Abstract
N-methyl-D-aspartate (NMDA) receptors and the expression of their different splice variants and subunits were previously characterized in the brain and spinal cord. However, knowledge on the NMDA receptor expression and function in the enteric nervous system is limited. Previous work suggested that NMDA receptors were involved in a rat model of visceral hypersensitivity. The aim of this study was to characterize the expression of the NMDA receptor NR1 splice variants and the NR2 subunit subtypes in the rat colon. We visualized the expression of NR1 protein in the rat submucosal and myenteric plexuses. The NR1 splice variants found in the colon of rats lacked the N1 and C1 cassettes and contained the C2 and C2′cassettes (NR1000 and NR1001). The NR2B and NR2D subunits were also found in the rat colon. Moreover, NMDA receptors in the rat colon were heteromeric, since NR1 was co-localized with NR2B and NR2D subunits using fluorescent immunohistochemistry. The identification of the NMDA receptors in the enteric nervous system could lead to the development of drugs that selectively modulate bowel function.
Keywords: glutamate receptor, NMDAR1, NMDAR2, myenteric plexus, submucosal plexus, immunohistochemistry
N-Methyl-D-aspartate (NMDA) receptors are the most clearly defined glutamate receptor subtype. These receptors have two subunit families designated NMDAR1 (NR1) and NMDAR2 (NR2) (Michaelis 1993). In mammals, the functional NMDA receptor is a heteromeric complex containing NR1 and NR2 subunits (McBain and Mayer 1994). However, the exact number of subunits in each heteromeric glutamate receptor channel is still unknown.
The NR1 subunit (Fig. 1) has only one gene which has three regions of alternative splicing, named the N1, C1 and C2 cassettes and results in eight possible splice variants. The N1 (exon 5) and C1 (exon 21) cassettes may be either present or absent without affecting the remaining NR1 protein. When the C2 cassette (exon 22) is spliced out the first stop codon is lost, resulting in the expression of the C2′ cassette. Therefore, either the C2 or C2′ cassette are present in all NR1 protein, but the two cassettes are not co-expressed within the same NR1 protein (Zukin and Bennett 1995). The N1 cassette is located extracellularly and interacts with various pharmacological modulators of the channel, including zinc, protons and polyamines (Dingledine et al. 1999). The C-terminal cassettes of the protein control cell-surface expression of NR1, where proteins with the shortest C-terminal region (lacking the C1 cassette and containing C2′; NR1x00) show the highest cell surface expression (Okabe et al. 1999).
Figure 1. Schematic representations of the proposed structure of the NMDA receptor channel subunits and the NR1 subunit splice variant composition.
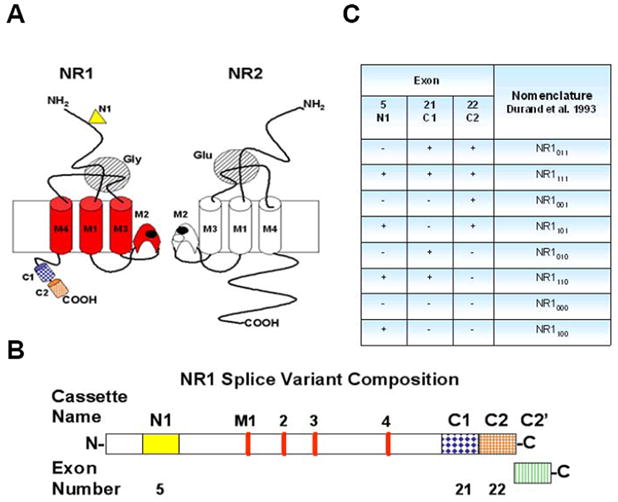
(A) Schematic representation of the proposed structure of the NMDA receptor channel subunits NR1 and NR2. The NR1 three transmembrane segment topology model shows the transmembrane domains in red and the alternatively spliced cassettes; N1 (yellow triangle), C1 (dark blue cylinder) and C2 (orange cylinder). Adapted from Yamakura and Shimoji 1999. (B) Detailed scheme of splice variant composition of NR1 protein. Colored boxes indicate the four alternatively spliced cassettes of NR1 protein, along with their name and exon number. The N1 cassette (exon 5) is extracellular at the N-terminal (solid yellow box). The red bars indicate the four transmembrane (M1, 3 and 4) or intramembrane (M2) domains of the NR1 protein. The three C-terminal cassettes; C1 (blue diamonds filled box), C2 (orange grid filled box) and C2′ (vertical green lines filled box) are located intracellularly (Prybylowski et al. 2001). (C) Splice variants of the NR1 subunit. The eight splice variants of the NR1 protein in the presence (+) or absence (−) of three alternatively spliced exons (N1, C1 and C2); NR1xyz, where x, y and z represent the cassettes N1, C1 and C2, respectively. The values of x, y and z are either 0 or 1 indicating the absence or presence of the respective cassette (Durand, Bennett and Zukin 1993).
The NR2 subunit includes four family members NR2A, 2B, 2C and 2D. Expression of different NR2 subunits determines the deactivation kinetics, antagonist sensitivity and ligand affinity of recombinant NR1/NR2 receptors (McBain and Mayer 1994, Mori and Mishina 1995).
The presence of L-glutamate receptors, presumably of the NMDA type, was documented in the myenteric plexus of the guinea pig (Moroni et al. 1986, Luzzi et al. 1988, Shannon and Sawyer 1989, Alesiani et al. 1990, Wiley et al. 1991). Furthermore, the expression of the NMDA receptor NR1 subunit was characterized by in situ hybridization and PCR techniques in both the rat and guinea-pig enteric plexuses (Burns et al. 1994, Broussard et al. 1994). Also glutamatergic receptors, including the NMDA type, were found in the enteric plexuses of the human colon (Giaroni et al. 2003). These findings together established the presence of NMDA receptors in the enteric nervous system. However, the roles of these enteric NMDA receptors are still not fully understood.
