Abstract
The trophic neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) increases in many different neuron types following injury; a response postulated to support cell survival and regeneration. In acutely isolated cardiac ganglia, approximately 1% of the cardiac neurons exhibited PACAP immunoreactivity whereas after 72 hours in culture, ~25% of the neurons were PACAP immunoreactive. In contrast, there was no increase in vasoactive intestinal polypeptide (VIP)-immunoreactive (IR) cells. Using a combination of immunocytochemical and molecular techniques, we have quantified PACAP expression, during explant culture of guinea pig cardiac ganglia. Using real time PCR, PACAP transcript levels increased progressively up to 48 hours in culture with no further increase after 72 hours. PACAP transcript levels were reduced by neurturin at 48 hours in culture but not after 24 or 72 hours in culture. In addition, neurturin partially suppressed the percentage of PACAP-IR neurons after 72 hours in culture, an effect mediated by activation of the phosphatidylinositol 3-kinase and mitogen activated protein kinase signaling pathways. The addition of different known regulatory molecules, including CNTF, Il-1β, TNFα, bFGF, TGF βand NGF did not increase the percentage of PACAP-IR neurons after 24 hours in culture; a result indicating that the generation and secretion of these factors did not stimulate PACAP expression. The presence of 20 nM PACAP or 10 μM forskolin increased the percentage of PACAP-IR cardiac neurons in 24 hour cultures, but not in 72 hour cultures. Neither treatment enhanced the number of VIP-IR neurons. The addition of the PAC1 receptor antagonist, M65 (100 nM) suppressed the 20 nM PACAP-induced increase in percentage of PACAP-IR cells in 24 hour cultures indicating the effect of PACAP was mediated through the PAC1 receptor. However, 100 nM M65 had no effect on the percentage of PACAP-IR cells in either 24 or 48 hour cultures not treated with exogenous PACAP, suggesting that endogenous release of PACAP likely did not contribute to the enhanced peptide expression. We postulate that the enhanced PACAP expression, which occurs in response to injury is facilitated in the explant cultured cardiac ganglia by the loss of a target-derived inhibitory factor, very likely neurturin. In intact tissues the presence of neurturin would normally suppress PACAP expression. Lastly, our results indicate that many common trophic factors do not enhance PACAP expression in the cultured cardiac neurons. However, the stimulatory role of an, as yet, unidentified factor can not be excluded.
Keywords: Parasympathetic neurons, trophic factors, choline acetyltransferase, vasoactive intestinal polypeptide, phosphatidylinositol 3-kinase, mitogen activated protein kinase, explant culture
Pituitary adenylate cyclase activating polypeptide (PACAP) is a potent trophic and intercellular signaling molecule, distributed widely throughout both the central and peripheral nervous systems (Arimura, 1998; Vaudry et al, 2000). In control peripheral nervous system structures, such as parasympathetic and sympathetic ganglia, dorsal root ganglia, vagal sensory ganglia and spinal and cranial motor nuclei, limited numbers of neurons exhibit PACAP immunoreactivity. However, PACAP expression increases markedly in these neurons following axotomy or explant culture (Zhang et al, 1995, 1996; Moller et al, 1997a, b; Zhou et al, 1999; Pettersson et al, 2004). It is hypothesized that the increased expression of PACAP may be a response to injury that supports cell survival and regeneration (Moller et al, 1997a, b; Armstrong et al, 2003; Boeshore et al, 2004; Pettersson et al, 2004; Suarez et al, 2006).
In acutely isolated guinea pig cardiac ganglia, all the intrinsic neurons are innervated by extrinsic PACAP-IR fibers, but very few of the cardiac neurons exhibit PACAP immunoreactivity (Braas et al, 1998; Calupca et al, 2000; Parsons et al, 2006). Following explant culture, the extrinsic PACAP-IR fibers degenerate and the percentage of cholinergic neurons expressing PACAP increases significantly (Calupca et al, 2000; Girard et al, 2006b).
Members of the glial derived family of trophic factors, particularly glial-derived neurotrophic factor (GDNF) and neurturin, are key regulatory molecules determining the migration and development of many parasympathetic ganglia, including the intrinsic cardiac ganglia (Enomoto et al., 2000; Hiltunen et al., 2000; Hashino et al., 2001; Airaksinen et al., 2002). In initial studies, we reported that the increase in PACAP-IR cardiac neurons is partially suppressed by two members of the glial-derived neurotrophic family, neurturin and GDNF (Girard et al, 2006b) although the intracellular signaling cascades mediating the effect of these two trophic factors were not identified.
In the present study, we have quantified using a combination of immunocytochemical and PCR techniques the time-dependent increase in PACAP expression that occurs when cardiac ganglia are maintained in explant culture. Since we previously demonstrated that adult guinea pig cardiac neurons preferentially expressed the neurturin specific receptor GFRα2 (Girard et al, 2006a), we also have analyzed potential signaling cascades underlying the neurturin modulation of PACAP expression in cultured cardiac neurons. Our results show that the neurturin-induced suppression of PACAP expression was mediated through the activation of phosphatidylinositol 3-kinase and mitogen activated protein kinase pathways. Furthermore, although a number of common regulatory or target-derived factors had no effect, the addition of PACAP or forskolin in 24 hour cultures could promote PACAP expression.
