mean EABR thresholds in each group at 3 days and weeks 1-6 after the onset of deafening. The vertical bars indicate one standard deviation. Mean EABR thresholds in the control group are stable throughout the experiment. EABR thresholds in treated group with high (10mg/kg/day Trolox and 200mg/kg/day ascorbic acid) and low dose (1mg/kg/day Trolox and 20mg/kg/day ascorbic acid) AOs decrease 3 weeks after deafening compared to those of untreated group. Asterisks indicate a significant difference (* p<0.05, **p<0.01).
In the control group there was a normal organ of Corti while both untreated and AO-treated deafened groups (Figs 7 B-D) had an elimination or severe destruction of hair cells and supporting cells extending from the base of the cochlea through the third turn reflecting the effectiveness of the intracochlear infusion of neomycin. Figure 7A-D shows representative sections through Rosenthal’s canal from each group of the Phase II (systemic treatment) study. In the normal hearing control group, where there was no hair cell loss, there is a clear highly dense packing of SGCs in Rosenthal’s canal, as expected (Figure 7A). A few SGCs are present in Rosenthal’s canal in the untreated deafened group (Fig 7B), with remaining SGC appearing more densely stained and somewhat smaller than those in 7A. On the other hand, systemic treatment with low (Fig 7C) and high (Fig 7D) AOs resulted in an enriched survival of SGCs compared with the untreated group. The SGC in the low dose (Fig 7D) treated ears while greater in number than in the untreated ears (Fig 7b), share with them a greater density of staining, not observed in the high dose AO treated animals (Fig 7C).
Fig. 7.
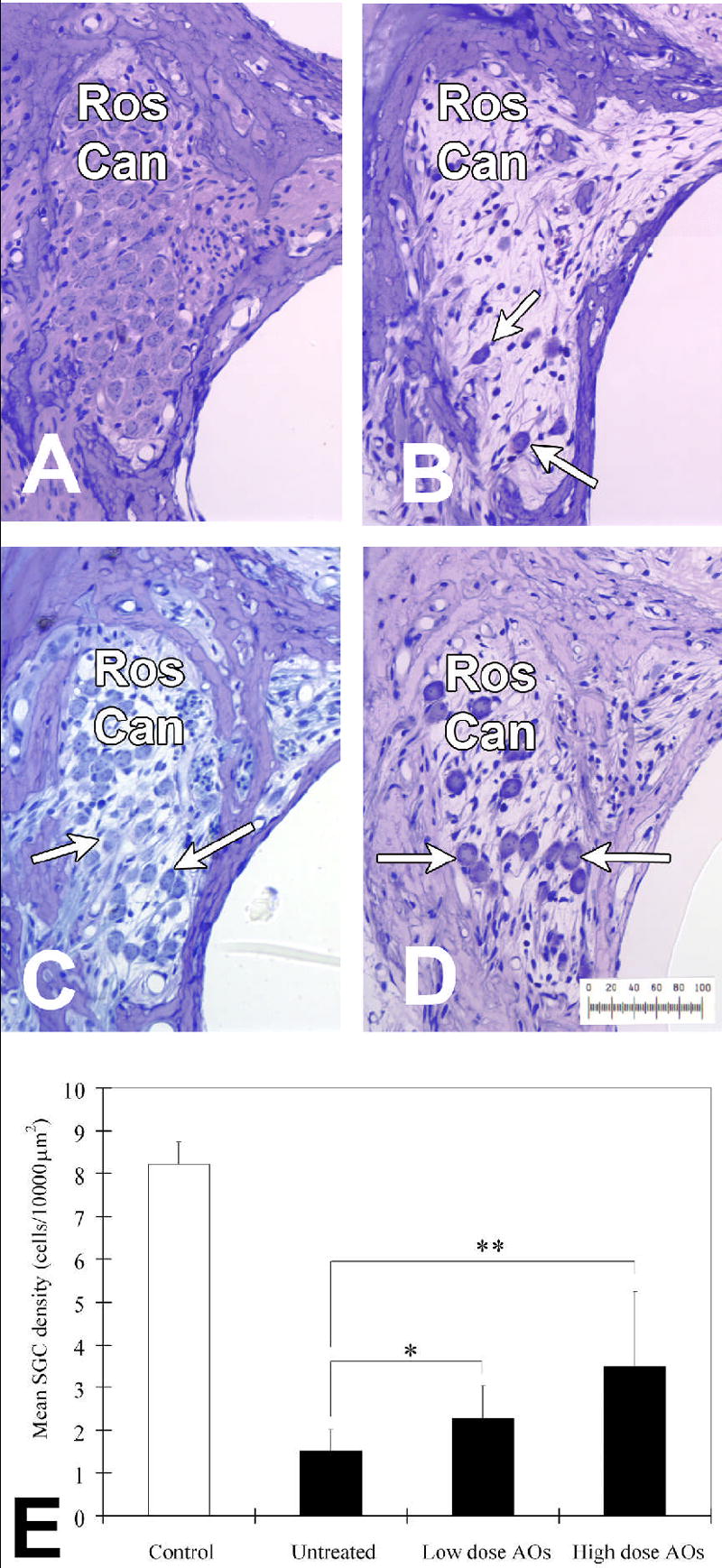
Representative sections of Rosenthal’s canal (abbreviated Ros Can in figure) in second turn of the cochlea from: (A) control, (B) untreated, (C) treated group with low dose (1mg/kg/day Trolox and 20mg/kg/day ascorbic acid) AOs, and (D) treated group with high dose (10mg/kg/day Trolox and 200mg/kg/day ascorbic acid) AOs. There is a severe loss of SGC in untreated group (B). SGC survival is observed in treated group with low and high dose AOs (C and D). The mean SGC density in each group is shown in (E). The vertical bars indicate one standard deviation. SGC density in treated group with low and high dose AOs is significantly higher than that of untreated group (* p<0.05, ** p<0.01, respectively).