Previous studies showed that the NR1 subunit was co-labeled with the vasoactive intestinal peptide (VIP) on the inhibitory motor neurons of the ENS using double in situ hybridization (Burns and Stephens 1995). Moreover, glutamate and NMDA enhance acetylcholine and noradrenaline release from the ENS. Colonic peristaltic reflex was inhibited in vitro when NMDA was added and these effects were antagonized by 2-Amino-5-phosphonovalerate (APV) (Cosentino et al. 1995, Giaroni et al. 2003). The presence of the NR1 subunit was also characterized in the rat extrinsic primary afferents (EPANs) and the intravenous administration of memantine inhibited pain responses to noxious mechanical stimuli (McRoberts et al. 2001). Intrathecal administration (10 nmol) of the NMDA receptor antagonist; MK-801 were sufficient to significantly attenuate the hyperalgesic visceromotor responses to colonic distention after zymosan-induced inflammation (Coutinho et al. 2001). In a similar inflammation model, the administration of both intrathecal MK-801 (1.5 nmol) and intraperitoneal MK-801 (0.15 mg/kg) completely abolished the colorectal distension-induced hypersensitivity of both noxious and innocuous stimuli (Gaudreau and Plourde 2004). Even though it is uncertain whether these NMDA receptor antagonists were acting at the level of the colon, taken together these studies suggested that NMDA receptors were involved in these models of visceral hypersensitivity. Whether visceral hypersensitivity is produced by NMDA receptors on nociceptive neurons or through the receptor’s actions on visceral motor neurons is currently not known. However, the identification and characterization of these enteric NMDA receptors could provide useful information for the development of drugs that selectively modulate bowel function. Here we studied the expression of the NMDA receptor NR1 protein splice variants and the NR2 subunit subtypes in the rat colon by using RT-PCR, western blot and immunohistochemistry.
Experimental Procedures
Animals
Sprague Dawley rats (200–300 g, N=9) were maintained on a 12 hour light/dark cycle and fed standard rodent chow and water ad libitum. All experiments were approved by the University of Florida Institutional Animal Care and Use Committee.
Reverse transcription-PCR (RT-PCR)
Animals were euthanized with CO2 and the brain and intestine were quickly removed. Total RNA was isolated from rat brain and intestine tissues using RNeasy Mini Kit from Qiagen (Valencia, CA.). Target transcripts were amplified with PCR primers from GenoMechanix (Gainesville, Fl) listed on Table 1. RT-PCR reactions were carried out using Access RT-PCR System from Promega (Madison, WI) and the following cycle conditions: 1 cycle at 48°C for 45 minutes, 94°C for 2 minutes and 72°C for 1 minutes, 35 cycles of PCR (94°C for 30 sec, 60°C for 1 minutes, 72°C for 2 minutes) and a final elongation period of 7 minutes at 72°C. PCR products were separated on 1.2% agarose gel with 1X TBE buffer, viewed with ethidium bromide and analyzed with Bio-Rad Gel Doc EQ Gel Documentation System, Bio-Rad Laboratories (Hercules, CA). All the RT-PCR products were sequenced at the Genome Sequencing Services Laboratory (GSSL), part of the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida, Gainesville, FL.
Table 1. RT-PCR Primers Sequences.
All primers were purchased from GenoMechanix (Gainesville, Fl). RT-PCR reactions were carried out using Access RT-PCR System from Promega (Madison, WI) with the following cycle conditions: 1 cycle at 48°C for 45 min, 94°C 27 for 2 min and 72°C for 1 min, 35 cycles of PCR (94°C for 30 sec, 60°C for 1 min, 72°C for 2 min) and a final elongation period of 7 min at 72°C.
| Primer name | Forward sequence | Fwd Tm | Reverse sequence | Rev Tm | Product length |
|---|---|---|---|---|---|
| General | |||||
| NR1 general | cagaaacccctcagacaagttc | 59°C | cttctgtgaagcctcaaactcc | 59°C | 213bp |
| NR1 generic | ccctcagacaagttcatctacgc | 61°C | aggttcttcctccacacgttcac | 61°C | 563bp |
| GAPDH | ccttcattgacctcaactacatggtcta | 63°C | tagcccaggatgcccttt | 55°C | 720bp |
| Exon 5 or N1 | |||||
| NR1a Exon 5 (−) | gcgagtctacaactggaaccac | 61°C | ctcgcttgcagaaaggatgatg | 59°C | 170bp |
| NR1b Exon 5 (+) | 235bp | ||||
| Exon 5+ inside | ggaactatgaaaacctcgacc | 58°C | NR1b Exon 5 Reverse | 59°C | 158bp |
| N1+ (ζ1 N1+) | accacttcactcccacccctgtctcctac | 72°C | gtccgcgcttgttgtcata | 61°C | 330bp |
| N1− (ζ1 N1−) | agctcaacgccacttctgtc | 61°C | caccttctctgccttggactcac | 65°C | 407bp |
| Exon 21 or C1 | |||||
| C1 | UseNR1 general Fwd or | 59°C | gttttgcaaagcgccgcgtcca | 63°C | 717bp |
| NR1 generic Fwd | 61°C | 710bp | |||
| C1+ (ζ1 C1+) | tgtgtccctgtccatactcaag | 60°C | gtcgggctctgctctaccac | 62°C | 307bp |
| Exon 22 or C2 | |||||
| NR1 Exon-22 | catggcaggggtcttcatgctg | 63°C | gaacacagctgcagctggccct | 65°C | 318bp |
| NR2 | |||||
| NR2A | tatagagggtaaatgttgga | 50°C | agaaactgtgaggcatttct | 52°C | 322bp |
| NR2B | actgtgacaacccacccttc | 58°C | cggaactggtccaggtagaa | 58°C | 400bp |
| NR2C | tgtgtcaggccttagtgaca | 56°C | ccacactgtctccagcttct | 58°C | 402bp |
| NR2D | aagaagatcgatggcgtctg | 56°C | ggatttcccaatggtgaagg | 56°C | 353bp |
All primers from GenoMechanix, Gainsville, Fl, USA.
Western blots
Samples of tissue were taken from rat brain and descending colon. Tissue was homogenized in cold Cell Lysate Buffer [1mM Sodium Ortho-Vanadate, 10 mM Tris and 1% SDS] using a Sonics Vibra-Cell Sonicator. Lysates were boiled for 5 minutes and then centrifuged at 16,000 g for 5 minutes and the supernatant was collected. Protein concentration was determined by standard spectrophotometer method. Proteins were separated using 4–20% Tris-Glycine Gel from Invitrogen (Carlsbad, CA), each lane was loaded with 15 μg of protein extract. Proteins in the gel were then transferred to a Millipore (Bedford, MA) Immobilon-P polyvinylidene fluoride (PVDF) membrane using a semi-dry transfer device (Bio-Rad Laboratories, Hercules, CA). The transfer buffer used contains 20% methanol, 48 mM Tris pH 9.2, and 39 mM glycine. The membrane was then placed in TTBS buffer [20 mM Tris pH 7.6, 0.9% NaCl, and 0.05% Tween-20, pH 7.4] containing 5% non-fat dry milk for 1 hour to block non-specific binding of antibodies. NR1 splice variant specific primary antibodies were provided by Dr. Michael Iadarola from The National Institute of Dental and Craniofacial Research (NIDCR), Bethesda, MD (Antibodies’ selectivity was assessed in Caudle et al. 2005). Antibodies against Actin, NR1, NR2B and NR2D were purchased from different vendors (Table 2). After overnight incubation with primary antibody at 4°C, the membrane was washed three times in TTBS (5 minutes each) and then placed in fresh TTBS containing 5% non-fat dry milk and secondary antibody [dilution 1:4000] for rabbit or mouse IgG coupled to horse radish peroxidase (HRP) for 1 hour. The membrane is then washed three times with TTBS (5 minutes each) and placed in chemiluminescence substrate (LumiGLO®, Cell Signaling Technology, Beverly, MA) and exposed to film at variable times points (15 seconds to 10 minutes) to ensure best resolution.