A preliminary account of some aspects of this study was presented at the VIIth International Symposium on VIP, PACAP and Related Peptides (Girard et al, 2006b).
EXPERIMENTAL PROCEDURES
Experiments were performed in vitro on atrial whole mount preparations containing the cardiac ganglia from Hartley guinea pigs (either sex; 250–400 g) that were euthanized and exsanguinated. Protocols for use of guinea pigs were approved by the University of Vermont Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering. The heart was quickly removed and placed in cold standard Krebs’ solution (in mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2CO4, 8 glucose). The pH was maintained at 7.4 by aeration with 95% O2 - 5% CO2.
Cardiac ganglia preparations were prepared as described previously (Mawe et al, 1996; Braas et al, 1998; Calupca et al, 2000). The preparations were maintained in Sylgard-lined dishes with oxygenated Krebs solution at 35 – 37°C. When prepared for immunocytochemistry, control and cultured preparations were fixed in 2% paraformaldehyde containing 0.2% picric acid for 2 hours at room temperature (Mawe et al, 1996; Braas et al, 1998; Lynch et al, 1999; Calupca et al, 2000; Parsons et al, 2006).
For culture, the cardiac ganglia explants were dissected under sterile conditions and then maintained at 37°C in culture media consisting of DMEM-F12 (1:1) containing 10% horse serum, gentamicin (10 μg/ml), amphotericin B (3.75 μg/ml), penicillin (100 units/ml) and streptomycin (100 μg/ml) (Sigma Chemical Company, St. Louis, MO, USA) (Lynch et al, 1999; Calupca et al, 2000; Braas et al, 2004; Girard et al, 2006a). The preparations were placed on a slowly rocking shaker table in a 37°C, 5% CO2 and 95% air incubator and kept for 24, 48, 72 or 96 hours with the culture media replaced every 24 hours.
Neurturin, Il-1β and TNFα were obtained from R&D Systems Inc. (Minneapolis, MN). Recombinant rat CNTF (expressed as [his]6CNTFyyy in a bacterial expression vector) (Heller et al, 1993), bFGF, TGF-β and NGF were generously supplied by Dr. Rae Nishi (Department of Anatomy and Neurobiology, University of Vermont). Wortmannin, PD980059, forskolin and veratridine (both from Calbiochem, La Jolla, CA) were prepared as stock solutions in DMSO and diluted in culture media just prior to use. PACAP27, referred to simply as PACAP throughout the text, was obtained from American Peptide Co, Sunnyvale CA. M65 was the generous gift of Dr. Ethan Lerner (Harvard Medical School).
Immunohistochemistry
The cardiac ganglia preparations were immunolabeled using procedures described previously (Mawe et al, 1996; Braas et al, 1998; Calupca et al, 2000; Parsons et al, 2006; Girard et al, 2006a, b). The fixed atrial tissue was washed in phosphate buffered saline, permeabilized with 0.5% Triton X-100 and incubated at 4°C overnight with primary antiserum (mouse monoclonal anti-PACAP 1:10, from Dr. Jan Fahrenkrug, Copenhagen, Denmark; rabbit anti-VIP 1:100 from CURE Research Center, UCLA, Los Angeles, CA; goat anti-choline acetyl transferase (ChAT) 1:100, obtained from Chemicon International, Temecula, CA; and rabbit anti-PGP 9.5 1:1000 from Ultraclomne Ltd, Isle of Wright, UK). All primary antisera have been used extensively in our prior studies where specificity of staining was determined using the appropriate control procedures (Mawe et al, 1996; Calupca et al, 2000; Parsons et al, 2006). The preparations were washed and incubated for 2 hours at room temperature with FITC-conjugated or Cy3-conjugated secondary antiserum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Each cardiac ganglia preparation was washed, mounted with Citifluor (UKA Chemical Laboratory, Canterbury, England) and viewed with a Nikon fluorescence photomicroscope with HBO 100 watt UV light source and filters for FITC and Cy3.
Quantification of peptide-IR cardiac neurons
Quantification of PACAP-IR parasympathetic neurons present in the cardiac ganglia followed procedures similar to those published previously (Lynch et al, 1999; Braas et al, 2004; Parsons et al, 2006). The number of cardiac neurons in each preparation was determined by counting ChAT-IR or PGP-9.5-IR cells (Mawe et al, 1996; Braas et al, 1998; Kennedy et al, 1998; Lynch et al, 1999; Calupca et al, 2000; Braas et al, 2004, Parsons et al, 2006). The percentage of cardiac ganglia neurons that expressed PACAP was then determined as a fraction of the total number of neurons that were ChAT or PGP-95 immunoreactive: [(PACAP-IR neurons / ChAT-IR or PGP-95-IR neurons) X 100 = %]. This allowed comparison between different whole mount preparations that contained varying numbers of neurons (Lynch et al, 1999).