Table 1 provides the quantitative results of the histological measurements made across treatment groups at each measurement site along the length of the cochlea (where P1 is the most basal portion of the cochlea). Mean values for each group for the area of Rosenthal’s canal, the number of SGC observed, and the calculated density of SGCs for each measurement site are given. Mean SGC survival among groups are shown in Figure 7E. Across the whole cochlea, mean SGC density for the control group was 7.9 (±0.5) per 10,000 μm2. Mean SGC survival for the untreated-deafened group was 1.5 (±0.5) per 10,000 μm2, whereas for high and low dose antioxidant treated groups SGC survival was 3.5 (±1.6) and 2.3 (±0.7) per 10,000 μm2, respectively. There was a statistically significant enhanced survival of SGC in the treated groups with high dose (p<0.01) and low dose (p<0.05) AOs compared with the untreated group. However, the difference in SGC density was not significant between the two (low and high dose) treated groups in SGC survival.
Table 1.
Summary of histological findings
| Number of SGCs | Mean±SD | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|---|
| Normal Hearing | 39.4±7.8 | 51.5 | 41.9 | 38.6 | 34.7 | 34.2 | 33.3 |
| Deafened Control | 7.4±2.9 | 7.8 | 6.0 | 7.0 | 7.1 | 8.5 | 8.2 |
| Low dose AO 1mg/kg/day Trolox and 20mg/kg/day ascorbic acid | 13.5± 3.5 | 19.1 | 10.6 | 12.5 | 12.7 | 12.6 | 13.2 |
| High dose AO 10mg/kg/day Trolox and 200mg/kg/day ascorbic acid | 17.7± 7.3 | 22.8 | 12.7 | 15.7 | 18.5 | 18.5 | 17.7 |
| Area | Mean±SD | P1 | P2 | P3 | P4 | P5 | P6 |
|
| |||||||
| Normal Hearing | 49387±6463 | 70974 | 51336 | 45799 | 42123 | 41561 | 41260 |
| Deafened Control | 51325±4726 | 66460 | 56893 | 47988 | 46843 | 40387 | 53710 |
| Low dose AO 1mg/kg/day Trolox and 20mg/kg/day ascorbic acid | 56023±4626 | 82663 | 57639 | 50511 | 47574 | 43017 | 54734 |
| High dose AO 10mg/kg/day Trolox and 200mg/kg/day ascorbic acid | 53560±4260 | 84010 | 55141 | 50258 | 43780 | 41698 | 46470 |
| Density | Mean±SD | P1 | P2 | P3 | P4 | P5 | P6 |
|
| |||||||
| Normal Hearing | 7.9±0.5 | 6.3 | 8.2 | 8.5 | 8.4 | 8.2 | 8.1 |
| Deafened Control | 1.5±0.5 | 1.0 | 1.0 | 1.5 | 1.5 | 2.1 | 1.4 |
| Low dose AO 1mg/kg/day Trolox and 20mg/kg/day ascorbic acid | 2.5±0.7 | 2.3 | 1.8 | 2.5 | 2.7 | 3.0 | 2.5 |
| High dose AO 10mg/kg/day Trolox and 200mg/kg/day ascorbic acid | 3.5±1.6 | 2.9 | 2.3 | 3.2 | 4.3 | 4.5 | 4.1 |
AO: antioxidants Trolox and ascorbic acid. For each value of SGC number and area of Rosenthal’s canal 6 sections were evaluated at each site along the cochlea (P1 – P6) times an n = 8 animals in each group: Normal, Deaf-untreated, Deaf-low dose AO treated, & Deaf- high dose AO treated.