Table 2. Antibodies used for Western Blots and Immunohistochemical Assays.
Antibodies were purchased from different vendors and dilutions were prepared according to vendors’ specifications. Incubation time for primary antibodies was 24 hours and 1 hour for secondary antibodies.
| Antibody | Host | Company | Cat. No. |
|---|---|---|---|
| Primary Antibodies | |||
| Actin | Mouse | Chemicon, Temecula, CA. | MAB1501 |
| Neurofilament | Mouse | Sigma, Saint Louis, Missouri. | N-5389 |
| NR1 | Mouse | BD Biosciences Pharmigen, San Diego, CA. | 556308 |
| NR1 | Rabbit | Santa Cruz Biotechnology, Santa Cruz, CA. | SC-9058 |
| NR1 splice variants | Rabbit | Provided by Dr. Michael Iadarola, The National Institute of Dental and Craniofacial Research NIDCR, Bethesda, MD. | N/A |
| NR2B | Rabbit | Santa Cruz Biotechnology, Santa Cniz, CA. | SC-9057 |
| NR2B | Mouse | BD Biosciences Pharmigen, San Diego, CA. | 610417 |
| NR2D | Rabbit | Santa Cruz Biotechnology, Santa Cruz, CA. | SC-10727 |
| Protein Gene Product 9.5 | Rabbit | Chemicon, Temecula, CA. | AB1761 |
| Fluorescent Secondary Antibodies | |||
| Alexa Fluor 488 | Mouse | Molecular Probes, Eugene, OR. | A21202 |
| Alexa Fluor 488 | Rabbit | Molecular Probes, Eugene, OR. | A21206 |
| Alexa Fluor 594 | Mouse | Molecular Probes, Eugene, OR. | A21201 |
| Alexa Fluor 594 | Rabbit | Molecular Probes, Eugene, OR. | A21442 |
Immunohistochemistry (IHC)
Perfusion fixation
Rats were given a lethal dose of pentobarbital and perfused through the heart with cold 0.9% saline followed immediately with cold 4% Paraformaldehyde. After fixation the colon of the animal was removed, post-fixed in Paraformaldehyde for 24h at 4°C, and then stored in 30% sucrose at 4°C for at least 24 hours.
Cryostat sections
Tissue was sectioned at 10 μm on a cryostat, serially mounted on a glass slide, and air-dried for 1 hour. All preparations were washed 3 times (10 minutes each) in Phosphate Buffered Saline (PBS) [10 mM sodium phosphate, pH 7.4, 0.9% NaCl] and placed in blocking buffer containing 3% Normal Goat Serum (NGS) with PBS for 1 hour, and incubated in primary antibody in 3% NGS/0.3% tween-20/PBS for 24 hours at 4°C. Most of primary antibodies mentioned above in the Western blots section were used. In addition antibodies for neuronal markers; Protein Gene Product 9.5 (PGP 9.5) and Neurofilament (NF) were used (Table 2). The sections were then washed 3 times in PBS (10 minutes each) followed by 1 hour incubation in secondary antibodies, either Alexa Fluor 488 or 495 (1:1000; Molecular Probes, Boston, MA) in 3%NGS/0.3% tween-20/PBS. Negative controls were performed by incubating samples with only secondary antibodies and omitting primary antibodies.
After incubation with secondary antibodies, tissue was washed 3 times (10 minutes each) and coversliped with ProLong® Antifade Kit mounting media (Molecular Probes, Boston, MA). The sections were visualized with filters for red and green excitation. Images were photographed on an Olympus BX51 Fluorescence microscope (Olympus, Center Valley, PA).
Whole mount
Segments of tissue from the descending colon (2–3cm) were removed and cut longitudinally. The segments were spread and mounted onto slides containing a silicone base to enable pinning of the tissue to the slides. To defat the tissue, the segments were washed through a series of ethanol dilutions starting with 2 washes in 100% ethanol followed by single washes in 95%, 70%, and 50% ethanol for 20 minutes each. Sections were incubated in distilled water overnight at 4°C. The mucosa, submucosal plexus and circular muscle layers were then pealed from each tissue section and the remaining myenteric plexus layer placed in 4% paraformaldehyde overnight followed by 3–5 washes (5 minutes each) in PBS. Tissue sections were cleared by placing them in KOH (in PBS) and glycerin serial incubations: 3:1 0.5% KOH: glycerin for 1 hour, 1:1 0.5% KOH: glycerin for 1 hour, 1:3 0.5% KOH: glycerin for 1 hour, and 100% glycerin overnight. A few drops of 30% H2O2 were added to both 1:1 and 1:3 KOH: glycerin incubations. After 3–5 washes (5 minutes each) in PBS, the tissue was placed in blocking buffer containing 3% Normal Goat Serum (NGS) with PBS for 30 minutes, then incubated in the primary antibody [1:500 – 1:100] in 3% NGS/PBS, 1% Triton X-100 overnight at 4°C. After 5 washes (10 minutes each) in PBS, the tissue was incubated for 60 minutes in secondary antibodies, either Alexa Fluor 594 or Alexa Fluor 488 (1:2000, Molecular Probes, Boston, MA) with 1% NGS/PBS, 0.3% Triton X-100. The tissue was then washed 6 times (5 minutes each) in PBS and placed in distilled water. Tissues were then mounted into clean slides and coversliped with ProLong® Antifade Kit mounting media (Molecular Probes, Boston, MA) (Rosa-Molinar et al. 1999). Slides were visualized with filters for red and green excitation. Images were photographed on an Olympus BX51 Fluorescence microscope (Olympus, Center Valley, PA).