Real time Quantitative Reverse transcription-polymerase chain reaction (Q-PCR)
Cardiac ganglia preparations were dissected under RNase-free conditions, and total RNA was extracted from individual preparations using TRI REAGENT (Sigma, Saint Louis, MO, USA). The total RNA quantity for each whole mount preparation was determined using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). 1 μg of RNA per sample was used to synthesize complementary DNA with the Omniscript reverse transcription (QIAGEN Inc., Valencia CA) and oligo-dT primers.
Real time PCR to quantify PACAP transcripts was performed as described previously (Girard et al, 2002a; Braas et al, 2004; Girard et al, 2006a). The primers used were identical to those published in our preliminary report (Girard et al, 2006b). Briefly, amplified guinea pig PACAP DNA product from specific primers was ligated into pCR2.1 TOPO using TOPO TA cloning kit (Invitrogen, Carlsbad, CA) to generate plasmids for peptide transcript standards. The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility) and 10-fold serial dilutions of stock plasmid were prepared to generate assay standard curves. Amplification of the guinea pig cardiac ganglia cDNA templates and plasmid standards was performed using SYBR Green Jumpstart Taq ReadyMix (Sigma, Saint Louis, MO, USA). Briefly, 1X buffer was used containing 5 mM MgCl2, 200 μM dATP, dGTP, dTTP and dCTP, 0.625 U AmpliTaq Gold and 300 nM of each primer in a final 25 μl reaction. Real-time quantitative PCR was performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Norwalk, CT) after optimization of cycling parameters which included SYBR Green I melting point analyses (Girard et al, 2002a). Data were analyzed at the termination of each assay using Sequence Detector 1.3 software (Applied Biosystems, Norwalk, CT). The increase in dye fluorescence intensity (∆Rn) was plotted as a function of cycle number and the threshold cycle (CT) was determined by the software as the amplification cycle at which ∆Rn first intersected the default baseline setting. Transcript levels in each sample were calculated from the CT by interpolation from the standard curve to yield the relative change in expression. All quantitative data were normalized to the starting amount of RNA present in each cardiac ganglia whole mount preparation. Data represent mean percent change compared to the average 0 day time point level.
Statistical analysis
Student’s t-test or one-way analysis of variance (ANOVA) was used to evaluate differences among groups. Differences were considered statistically significant if p<0.05.
RESULTS
PACAP-IR neurons increase during culture of cardiac ganglia
The percentage of neurons exhibiting PACAP immunoreactivity increases when cardiac ganglia are cultured (Calupca et al, 2000; Girard et al, 2006b). In acutely isolated preparations, very few of the intrinsic cardiac neurons exhibited immunoreactivity for PACAP (1.3 ± 0.4 %, n = 4 whole mount preparations) whereas by 24 hours there was an approximately four-fold increase and an approximately twenty fold increase in the percentage of PACAP-IR neurons after 48 hours in culture (Figure 1A, D). No significant increase was evident up to 96 hours (Figure 1D).
Figure 1.

The percentage of PACAP-IR cardiac neurons increases over time in culture. A: Many PACAP-IR neurons were present within cardiac ganglia in 72 hour cultures. The arrows point to neurons that exhibit PACAP immunoreactivity whereas asterisks mark neurons that are not peptide positive. The arrowheads point to PACAP-IR fibers in an adjacent nerve bundle. B: PACAP-IR fibers in a nerve connective. Note the intense PACAP staining at the ends of the fibers. C: An example of a single VIP-IR neuron in a 72 hour culture. Note that both the cell body and axon exhibited VIP immunoreactivity. The scale bars in A-C equal 50 μm. D: The percentage of PACAP-IR neurons increases progressively over time in culture. Asterisks indicate significant (p < 0.001, one way ANOVA) increases in PACAP-IR cells above controls, t = 0. In contrast, over the same time in culture, there is no significant increase in the percentage of VIP-IR cardiac neurons.
In the cultured cardiac ganglia preparations, PACAP immunoreactivity was not restricted to the neuronal cell bodies, but also was present in axonal projections. As the percentage of PACAP-IR neurons increased, the density of the peptide-IR fibers also increased. PACAP immunoreactivity was evident in fibers emanating from the cells (Figure 1A) and PACAP-IR fibers were present in both the small and large nerve bundles. Generally, the PACAP-IR fibers entered nerve bundles and traversed for long distances with the fluorescence more intense at the ends of regenerating fibers, suggesting an accumulation of PACAP in growth cones (Figure 1B). By 72 hours in culture, numerous ChAT-IR nerve fibers were evident extending out from the cut ends of fiber bundles; some of these regenerating nerve fibers also were immunoreactive for PACAP with the fluorescence commonly very intense at the ends of the fibers (Girard et al, 2006b).