Discussion
This study demonstrates in vivo that free radical scavengers, a combination of vitamin E and C, not only enhance SGC survival but also increases electrical sensitivity in the cochlear nerve after deafness. It is especially interesting to note that EABR thresholds and rescue of SGC in the treated groups were improved compared to untreated groups, not only when AOs were applied locally in the scala tympani but also when administered systemically. Significant decreases of EABR thresholds were observed 2 weeks after deafening in the group treated with local AOs and 3 weeks after deafening in groups treated with systemic AOs. Decreases of EABRs in treated groups with local AOs occurred more rapidly than those of treated group with systemic antioxidants. AOs applied into the scala tympani may work rapidly on the cochlear nerve with access via the habenula perforate and fenestrae in the modiolar bone throughout the basal and first turn of the human and guinea pig cochlea (Lim and Kim, 1983; Kücük et al., 1991; Glueckert et al., 2005). However, using this pathway AOs may also reach the fluid surrounding the cells within the organ of Corti (e.g. Ulfendahl et al., 2000), and thus, by protecting the hair cells, prevent secondary degeneration of SGC. This mechanism could be of particular relevance and value in patients now receiving cochlear implants, with partial acoustic hearing preservation, in which a significant population of functioning hair cells must be present at the time of implantation (Gantz, et al., 2005). In all implant patients preservation of remaining auditory nerve is considered of value, for patients with remaining hearing the preservation of functioning sensory cells additionally benefits acoustic hearing provided by hybrid electrical-acoustic prostheses (Gantz, et al., 2005). Since AOs have been shown to protect the sensory cells from other stress factors, such as intense noise trauma (see Le Prell et al, 2007; Miller et al, 2006 for review), it is reasonable to suggest such treatment may protect remaining sensory cells from implant induced trauma.
Previous studies using EABRs demonstrated a relationship between the number of SGCs and electrophysiological findings. Shinohara et al. (2002) showed close relation between SGC survival and EABR threshold. Consistent with the findings of Smith and Simmons (1983) and Jyung et al. (1989), Hall (1990) reported that the P1 amplitude was highly correlated with the number of SGCs. Therefore, we evaluated both EABR threshold and P1 amplitude as assessment of auditory function in Phase II. Systemic treatment with AOs suppressed decreases of P1 amplitudes compared to the untreated group, which shows enhancement of SGC function after deafness. This enhanced function demonstrated itself as a treatment-induced increase in the dynamic range of auditory nerve responsiveness.
The EABR threshold for the systemically treated antioxidant group showed a rapid and significant elevation in threshold (Fig 6) immediately following the deafening procedure, which continued through week 1. We assume that this in part reflected the direct effect of the ototoxic antibiotic neomycin on the afferent nerve fibers. While the primary target of neomycin is the sensory hair cells, with direct delivery of a 10% solution to the scala tympani, the drug has direct access to the auditory nerve fibers (Lim and Kim, 1983; Kücük et al., 1991; Glueckert et al., 2005) and previous work has indicated that the drug may directly damage afferent nerve fibers (Leake-Jones, et al., 1980) as well as hair cells. We suggest that the rapid and dramatic elevation in ABR threshold observed immediately (compare all deafened groups EABR thresholds with the controls on the 1st measurement, Fig 6) and in the short run (at least through week 1) after this local drug treatment reflects this direct damaging effect of the drug on the auditory nerve. Thus auditory nerve cell dysfunction and eventual death reflects the direct action of the aminoglycoside antibiotic on the nerve tissues plus the neurotrophic deprivation due to destruction of hair cells. Both factors involve the formation of free radicals and subsequent direct and indirect destruction of elements of the cell. Local treatment with AOs (whether it be low or high dose) by scavenging free radicals, reducing the oxidative state change induced by neurotrophic deprivation and the direct effects of the neomycin, inhibits the auditory nerve cell death pathway preventing some of the consequent degeneration and damage to the auditory nerve. These findings are consistent with a previous report that sod1- null mice (reduced endogenous antioxidant enzyme) demonstrate an accelerated age-related loss in auditory nerve (Keithley, et al., 2005).