Results
Expression of NR1 protein in the rat colon
The expression of NR1 was examined in the rat colon (Fig. 2). We used two different sets of primers to amplify by RT-PCR (Fig. 2A) the RNA extracted from intestine and brain tissues. These primers (arbitrarily named NR1 general and generic) recognized areas in the NR1 outside the spliced exons. The expression of NR1 was evident in the colon tissue (gastrointestinal, GI) and the rat brain. Rat brain was used as a positive control. Sample integrity and reaction reliability were validated with the expression of the housekeeping gene, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in both the GI and the brain tissues. A negative control (−RT) that lacked reverse transcriptase using GAPDH primers was included to control for genomic DNA contamination.
Figure 2. Expression of NR1 in rat colon.
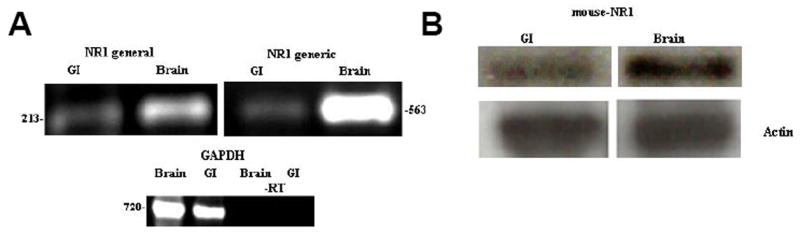
(A) RT-PCR analyses of NR1 in rat colon (named GI, for gastrointestinal) and brain tissues using two different set of primers; NR1 general and NR1 generic, that recognize areas in the NR1 outside the spliced exons. Assessment of housekeeping gene, GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) was included as a control for sample integrity and reaction fidelity. A negative control (−RT) was included which lacks Reverse transcriptase and uses GAPDH primers. (B) Top panel, western blot showing broad expression of NR1 protein in GI and brain tissues using general mouse-NR1 antibody that recognizes a site outside of the alternatively spliced cassettes. Bottom panel, an antibody against actin was use as a control for loading purposes.
Protein expression was confirmed by western blot analyses that showed the NR1 protein in GI and brain tissues using general mouse-NR1 antibody, which recognized a site outside of the alternatively spliced cassettes (Fig. 2B, Top panel). To control for sample loading purposes, an antibody against Actin was used.
Furthermore, we visualized the double label of the NR1 protein with the neuronal markers; Protein Gene Product 9.5 (PGP 9.5) (Fig. 3A) and neurofilament (NF) (Fig. 3B) in the rat myenteric and submucosal plexuses using immunohistochemistry. The co-labeling of NR1 and the neuronal markers PGP and neurofilament verified that NR1 is localized in enteric neurons.
Figure 3. Visualization of NR1 protein and neuronal markers; PGP and neurofilament in the rat colon.
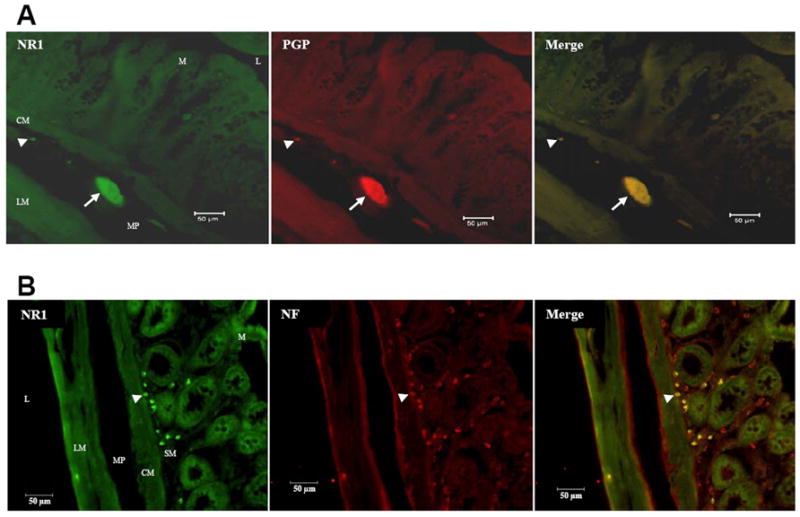
Immunohistochemistry with Alexa fluor-linked antibodies on transverse colon sections showing NR1 protein and neuronal marker protein gene product 9.5 (PGP 9.5) co-expression (A) and NR1 protein and neuronal marker Neurofilament (NF) co-labeling (B). Scale bar = 50 μm. Section thickness = 10 μm. L = lumen, M = mucosa, SM = submucosal plexus, CM = circular muscle, MP = myenteric plexus, LM = longitudinal muscle, Arrow = ganglia, Arrow head = single cells.
Expression of splice variants of NR1 protein
The NR1 subunit gene has a total of 22 exons, three of which (exons 5, 21 and 22) undergo alternative splicing to generate eight NR1 splice variants. The alternatively spliced cassettes of the NR1 protein were named N1, C1 and C2. A detailed scheme of splice variant composition of NR1 protein is presented in Fig. 1B. The four alternatively spliced cassettes of NR1 protein are represented as colored boxes. In this model, the N1 cassette (exon 5) is extracellular at the N-terminal, while the three C-terminal cassettes (C1, C2 and C2′) are located intracellularly. The resultant eight splice variants of the NR1 protein in the presence (+) or absence (−) of the three alternatively spliced exons (N1, C1 and C2) have several nomenclatures (Reviewed by Yamakura and Shimoji 1999). The nomenclature used here; NR1xyz, was proposed by Durand and colleagues (Durand et al. 1993), where x, y and z, represent the cassettes N1, C1 and C2, respectively. The values of x, y and z are either 0 or 1 indicating the absence or presence of the respective cassette (Fig. 1C).
After verifying the general expression of NR1 protein in the rat intestine, we examined the expression of the splice variants of NR1 using specific primers and antibodies with RT-PCR and western blots. Rat brain contains all splice variant isoforms (Sugihara et al. 1992), thus, this tissue was used as a control in all our assays.
Expression of the N1 cassette (exon 5) in the rat colon
NR1 protein containing and lacking the N1 cassette was known to be present in both rat brain (Sugihara et al. 1992) and spinal cord (Tolle et al. 1995, Prybylowski et al. 2001). To analyze the expression of the N1 cassette in rat colon we performed RT-PCR (Fig. 4A) using four different sets of primers (Table 1); “NR1a(b)”, which produced two different sized products, a 274 bp band in the presence of exon 5 or a 211 bp band in the absence of this cassette. Primers “exon 5+” and “N1+”, that were designed inside the N1 cassette and only showed a band if this cassette was present. Alternatively “N1−” primers, only showed a band if exon 5 was not present. While brain samples expressed both the N1 splice variants, the rat colon only expressed the variant that lacked N1 (NR10yz). To verify these findings at the protein level, western blot assays were performed. The expected NR1 band around 118 kDa was present in brain but it was lacking in GI tissues (Fig. 4B).