PACAP is a member of a family of neuropeptides, which includes VIP. Following axotomy or explant culture of rat sympathetic superior cervical ganglia (SCG), there is an increase in neuronal VIP as well as PACAP (Hyatt-Sachs et al, 1993; Sun et al, 1994; Sun and Zigmond, 1996; Moller et al, 1997a, b). A comparison of PACAP-IR and VIP-IR cells in the current study revealed that the percentage of VIP-IR cells did not increase over periods up to 96 hours in cultured guinea pig cardiac ganglia (Figure 1D). These results confirm our earlier analysis of VIP expression in cultured cardiac neurons (Parsons et al, 2006). In both freshly dissected and cultured ganglia preparations, when present, VIP immunoreactivity was evident in both the cell soma and throughout the axon (Figure 1C).
The increase in PACAP expression was not dependent on the presence of serum-derived factors. Two cardiac ganglia explants were cultured for 72 hours without added horse serum. In these two preparations the percentage of PACAP-IR cells was 24% and 22%, values similar to those in 72 hour cultured cardiac ganglia with added serum (25 ± 3 %, n = 5 preparations).
When the ganglia are isolated and maintained as explants in culture, the preganglionic drive to the ganglia is no longer present. As the guinea pig cardiac neurons are not spontaneously active, we tested whether the loss of stimulation might be a factor initiating the increase in PACAP expression. Cardiac ganglia whole mounts were chronically depolarized for 24 hours by the presence of veratridine (1.5 μM), which is an activator of voltage-gated sodium channels. In 3 veratridine-treated ganglia, the percentage of PACAP-IR neurons was 7 ± 2 %, a value similar to that noted in ganglia not exposed to veratridine (6 ± 2 %). Thus, chronic stimulation did not suppress PACAP expression.
PACAP transcript levels increase over time in culture
In our preliminary report, we showed that PACAP transcript levels were increased after 72 hours in culture (Girard et al, 2006b). In this study, we have determined, using real time PCR, the time course of the increase in PACAP transcript levels when cardiac ganglia are cultured. The same amplification parameters were applied to templates prepared from multiple acutely isolated and cultured preparations. The results showed that the PACAP transcript levels rose progressively over time, reaching a peak value at 48 hours (Figure 2A).
Figure 2.
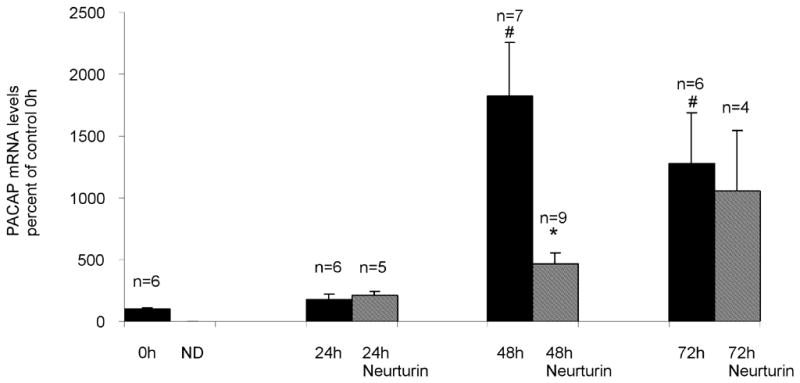
PACAP transcript levels increase during culture of cardiac ganglia. Real time PCR analysis of PACAP transcript levels normalized to RNA/whole mount determined for extracts from acutely isolated preparations and from extracts of cardiac ganglia explants at different times in culture. Neurturin (10 ng/ml) suppressed PACAP transcript levels after 48 hours, but not after 24 or 72 hours in culture. Data represents mean ± SEM of results from at least 5 untreated cardiac ganglia preparations at each time point and mean ± SEM of results from at least 4 neurturin treated cardiac ganglia preparations at 24, 48 and 72 hours in culture. For untreated preparations, asterisks indicate significant differences (p < 0.001) from control 0 hour values, as determined by one way ANOVA. The # indicates a significant difference as determined by Students’ non-paired t-test (p < 0.05) in PACAP transcript level between untreated and neurturin-treated 48 hour cultured ganglia.
Neurturin reduces PACAP transcript levels, but only after 48 hours in culture
We reported previously that neurturin (10 ng/ml) treatment reduced the percentage of PACAP-IR cardiac neurons after 72 hours in culture, but did not decrease the 72 hour PACAP transcript level (Girard et al, 2006b)
In the present study, we tested whether neurturin affected PACAP transcript levels over a range of times in culture. Ganglion explants were maintained in culture for 24, 48 and 72 hours in the presence and absence of neurturin (10 ng/ml). Extracts from at least 4 cardiac ganglia whole mounts were analyzed by real time PCR to determine relative levels of PACAP transcript after these times in culture. We confirmed our initial results that PACAP transcript levels were not significantly decreased after 72 hours in culture (Figure 2B). We also found that neurturin had no effect on PACAP transcript levels at 24 hours in culture. However, neurturin did significantly reduce PACAP transcript levels after 48 hours in culture (Figure 2B). Our results also showed that PACAP transcript levels continued to increase over time in culture when neurturin was present, an observation suggesting that other mechanisms, in addition to a loss of neurturin, must contribute to the stimulation of PACAP expression.