Approximately 50% of the SGC survived in deafened treated animals compared to controls; while approximately 20% survived in deafened untreated animals. Relative EABR thresholds were approximately 55 μA (controls), 135 μA (deaf-treated) and 260 μA (deaf-untreated). The immediate EABR threshold elevation (measurement 1, Fig 6) reflects pathophysiological changes in the auditory nerve, while degeneration has not yet occurred. While speculative, these data are consistent with the assumption that the AO treatment reversed this initial pathophysiology and prevented some of the subsequent degeneration yielding a threshold that was about midway between the control and untreated-deafened animals, and a 50% survival of SGC. Of course this speculation must be viewed in the light of a long standing issue of variability seen in measures of tissue survival and physiological responsiveness, not restricted to the auditory nerve. Certainly the reduction observed in EABR thresholds (Fig 6) beginning 3 weeks after antioxidant treatment indicates that the AOs systemically delivered have access to the tissues of the inner ear and are effective in reducing pathology induced by the ototoxic drugs and the neurotrophic factor deprivation that follows loss of sensory cells.
The antioxidant therapy was less effective in complete preservation of the SGC than previous work from this and other laboratories using neurotrophin therapy (Staecker et al., 1996; Ernfors et al., 1996; Miller et al., 1997; Ylikoski et al., 1998; Yagi et al., 2000; Shinohara et al., 2002; Yamagata et al., 2004). In the absence of neurotrophic input from the sensory cells, gene regulation is modified initiating events leading to cell death. Along this cascade cellular oxidative state changes occur with the formation of free radicals. Antioxidant therapy is an attempt at rescuing auditory nerve cells along the cell death pathway at a point defined by free radical formation. Neurotrophin factor replacement therapy blocks the initiating conditions that upregulate cell death pathways, interceding at the beginning of the apoptotic pathway and results in a greater preservation of auditory neurons. This difference suggests that a “replacement therapy” (ie. intervening before initial degenerative events occur) is more effective that a later stage intervention, which may not block all pathways to cell damage initiated by the neurotrophic factor deprivation. Our data suggest, however, that it is possible to intervene further downstream with antioxidant application and still effect a statistically significant enhancement of nerve preservation and function.
Efficacy of a cochlear implant is partly dependent on the survival and excitability of the auditory nerve and prevention of neural degeneration is thus of clinical relevance. In this study, only the high dose vitamin C (200mg/kg/day) is greater than a clinically approved dose whereas all other concentrations, producing equally positive results, are within recommended daily limits. Antioxidant treatment may, however, be of clinical interest with other conditions. For example, formation of reactive oxygen species (ROS) and nitric oxide (NO) play a central role in the development of intracranial complications and brain damage in bacterial meningitis (Koedel et al; 1995; Koedel and Pfister, 1997; Buster et al., 1995), and sensorineural hearing loss following acute bacterial meningitis can be caused by an inflammatory response/response mediated generation of free radicals. AOs as an adjunctive treatment in deafness and cochlear implantation may be beneficial in decreasing CNVIII nerve degeneration associated with meningitis (Ge, et al., 2004).