Figure 4. Expression of NR1 splice variants in the rat colon.
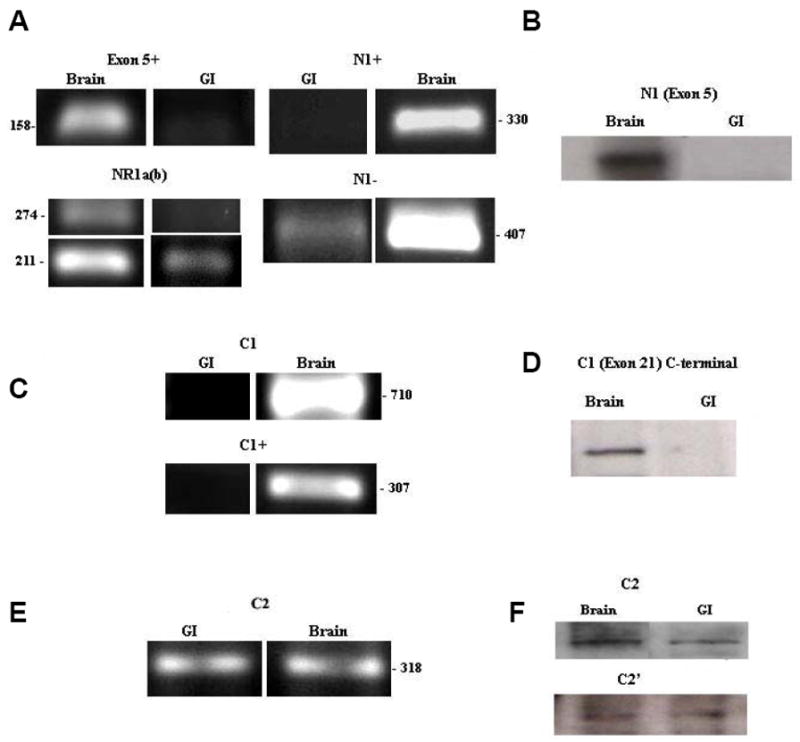
(A) Expression of N1 cassette (exon 5) in the rat colon. RT-PCR analyses of N1 cassette (exon 5) expression in rat colon and brain tissues using four different sets of primers; “NR1a(b)”, “exon 5+”, “N1+” and “N1−”. (B) Western Blot showing expression of N1 cassette containing NR1 protein in GI and brain tissues using antibody against N1. (C) Expression of C1 cassette (exon 21) in the rat colon. RT-PCR results of C1 cassette (exon 21) expression in rat colon and brain tissues using two different set of primers; C1 and C1+. (D) Western blot showing expression of C1 cassette containing NR1 protein using antibody targeted towards the C-terminal of the C1 cassette (exon 21). (E) Expression of C2 cassette (exon 22) in the rat colon. RT-PCR results of C2 cassette (exon 22) expression in rat colon and brain tissues using primers targeted to the C2 cassette. (F) Western blot showing probing of C2+ antibody which is targeted to the C2 cassette and C2− antibody directed against the C2′ cassette.
These results further suggested that only splice variants without the N1 cassette (NR10yz) were present in the rat colon.
Expression of C-terminal cassettes in the rat colon
The C-terminal cassettes were found to be critical in determining the cellular localization of the NR1 (Okabe et al. 1999). Here we analyzed the expression of the rat colon C1 cassette by RT-PCR, using two sets of primers; “C1” (with NR1 generic, see Table 1) and the “C1+” primers (Liesi et al. 1999). We found with both sets of primers that the expected C1 band was present in brain, but was absent from the rat colon (Fig. 4C).
To confirm these findings, we blotted using an antibody targeted towards the C1 cassette. We observed the expression of C1 containing NR1 protein in brain but not in GI (Fig. 4D).
Whereas bands corresponding to the C1 cassette in both RT-PCR and western blot analyses were present as expected in brain, none were visible in GI, suggesting that only the NR1 splice variants that lacked the C1 cassette (NR1x0z) were present in the rat colon.
When the C2 cassette (exon 22) is spliced out the first stop codon is removed, resulting in the expression of the C2′ cassette. Consequently, either the C2 or C2′ cassette is present in all NR1 protein, but the two cassettes are not co-expressed within one NR1 molecule (Zukin and Bennett 1995).
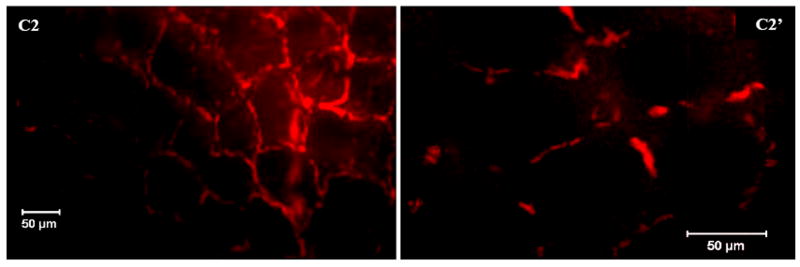
RT-PCR and western blot were used to assess the expression of the C2 and C2′cassettes. RT-PCR with primers for exon 22 showed a band for both GI and brain (Fig. 4E). For the western blot analyses we employed two different antibodies; the C2+ antibody, which targeted the C2 cassette and the C2- antibody directed against the C2′ cassette. These antibodies showed expression of both the C2 and C2′ cassettes in the rat brain and colon (Fig. 4F). These results suggested that both NR1 splice variants, the one that has the C2 cassette and the one that contains the C2′ cassette (NR1xx0 and NR1xx1) were present in the rat colon. These variants were visualized in the rat myenteric plexus by immunohistochemistry with Alexa fluor-linked antibodies (Fig 5).
Figure 5. Visualization of the C2 and C2′cassettes in the rat myenteric plexus.
Whole mount immunohistochemistry showing the C2 cassette expression and the C2′ cassette expression in the rat myenteric plexus using an Alexa fluor antibody. Scale bar = 50 μm.