The neurturin-induced suppression of PACAP-IR neurons is mediated by the phosphatidylinositol 3-kinase (PI3-kinase) and mitogen activated protein kinase (MAPK) signaling pathways
Neurturin binds to a membrane receptor GRFα2; an interaction that activates Ret receptor tyrosine kinase and potentially recruits multiple downstream intracellular signaling cascades including the PI3-kinase and the mitogen activated protein kinase (MAPK) pathways (Airaksinen and Saarma, 2002). Previously, we determined that the guinea pig cardiac neurons express GRFα2 (Girard et al, 2006a). Consequently, we tested whether treatment with inhibitors that preferentially interrupt the PI3-kinase and MAPK pathways could blunt the neurturin-induced inhibition of PACAP expression in cultured cardiac ganglia. For these experiments, we determined the percentage of ChAT-IR neurons that also exhibited PACAP immunoreactivity in cardiac ganglia that were cultured for 72 hours with neurturin (10 ng/ml) and either the PI3-kinase inhibitor wortmannin (100 nM ) or the MEK inhibitor PD 98059 (50 μM). When cardiac ganglia were cultured for 72 hours with neurturin and either wortmannin or PD 98059, the percentage of PACAP-IR cells was significantly greater than that in cultures kept in just neurturin plus vehicle (Figure 3). However in both cases, the percentage of PACAP-IR cells was less than in the absence of any drugs (compare results in Figures 1 and 3). To establish that the inhibitors by themselves did not affect PACAP expression, we determined in four additional 72 hour explants (two with each inhibitor) that PD 98059 or wortmannin in the absence of neurturin had no obvious effect on the percentage of PACAP-IR cells (PD 98059: PACAP-IR/ChAT-IR neurons = 22 % and 25 %; wortmannin: PACAP-IR/ChAT-IR neurons 31 % and 22 %).
Figure 3.
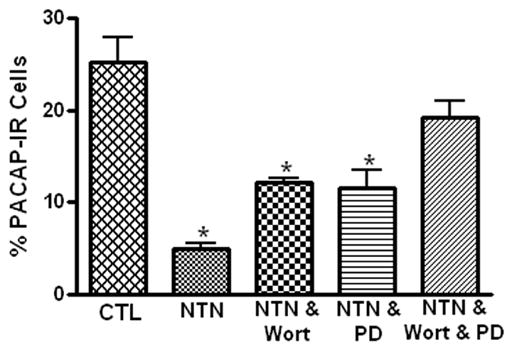
The neurturin-induced suppression of PACAP-IR neurons is mediated by activation of PI3-kinase and MAPK signaling pathways. Neurturin (NTN) significantly decreased (indicated by asterisks) the percentage of PACAP-IR cells after 72 hours in culture (CTL = control). The suppression of PACAP expression by neurturin was reduced by co-treatment with either 100 nM wortmannin (Wort), an inhibitor of PI-3 kinase, or 50 μM PD890059 (PD), an inhibitor of MEK and essentially eliminated in cultures treated with neurturin and both inhibitors. The results are presented as mean ± SEM for at least 3 preparations. Data analyzed with one way ANOVA with differences significant at p > 0.05.
The fact that both wortmannin and PD 98059 partially rather than completely suppressed the effect of neurturin suggested that these two pathways might act in parallel to reduce PACAP expression. Consequently, another series of experiments was completed in which both wortmannin and PD 98059 were applied with neurturin for 72 hours in culture. In these cardiac ganglia, the percentage of PACAP-IR cells approached that seen in the absence of any drugs (Figure 1).
Neurturin does not deplete cellular PACAP
Previously, we demonstrated that PACAP diminished cellular stores of somatostatin in the cardiac neurons (Braas et al, 2004). Consequently, we tested whether a neurturin-induced depletion of cardiac neuron cellular PACAP also might have contributed to the decrease in percentage of PACAP-IR neurons noted in neurturin-treated cultures. To assess this possibility, cardiac ganglia were cultured for 48 hours without neurturin in order to allow PACAP production to occur and the percentage of PACAP-IR neurons to reach maximum levels. The preparations were then treated with neurturin (10 ng/ml) for a subsequent 48 hours and the percentage of PACAP-IR cells was compared to the percentage of PACAP-IR neurons evident in preparations cultured for 96 hours in the absence of neurturin. As shown in Figure 4, the percentage of PACAP-IR cells was similar in both cases. Thus, when PACAP production had been allowed to proceed, neurturin treatment did not reduce PACAP levels below immunocytochemically detectable levels. Thus, we conclude that a neurturin-induced depletion of peptide was not a factor contributing to the decreased percentage of PACAP-IR cardiac neurons.