Antioxidant therapies such as vitamin E and vitamin C have been considered to be effective clinically for neurodegenerative disease such as Alzheimer’s and Parkinson’s disease (Fahn, 1992; Sano et al., 1997; Kontush et al., 2001). Fahn (1992) suggested that a high dose of alpha-tocopherol (3200 IU/day) and vitamin C (3000 mg/day) delayed the progression of Parkinson’s disease. Sano et al. (1997) also showed that 2000 IU (approximately 1350 mg) alpha-tocopherol was beneficial in delaying the primary outcome of Alzheimer’s disease progression in a double-blind, placebo-controlled study. Furthermore, Kontush et al. (2001) reported that supplementation of vitamin E (400 IU/day) and vitamin C (1000 mg/day) for 1 month increased their concentrations in cerebrospinal fluid in patients with Alzheimer’s disease. In human, vitamin E (a dosage up to 1000 mg/day) is considered to be entirely safe and without side effects (Diplock, 1998). Vitamin C is also relatively safe and free from side effects, indeed, even very high doses of vitamin C (up to 2000mg/day) only occasionally results in side effects (Diplock, 1998).
In animal experiment, antioxidant therapy such as free radical scavengers has successfully attenuated noise and drug-induced ototoxicity, which cause production of ROS. Especially, free radical scavengers such as L-N-Acetyl-Cysteine, alpha-lipoic acid and vitamin E are in the spotlight for therapeutic potential because they have essentially no side effects in human. Feghali et al. (2001) showed that L-N-Acetyl-Cysteine (NAC) protected both hair cells and auditory neurons against cisplatin-induced ototoxicity in vitro. Robust formation of ROS in the organ of Corti with noise exposure has been shown (Ohinata et al., 2000a), which is followed by an upregulation of endogenous antioxidant glutathione (GSH) (Yamasoba et al., 1998). Decreasing endogenous GSH increases noise damage, while providing supplementary exogenous AOs (NAC, GSH monoethyl ester) or blockers of excitotoxity decreases noise-induced ABR threshold shifts and hair cell loss (Ohinata et al., 2000b). Kopke et al. (2000) reported that intraperitoneal injection with a combination of NAC and salicylate reduced threshold shifts on ABR following noise exposure in the chinchilla. They also showed that the antioxidant treatment prior to noise exposure was effective in prevention of hair cell loss. According to Rybak et al. (1999) and Conlon et al. (1999), lipoic acid attenuated threshold shifts of ABRs or compound action potentials against drug-induced ototoxicity such as cisplatin and aminoglycoside. Recently, Teranishi et al. (2001) showed that α-tocopherol injected intraperitoneally suppressed not only threshold shifts of ABRs but also hair cell loss against cisplatin-induced ototoxicity. Antioxidant therapy has been well-established in animal experiments and can prevent hair cell damages and hearing loss against noise and drug-induced ototoxicity. However, until this investigation, there is no report that addresses in vivo effects of antioxidant on degeneration of the auditory nerve and electrical sensitivity of the cochlear nerve following deafness.
In conclusion, this study shows that AOs enhanced both CNVIII cell survival and electrical sensitivity following deafferentation when applied locally and systemically. These results suggest that AOs have therapeutic potential in prevention of degeneration of SGC in human, which may lead to a decrease in auditory nerve cell loss following deafness, particularly meningitis, and increase the benefits of cochlear implants as a treatment for deafness.
Acknowledgments
The authors would like to thank Ms P Mannström for technical support. This work was supported by the European Union Biotechnology Research Program (QLG3-CT-2002-01563), the Swedish Research Council, the Foundation Tysta Skolan, NIH Grant DC03820, the Ruth and Lynn Townsend Professorship and by an institutional grant from the Swedish Foundation for International Cooperation in Research and Higher Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergstrom L. Some pathology of sensory and neural hearing loss. Canadian J Otolaryngol. 1975;4(2):1–28. [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G, Larky J, Lindstrom B, Nedzelski J, Peterson A, Shipp D, Staller S, Whitford L. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neuro-otol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- Buster BL, Weintrob AC, Townsend GC, Scheld WM. Potential role of nitric oxide in the pathophysiology of experimental bacterial meningitis in rats. Infect Immun. 1995;63:3835–3839. doi: 10.1128/iai.63.10.3835-3839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tappel AL. Protection of vitamin E, selenium, trolox C, ascorbic acid palmitate, acetylcysteine, coenzyme Q0, coenzyme Q10, beta-carotene, canthaxanthin, and (+)-catechin against oxidative damage to rat blood and tissues in vivo. Free Radic Biol Med. 1995;18:949–953. doi: 10.1016/0891-5849(94)00238-f. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Conlon BJ, Aran JM, Erre JP, Smith DW. Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res. 1999;128:40–44. doi: 10.1016/s0378-5955(98)00195-6. [DOI] [PubMed] [Google Scholar]