Expression of NR2 protein subtypes
The second subunit family of the rat NMDA receptor, named NMDAR2 (NR2), has four members the NR2A, NR2B2, NR2C, and NR2D subunits. In mammals functional NMDA receptor channels are produced when the NR1 subunit is expressed together with one or more of the four NR2 subunit subtypes (McBain and Mayer 1994). All four NR2 subunit subtypes are expressed in brain therefore we compared the expression of NR2 in the rat colon with this tissue.
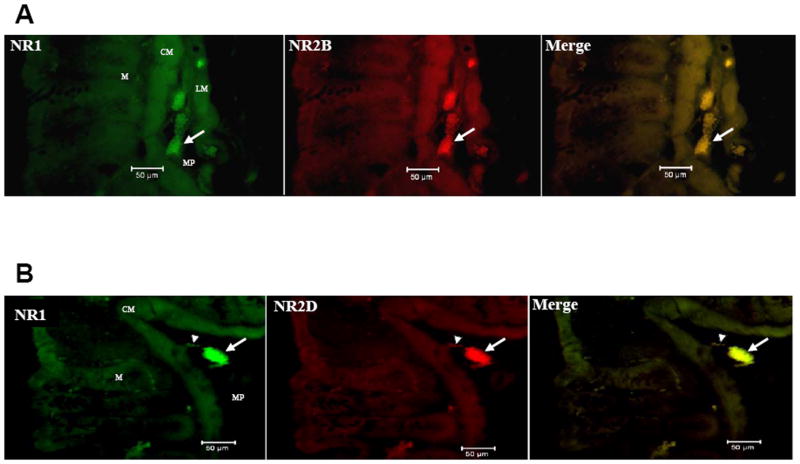
We used RT-PCR with four different sets of primers to assess the expression the NR2 subunits (Fig. 6A). Analyses of NR2A and NR2C demonstrated no bands for GI, while both NR2B and NR2D were evident in GI and brain tissues. These results suggested that only NR2B and NR2D were present in the rat colon. These subunits were visualized in the rat myenteric plexus by immunohistochemistry with Alexa fluor-linked antibodies (Fig 6B). Moreover, we were able to visualize the double label of the NR2B protein with the neuronal marker; Protein Gene Product 9.5 (PGP 9.5) (Fig. 6C) using immunohistochemistry. The co-labeling of NR2B and the neuronal marker PGP further verified that NR2B was localized in enteric neurons.
Figure 6. Expression of NR2 subtypes in the rat colon.
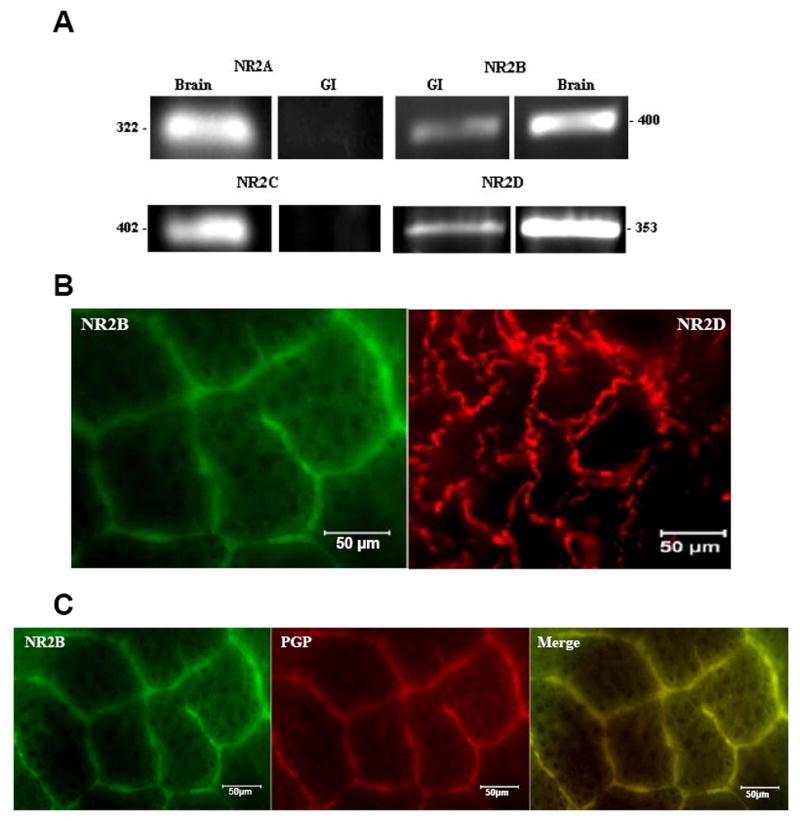
(A) RT-PCR analyses of NR2A, NR2B, NR2C and NR2D expression in the rat colon and brain tissues using primers to detect the presence of each different NR2 subtype respectively. (B) Whole mount immunohistochemistry showing the presence of the NR2B and NR2D proteins in the rat myenteric plexus using Alexa fluor-linked antibodies. (C) Double-labeling of NR2B and neuronal marker PGP 9.5. Scale bar = 50 μm.
Co-labeling of NR1 with NR2B and NR2D in the rat colon
The functional NMDA receptor is a heteromeric complex containing NR1 and NR2 subunits (McBain and Mayer 1994). In order to verify if NR2B and NR2D were co-localized with NR1 in the rat colon we used specific antibodies targeted to NR1, NR2B and NR2D. We visualized the double labeling of NR1 with both NR2B and NR2D with immunohistochemistry using Alexa fluor-linked secondary antibodies (Fig. 7). The co-localization of NR1 with NR2B and NR2D suggested there were heteromeric complexes of these NR2 subtypes with NR1 in the rat colon.
Figure 7. Co-labeling of NR1 with NR2B and NR2D subunits in the rat colon.
Transverse colon sections showing co-labeling of the NR1 and NR2B (A) and the NR1 and NR2D proteins (B) in the rat colon using Alexa fluor-linked antibodies. Scale bar = 50 μm. M = mucosal crypts, CM = circular muscle, MP = myenteric plexus, LM = longitudinal muscle, Arrow = ganglia, Arrow head = single cells.
Discussion
Our studies confirmed the expression of NR1 protein in the rat myenteric and submucosal plexuses. We found that only the NR1 splice variants that contained the C2 or the C2′ cassette (NR1000 and NR1001) were present in the rat colon. Also, the NR2B and NR2D subunits were the only NR2 subunit subtypes found in the rat colon. Lastly, the NMDA receptors in the rat colon were arranged in heteromeric complexes including the NR1 with NR2B and NR2D subunits.