Figure 4.
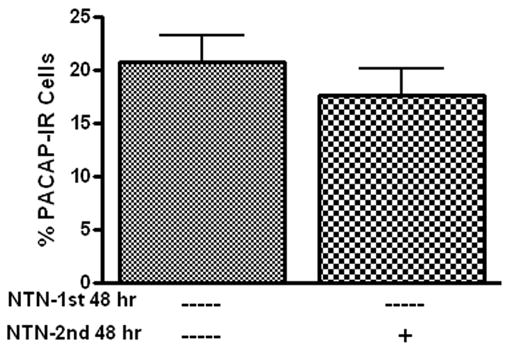
Neurturin does not deplete cellular PACAP. Results from two groups of cardiac ganglia explants (n = 3 in each group) cultured for 96 hours. The first group was cultured for 96 hours without neurturin (NTN). The second group was cultured for an initial 48 hours without neurturin and then the subsequent 48 hours with 10 ng/ml neurturin. There was no difference in percentage of PACAP-IR neurons in these two groups after 96 hours.
Members of common regulatory factor families do not stimulate PACAP expression
In rat SCG neurons, one key mechanism stimulating a change in transmitter phenotype in ganglia explants or after axotomy is the production and secretion of a regulatory factor, leukemia inhibitory factor (LIF), by non-neural cells (Sun et al, 1994; Lewis et al, 1994; Sun and Zigmond, 1996; Cheng et al, 1997; Boeshore et al, 2004). Consequently, we tested, using an immunocytochemical assay, whether members of a number of common regulatory factor and/or target-derived factor families enhanced PACAP expression. For these experiments, the percentage of PACAP-IR neurons was compared in ganglia preparations cultured for 24 hours either with or without different regulatory factors. The regulatory factors CNTF (50 ng/ml), Il-1β (20 ng/ml), TNFα (20 ng/ml), bFGF (50 ng/ml), TGF-β (50 ng/ml) and NGF (100 ng/ml) were each tested in 3 whole mount preparations. None enhanced PACAP expression during 24 hours in culture (Figure 5). These results suggested that generation of these common regulatory factors most likely did not stimulate PACAP expression in cultured cardiac ganglia during this time frame.
Figure 5.
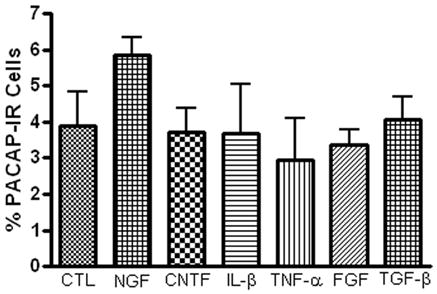
The presence of a number of established regulatory factors does not affect the percentage of PACAP-IR neurons in 24 hour cultured ganglia. Cardiac ganglia explants were cultured for 24 hours in the presence of CNTF (50 ng/ml), Il-1β (20 ng/ml), TNFα (20 ng/ml), FGF (50 ng/ml), TGF-β (50 ng/ml) or NGF (100 ng/ml), then fixed and immunostained using antiserum directed against PACAP and antiserum+ against ChAT.
PACAP enhances PACAP expression in cardiac ganglia cultures
PACAP is a trophic factor, which can enhance neuropeptide expression in PC12 cells, chromafin cells and rat SCG neurons (Hashimoto et al, 2000; Girard, et al, 2002b; Vaudry and Taupenot, 2002). Thus, we tested whether the addition of PACAP ( to the culture media would enhance PACAP expression in the cardiac neurons. In the initial experiments, we tested whether treatment with 20 nM PACAP increased the percentage of PACAP-IR neurons evident after 24 hours in culture. As evident from the results presented in Figure 6A, the percentage of PACAP-IR neurons in 24 hour cultures was enhanced by the presence of PACAP. In additional experiments, we tested whether PACAP also increased the percentage of PACAP-IR neurons during 72 hours in culture. In contrast to what was found for 24 hour cultures, treatment with 20 nM PACAP did not increase the percentage of PACAP-R neurons in 72 hour cultures (Figure 6B).
Figure 6.
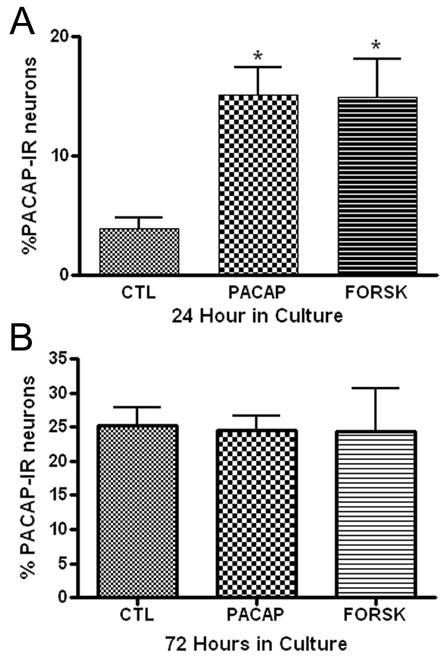
Treatment with PACAP or forskolin can enhance PACAP expression. Cardiac ganglia explants were cultured for 24 hours (A) or 72 hours (B) in the presence of PACAP (20 nM) or forskolin (10 μM) or without either drug. Both PACAP and forskolin treatment increased the percentage of PACAP-IR cells in 24 hour cultures, but not in 72 hour cultures. At least 3 preparations were analyzed under each condition. The asterisks in A indicate significant difference (p < 0.05) from untreated value as determined by ANOVA.