- Davies AM. The neurotrophic hypothesis: where does it stand? Philosophical Transactions of the Royal Society of London - Series B. Biolog Sci. 1996;351:389–394. doi: 10.1098/rstb.1996.0033. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM., Jr Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation-induced death of sympathetic neurons. Molecular Pharmacol. 1997;51(6):897–906. doi: 10.1124/mol.51.6.897. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM., Jr Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21(4):695–705. doi: 10.1016/s0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- Diplock AT. Defense against reactive oxygen species. Free Radic Res. 1998;29(6):463–467. doi: 10.1080/10715769800300521. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Creedon DJ, Johnson EM, Jr, Holtzman DM. Rapid suppression of free radical formation by nerve growth factor involves the mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 1997;94:4086–4091. doi: 10.1073/pnas.94.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2:63–67. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Fahn S. A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson’s disease. Ann Neurol. 1992;32(Suppl):128–132. doi: 10.1002/ana.410320722. [DOI] [PubMed] [Google Scholar]
- Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope. 2001;111:1147–1155. doi: 10.1097/00005537-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Gabaizadeh R, Staecker H, Liu W, Van De Water TR. BDNF protection of auditory neurons from cisplatin involves changes in intracellular levels of both reactive oxygen species and glutathione. Brain Res Mol Brain Res. 1997;50:71–78. doi: 10.1016/s0169-328x(97)00173-3. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Ge NN, Brodie SA, Tinling SP, Brodie HA. The effects of superoxide dismutase in gerbils with bacterial meningitis. Otolaryngol Head Neck Surg. 2004;131:563–72. doi: 10.1016/j.otohns.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Glueckert R, Pfaller K, Kinnefors A, Rask-Andersen H, Schrott-Fischer A. The human spiral ganglion: new insights into ultrastructure, survival rate and implications for cochlear implants. Audiol Neuro-otol. 2005;10:258–273. doi: 10.1159/000086000. [DOI] [PubMed] [Google Scholar]
- Greenlund LJ, Deckwerth TL, Johnson EM., JR Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res. 1990;49:155–168. doi: 10.1016/0378-5955(90)90102-u. [DOI] [PubMed] [Google Scholar]
- Incesulu A, Nadol JB., Jr Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1998;107:906–911. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- Jyung RW, Miller JM, Cannon SC. Evaluation of eighth nerve integrity by the electrically evoked middle latency response. Otolaryngol Head Neck Surg. 1989;101:670–682. doi: 10.1177/019459988910100610. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kileny PR, Zimmerman-Phillips S, Kimink JL, Schmaltz SP. Effects of preoperative electrical stimulability and historical factors on performance with multichannel cochlear implant. Ann Otol Rhinol Laryngol. 1991;100:563–568. doi: 10.1177/000348949110000708. [DOI] [PubMed] [Google Scholar]
- Koedel U, Bernatowicz A, Paul R, Frei K, Fontana A, Pfister HW. Experimental pneumococcal meningitis: cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann Neurol. 1995;37:313–323. doi: 10.1002/ana.410370307. [DOI] [PubMed] [Google Scholar]
- Koedel U, Pfister HW. Protective effect of the antioxidant N-acetyl-L-cysteine in pneumococcal meningitis in the rat. Neurosci Lett. 1997;225:33–36. doi: 10.1016/s0304-3940(97)00177-8. [DOI] [PubMed] [Google Scholar]
- Kontush A, Mann U, Arlt S, Ujeyl A, Luhrs C, Muller-Thomsen T, Beisiegel U. Influence of vitamin E and C supplementation on lipoprotein oxidation in patients with Alzheimer’s disease. Free Radic Biol Med. 2001;31:345–354. doi: 10.1016/s0891-5849(01)00595-0. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, Lambert DC, Charon CC, Ding DL, McBride D. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149:138–146. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- Kücük B, Abe K, Ushiki T, Inuyama Y, Fukuda S, Ishikawa K. Microstructures of the bony modiolus in the human cochlea: a scanning electron microscopic study. J Electron Microsc (Tokyo) 1991;40:193–197. [PubMed] [Google Scholar]