The presence of the N1 cassette caused a decrease in the open time of the NMDA receptor channel (Rumbaugh et al. 2000) and decreased the ability of spermine to potentiate NMDA mediated currents (Mott et al. 1998). Since these enteric receptors lacked the N1 cassette, they would show an increased pH, Zn2+, and spermine sensitivity similar to receptors with mutations at the N1 cassette (Traynelis et al. 1998).
NR1 proteins with the shortest C-terminal region (lacking the C1 cassette and containing C2′; NR1x00) showed the highest cell surface expression (Okabe et al. 1999). The lack of the C1 cassette also affected the PKC potentiation (Durand et al. 1993, Logan et al. 1999, Zheng et al. 1997) and the calmodulin-dependent inhibition (Ehlers et al. 1996) of these receptors’ currents. Receptor clustering may be affected also, since the C1 cassette was shown to have specific interactions with multiple intracellular proteins, including neurofilament-L (Ehlers et al. 1998) and the cytoskeletal protein yotiao (Lin et al. 1998). Therefore, these enteric NMDA receptors which lacked both the N1 and C1 cassette may have an increased cell-surface expression but poor clustering properties. Moreover, prior studies found that NR1000 receptors were markedly less active than other splice forms like NR1111 (Durand et al. 1993), indicating that the NMDA receptors in the colon may not produce large currents in the neurons when activated.
Functional NMDA receptor channels in mammals are considered to be produced only when the NR1 subunit is expressed together with one or more of the four NR2 subunits (McBain and Mayer 1994). While the NR1 is widely expressed throughout the whole brain, the expression of the NR2 subunits is highly regulated. The distributions of NR2A and NR2C have temporal and spatial similarities to that of NR1, while the expression of NR2B shows differences in the intensity and distribution (Takai et al. 2003). NR2D expression in the brain is very faint compared to other NR2s, and mutant mice defective in NR2D expression appear to develop normally (Ikeda et al. 1995). Not only the intensity and distribution of expression of the NR2 subunits is unique, but their functional behavior differs. The offset decay time constant of the NRl/NR2B channel is ~400 msec, while the NR1/NR2D channel shows a very long offset decay time constant (~5000 msec) (Reviewed by Mori and Mishina 1995).
The NR2B subunit has been the object of great interest as a therapeutic target in a wide range of pathologies, including acute and chronic pain. Therefore, a remarkable compilation of drugs were developed which target the NR2B subunit (Chazot 2004).
In conclusion we found that enteric NMDA receptors were heteromeric complexes of either NR1000 or NR1001 with the NR2B and NR2D subunits. Moreover, in our studies with a 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced model of colitis of the rat colon, both the expression of the N1 and C1 cassettes are enhanced 14 days after inflammation (Zhou et al. 2006). Therefore, selective changes in the expression of the NR1 splice variants and possibly the NR2 subunit subtypes of the NMDA receptors may be an element for the ongoing visceral hypersensitivity in conditions such as irritable bowel syndrome (IBS). It is possible that the enteric NMDA receptors have low activity in the normal healthy intestines and their activity is increased after alternative splicing events that occur during pathophysiological states. Previous studies using NMDA receptor antagonists showed promising inhibitory effects on models of visceral hypersensivity (Coutinho et al. 2001, McRoberts et al. 2001, Giaroni et al. 2003, Gaudreau and Plourde 2004). The drugs in these studies seemed to be working at the levels of the spinal cord and primary afferent neurons. Whether these NMDA receptor antagonists could have an effect at the enteric level remains uncertain. To our knowledge this is the first comprehensive analysis of the expression of the NMDA receptor NR1 protein splice variants and the NR2 subunit subtypes in the rat colon. Therefore, more studies are needed to determine the specific properties of these receptors both in normal and pathological conditions. A better understanding of the expression, physiology and pharmacological properties of the NMDA receptors present in the enteric nervous system could lead to the development of drugs that selectively modulate the bowel function.
Acknowledgments
The authors thank Dr. Michael Iadarola, from The National Institute of Dental and Craniofacial Research (NIDCR) in Bethesda, Maryland for supplying the NR1 splice variants specific antibodies. We like to thank Alan Jenkins from the Laboratory of Dr. John Neubert at the University of Florida, Department of Orthodontics for providing technical assistance with the immunohistochemistry protocol. We also like to thank Dr. Eduardo Rosa-Molinar and Jose Serrano from the Department of Biology at the University of Puerto Rico in San Juan, Puerto Rico for providing us with the whole mount immunohistochemistry protocol. The work summarized here was supported by The National Institutes of Health NS045614.
Comprehensive List of Abbreviations
- APV
2-Amino-5-phosphonovalerate
- ENS
Enteric Nervous System
- EPAN
Extrinsic primary afferents
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GI
Gastrointestinal
- HRP
Horse radish peroxidase
- IBS
Irritable Bowel Syndrome
- IHC
Immunohistochemistry
- NMDA
N-methyl-D-aspartate
- NF
Neurofilament
- NGS
Normal Goat Serum
- PBS
Phosphate Buffered Saline
- PGP 9.5
Protein gene product 9.5
- PVDF
Polyvinylidene fluoride
- RT- PCR
Reverse transcriptase polymerase chain reaction
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- VIP
Vasoactive intestinal peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, Fukaya M, Yagi T, Mishina M, Watanabe M, Sakimura K. NMDA receptor GluRepsilon/NR2 subunits are essential for postsynaptic localization and protein stability of GluRzeta1/NR1 subunit. J Neurosci. 2004;24(2):7292–7304. doi: 10.1523/JNEUROSCI.1261-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alesiani M, Pellegrini Giampietro DE, Cherici G, Galli A, Moroni F. The guinea pig myenteric plexus as a tool to characterize drugs active at the glycine recognition site of the NMDA receptors. Pharmacol Res. 1990;22(Suppl 1):15–16. doi: 10.1016/1043-6618(90)90785-c. [DOI] [PubMed] [Google Scholar]
- Ascher P, Nowak L. Calcium permeability of the channels activated by N,-methyl-D-aspartate (NMDA) in isolated mouse central neurones. J Physiol. 1986;377:35P. [Google Scholar]
- Broussard DL, Li X, Pritchett DB, Lynch D, Bannermann PG, Pleasure D. The expression of a NMDA receptor gene in guinea-pig myenteric plexus. Neuroreport. 1994;5(2):973–976. doi: 10.1097/00001756-199404000-00030. [DOI] [PubMed] [Google Scholar]