Guinea pig cardiac neurons express the PAC1 receptor (Braas et al, 1998) and activation of PAC1 receptors is commonly coupled to an increase in adenylate cyclase activity (Braas and May, 1999). If the effect of PACAP on PACAP expression was mediated through activation of adenylyl cyclase, then a similar enhancement of PACAP would be expected following treatment with the potent adenylyl cyclase activator forskolin. Therefore, we also tested in a separate series of experiments whether the presence of forskolin (10 μM) in 24 hour cultures or in 72 hour cultures could increase the percentage of PACAP-IR cardiac neurons. Forskolin enhanced PACAP expression when present during 24 hours in culture (Figure 6A), but not during 72 hours in culture (Figure 6B). The similar results with PACAP and forskolin treatment are consistent with the effect of PACAP being mediated at least in part by activation of adenylyl cyclase.
In separate experiments, we tested whether either PACAP or forskolin could enhance VIP expression in the cultured guinea pig cardiac neurons. These experiments were done using 24 hour cultures. Neither 20 nM PACAP nor 10 μM forskolin increased the percentage of VIP-IR neurons (Figure 7).
Figure 7.
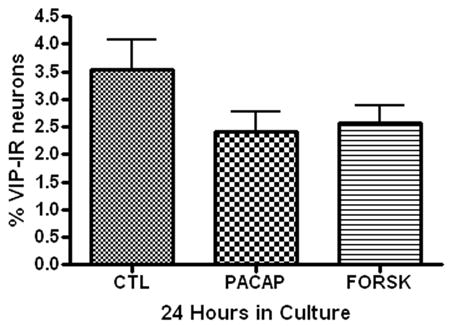
Treatment with PACAP or forskolin has no effect on VIP expression in cultured cardiac neurons. Cardiac ganglia explants were cultured for 24 hours (A) or 72 hours (B) in the presence of PACAP (20 nM) or forskolin (10 μM) or without either drug. Neither treatment affected the percentage of VIP-IR neurons. At least 3 preparations were analyzed under each condition.
Because exogenous PACAP could promote PACAP expression in 24 hour cultures, we tested whether endogenous PACAP released from the cardiac neurons was a factor contributing to the stimulation of peptide expression during culture. To test this hypothesis, we treated 24 or 48 hour cultures with the PAC1 receptor antagonist M65 (100 nM) (Moro et al, 1999). As summarized in Figure 8, the presence of 100 nM M65 did not suppress PACAP expression when present in either 24 or 48 hour cultures.
Figure 8.
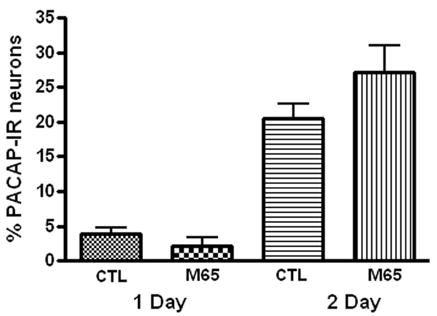
The presence of the PAC1 receptor antagonist M65 did not suppress PACAP expression. Cardiac ganglia explants were cultured for 24 hours or 48 hours in the presence of 100 nM M65. The percentage of PACAP-IR cells was not significantly affected by the presence of the antagonist after either time in culture.
To ensure that M65 at a concentration of 100 nM would be expected to suppress an effect of endogenous PACAP, we tested whether 100 nM M65 diminished the effect of exogenous PACAP on the percentage of PACAP-IR cells in 24 hour cultures. Ganglia cultures were cultured in the presence of 100 nM M65 and 20 nM PACAP for 24 hours and the percentage of PACAP-IR cells determined. When 100 nM M65 was included along with 20 nM PACAP in 24 hour cultures, the percentage of PACAP-IR cells (9 ± 0.4, n = 4 whole mounts) was significantly (P= 0.03) less than when only 20 nM PACAP was present ( 15 ± 2 %, 4 whole mounts). Thus, we conclude that at 100 nM, M65 should effectively antagonize the effect of endogenously released PACAP.