- Leake-Jones PA, O’Reilly BF, Vivion MC. Neomycin ototoxicity: ultrastructural surface pathology of the organ of Corti. Scan Electron Microsc. 1980;3:427–34. [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami S, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DJ, Kim HN. The canaliculae perforantes of Schuknecht. Adv Otorhinolaryngol. 1983;31:85–117. doi: 10.1159/000407858. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuroprotective strategies based on targeting of postreceptor signaling events. In: Mattson MP, editor. Neuroprotective Signal Transduction. Vol. 345 Humana Press; New Jersey: 1998. pp. 301–335. [Google Scholar]
- Miller AL, Morris DJ, Pfingst BE. Effects of time after deafening and implantation on guinea pig electrical thresholds. Hear Res. 2000;144:175–186. doi: 10.1016/s0378-5955(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Miller J, Yamashita S, Minami S, Yamasoba T, Le Prell C. Mechanisms and prevention of noise induced hearing loss. Otol Jpn. 2006;16:139–153. [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Histopathology of residual and recurrent conductive hearing loss after stapedectomy. Otol Neurotol. 2001;22(2):162–169. doi: 10.1097/00129492-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000a;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000b;146:28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Otte J, Schunknecht HF, Kerr AG. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope. 1978;88:1231–1246. doi: 10.1288/00005537-197808000-00004. [DOI] [PubMed] [Google Scholar]
- Pfingst BE. Changes over time in thresholds for electrical stimulation of the cochlea. Hear Res. 1990;50:225–236. doi: 10.1016/0378-5955(90)90047-s. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Shivapuja BG, Schwimmer CL, Seidman MD. Lipid peroxidation inhibitor attenuates noise-induced temporary threshold shifts. Hear Res. 1994;74:217–220. doi: 10.1016/0378-5955(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 1995;76:386–394. doi: 10.1111/j.1600-0773.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Ravi R, Somani SM. Mechanism of protection by diethyldithiocarbamate against cisplatin ototoxicity: antioxidant system. Fundam Appl Toxicol. 1995;26:293–300. doi: 10.1006/faat.1995.1100. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Husain K, Whitworth C, Somani SM. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Toxicol Sci. 1999;47:195–202. doi: 10.1093/toxsci/47.2.195. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Sato K, Saito H, Katsuki H. Synergism of tocopherol and ascorbate on the survival of cultured brain neurones. Neuroreport. 1993;4:1179–1182. [PubMed] [Google Scholar]
- Seidman MD, Shivapuja BG, Quirk WS. The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol Head Neck Surg. 1993;109:1052–1056. doi: 10.1177/019459989310900613. [DOI] [PubMed] [Google Scholar]
- Sha S-H, Schacht J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res. 1999;128:112–118. doi: 10.1016/s0378-5955(98)00200-7. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykkö I, Olivius NP, Kaksonen R, Lindström B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99:1657–60. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MW, Binzer SM, Holden LK, Holden TA. Hearing changes in adults with cochlear implants. Seminars in Hearing. 1995;16:228–238. [Google Scholar]
- Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. Ann Otol Rhinol Laryngol. 1983;92:19–23. doi: 10.1177/000348948309200105. [DOI] [PubMed] [Google Scholar]
- Song B-B, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res. 1996;94:87–93. doi: 10.1016/0378-5955(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebrev P, Van De Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Stocker R. Lipoprotein oxidation: mechanistic aspects, methodological approaches and clinical relevance. Curr Opin Lipidol. 1994;5:422–433. [PubMed] [Google Scholar]
- Teranishi M, Nakashima T, Wakabayashi T. Effects of alpha-tocopherol on cisplatin-induced ototoxicity in guinea pigs. Hear Res. 2001;151:61–70. doi: 10.1016/s0300-2977(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M, Scarfone E, Flock A, Le Calvez S, Conradi P. Perilymphatic fluid compartments and intercellular spaces of the inner ear and the organ of Corti. Neuroimage. 2000;12:307–313. doi: 10.1006/nimg.2000.0617. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J, Polak M, Malgrange B, Lefebvre PP, Staecker H, Balkany TJ. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol. 2004;25(4):627–632. doi: 10.1097/00129492-200407000-00035. [DOI] [PubMed] [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykkö I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang H-Y, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Harris C, Shoji F, Lee RJ, Nuttall AL, Miller JM. Influence of intense sound exposure on glutathione synthesis in the cochlea. Brain Res. 1998;804:72–78. doi: 10.1016/s0006-8993(98)00660-x. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]