- Burns GA, Stephens KE, Benson JA. Expression of mRNA for the N-methyl-D-aspartate (NMDAR1) receptor by the enteric neurons of the rat. Neurosci Lett. 1994;170(2):87–90. doi: 10.1016/0304-3940(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Burns GA, Stephens KE. Expression of mRNA for the N-methyl-D-aspartate (NMDAR1) receptor and vasoactive intestinal polypeptide (VIP) co-exist in enteric neurons of the rat. J Auton Nerv Syst. 1995;55(2):207–210. doi: 10.1016/0165-1838(95)00043-w. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Perez FM, Del Valle-Pinero AY, Iadarola MJ. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol Pain. 2005;21:25. doi: 10.1186/1744-8069-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazot PL. The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr Med Chem. 2004;11(2):389–396. doi: 10.2174/0929867043456061. [DOI] [PubMed] [Google Scholar]
- Cosentino M, De Ponti F, Marino F, Giaroni C, Leoni O, Lecchini S, Frigo G. N-methyl-D-aspartate receptors modulate neurotransmitter release and peristalsis in the guinea pig isolated colon. Neurosci Lett. 1995;183(1–2):139–142. doi: 10.1016/0304-3940(94)11134-5. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. The role of CNS NMDA receptors and nitric oxide in visceral hyperalgesia. Eur J Pharmacol. 2001;429(1–3):319–325. doi: 10.1016/s0014-2999(01)01331-0. [DOI] [PubMed] [Google Scholar]
- Davies J, Francis AA, Jones AW, Watkins JC. 2-amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981;21:77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Durand GM, Bennett MV, Zukin RS. Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci (USA) 1993;90:6731–6735. doi: 10.1073/pnas.90.14.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Fung ET, O’Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998;18:720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84(2):745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Gaudreau GA, Plourde V. Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity. Behav Brain Res. 2004;150(1–2):185–189. doi: 10.1016/j.bbr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Giaroni C, Zanetti E, Chiaravalli AM, Albarello L, Dominioni L, Capella C, Lecchini S, Frigo G. Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur J Pharmacol. 2003;476(1–2):63–69. doi: 10.1016/s0014-2999(03)02147-2. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Araki K, Takayama C, Inoue Y, Yagi T, Aizawa S, Mishina M. Reduced spontaneous activity of mice defective in the epsilon 4 subunit of the NMDA receptor channel. Brain Res Mol Brain Res. 1995;33(2):61–71. doi: 10.1016/0169-328x(95)00107-4. [DOI] [PubMed] [Google Scholar]
- Liesi P, Stewart RR, Akinshola BE, Wright JM. Weaver cerebellar granule neurons show altered expression of NMDA receptor subunits both in vivo and in vitro. J Neurobiol. 1999;38(2):441–454. [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997;17(2):4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Rivera FE, Leonard JP. Protein kinase C modulation of recombinant NMDA receptor currents: roles for the C-terminal C1 exon and calcium ions. J Neurosci. 1999;19(2):974–986. doi: 10.1523/JNEUROSCI.19-03-00974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Zilletti L, Franchi-Micheli S, Gori AM, Moroni F. Agonists, antagonists and modulators of excitatory amino acid receptors in the guinea-pig myenteric plexus. Br J Pharmacol. 1988;95(2):1271–1277. doi: 10.1111/j.1476-5381.1988.tb11764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120(2):1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Michaelis EK. Two different families of NMDA receptors in mammalian brain: physiological function and role in neuronal development and degeneration. Adv Exp Med Biol. 1993;341:119–128. doi: 10.1007/978-1-4615-2484-7_11. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34(2):1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Moroni F, Luzzi S, Franchi-Micheli S, Zilletti L. The presence of N-methyl-D-aspartate-type receptors for glutamic acid in the guinea pig myenteric plexus. Neurosci Lett. 1986;68(2):57–62. doi: 10.1016/0304-3940(86)90229-6. [DOI] [PubMed] [Google Scholar]
- Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R. Enhancement of proton inhibition: a novel mechanism of inhibition of NMDA receptors by phenylehanolamines. Nat Neurosci. 1998;1:659–667. doi: 10.1038/3661. [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40(2):581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. J Neurosci. 1999;19:7781–7792. doi: 10.1523/JNEUROSCI.19-18-07781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski KL, Grossman SD, Wrathall JR, Wolfe BB. Expression of splice variants of the NR1 subunit of the N-methyl-D-aspartate receptor in the normal and injured rat spinal cord. J Neurochem. 2001;76(2):797–805. doi: 10.1046/j.1471-4159.2001.00069.x. [DOI] [PubMed] [Google Scholar]
- Rosa-Molinar E, Proskocil BJ, Ettel M, Fritzsch B. Whole-mount procedures for simultaneous visualization of nerves, neurons, cartilage and bone. Brain Res Brain Res Protoc. 1999;4(2):115–123. doi: 10.1016/s1385-299x(99)00007-0. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JF, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophys. 2000;83:1300–1307. doi: 10.1152/jn.2000.83.3.1300. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Sawyer BD. Glutamate receptors of the N-methyl-D-aspartate subtype in the myenteric plexus of the guinea pig ileum. J Pharmacol Exp Ther. 1989;251(2):518–523. [PubMed] [Google Scholar]
- Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992;185:826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Takai H, Katayama K, Uetsuka K, Nakayama H, Doi K. Distribution of N-methyl-D-aspartate receptors (NMDARs) in the developing rat brain. Exp Mol Pathol. 2003;75(2):89–94. doi: 10.1016/s0014-4800(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Tolle TR, Berthele A, Laurie DJ, Seeburg PH, Zieglgansberger W. Cellular and subcellular distribution of NMDAR1 splice variant mRNA in the rat lumbar spinal cord. Eur J Neurosci. 1995;7(2):1235–1244. doi: 10.1111/j.1460-9568.1995.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Watkins JC. The synthesis of some acidic amino acids possessing nemopharmacological activity. J Med Pharm Chem. 1962;5:1187–1199. doi: 10.1021/jm01241a010. [DOI] [PubMed] [Google Scholar]
- Wiley JW, Lu YX, Owyang C. Evidence for a glutamatergic neural pathway in the myenteric plexus. Am J Physiol. 1991;261(4 Pt 1):G693–700. doi: 10.1152/ajpgi.1991.261.4.G693. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59(2):279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Ca2+ influx amplifies protein kinase C potentiation of recombinant NMDA receptors. J Neurosci. 1997;17(2):8676–8686. doi: 10.1523/JNEUROSCI.17-22-08676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Caudle RM, Price DD, Del Valle-Pinero AY, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006;2(1):3. doi: 10.1186/1744-8069-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDAR1 receptor subunit. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]