DISCUSSION
PACAP expression may be a mechanism supporting cardiac neuron survival and regeneration following injury in vivo (Moller et al, 1997a,b; Armstrong et al, 2003; Boeshore et al, 2004; Suarez et al, 2006). In the present study we used explant cultured cardiac ganglia as a model system to quantify the PACAP expression which occurs when cardiac neurons are injured. During culture, the percentage of neurons expressing PACAP increases over time. In addition, PACAP appears to accumulate in the ends of the regenerating axons. We postulate that during culture, one mechanism facilitating PACAP expression is the loss of the target-derived factor neurturin, which in vivo would suppress PACAP expression.
In explant culture, guinea pig cardiac neurons also progressively increase expression of cocaine- and amphetamine-regulated transcript protein (CARTp) (Calupca et al, 2002; Girard et al, 2006a). In our recent study, we found that a subpopulation of the cultured cardiac neurons express both CARTp and PACAP whereas other populations of responsive neurons express only PACAP or CARTp (Girard et al, 2006a). The present results suggest that regulation of PACAP expression is more complex than that of CARTp expression, which primarily results from the loss of neurturin (Girard et al, 2006a). Thus, in the case of CARTp, neurturin normally inhibits CARTp expression, but during culture the loss of neurturin releases this inhibition. In contrast for PACAP, the loss of neurturin appeared to be only one of a number of mechanisms regulating its expression as PACAP expression continued to increase, albeit at a lower level, even when neurturin was present in the culture media.
In the rat SCG, synthesis and secretion of LIF by non-neuronal cells after axotomy or during explant culture is thought to be an important stimulator of neuropeptide expression (Sun et al, 1994; Lewis et al, 1994; Sun and Zigmond, 1996; Cheng et al, 1997; Boeshore et al, 2004). We postulated that a similar mechanism might contribute to the stimulation of PACAP expression in cultured cardiac neurons. Consequently, we tested a number of different regulatory factors and/or target-derived factors, but none stimulated PACAP expression following 24 hours in culture. In addition, other recently completed experiments indicated that the addition of 10 μM AG490, a potent inhibitor of the JAK-Stat signaling pathway, did not suppress PACAP expression in 72 hour cultures (Young and Parsons, unpublished observations). This observation supported our conclusion that generation and secretion of a cytokine did not stimulate PACAP expression under the conditions of our experiments. Results of an earlier study, indicated that the increased expression of PACAP in mouse facial motor neurons following axotomy apparently is dependent on inflammatory factors, but not LIF, Il-6 or TNFα. Thus, although none of the regulatory factors tested apparently was involved in stimulating cardiac neuron PACAP production, the possibility that the generation of an undefined regulatory factor participated in the stimulation of PACAP expression can not be ruled out.
The guinea pig cardiac neurons express the PAC1 receptor (Brass et al, 1998). As PACAP can act as a trophic factor and can stimulate expression of neuropeptides in sympathetic neurons (Girard, et al, 2002b), we determined whether PACAP enhanced neuronal PACAP expression during culture of the cardiac ganglia. The addition of 20 nM PACAP during 24 hours in culture enhanced the percentage of PACAP-IR cardiac neurons. A similar effect was obtained with forskolin treatment. In contrast, neither PACAP nor forskolin affected the percentage of PACAP-IR present in 72 hour cultures. These observations suggest that both may accelerate PACAP production in responsive cells, but neither increased the maximum number of cells expressing PACAP. Furthermore, the similar action of PACAP and forskolin indicated that their effect is very likely to have been mediated by the activation of adenylyl cyclase and generation of cAMP. The rat PACAP promoter has been shown to be activated by adenylate cyclase through a cAMP response element, enabling stimulation of transcription of proPACAP (Fukuchi et al, 2004).
Although exogenous PACAP enhanced peptide expression, the inability of the PAC1 receptor antagonist M65 (Moro et al, 1999) to suppress PACAP expression suggested that endogenous PACAP released from the cardiac neurons under the conditions of this study very likely did not contribute to the enhanced peptide expression. Because 100 nM M65 suppressed the effect of exogenous PACAP, we suggest that at 100 nM, M65 would have antagonized any effects of PACAP released from the cardiac neurons during culture.
In many cell types, VIP transcription is enhanced by activation of CREB, through a cAMP response element binding site (Eiden and Hotchkiss, 1983; Tsukada et al, 1987; Fink et al, 1991). Consequently, we were surprised that neither PACAP nor forskolin treatment during culture increased the percentage of VIP-IR cardiac neurons. Apparently in the case of guinea pig cardiac neurons, generation of the cAMP-phosphoCREB signaling pathways does not promote VIP expression.
In the rat SCG, loss of the preganglionic input to the SCG is not thought to be a key factor regulating changes in transmitter phenotype (Hyatt-Sachs et al, 1993; Moller et al, 1997b). During isolation of the cardiac ganglia, the preganglionic and other extrinsic inputs are severed and many of the cardiac neurons are axotomized. The extrinsic fibers degenerate in culture. However, the degeneration of preganglionic and other extrinsic fibers follows rather than precedes the increase in PACAP-IR intrinsic cardiac neurons during explant culture (Young and Parsons, unpublished observations). Thus, we postulate that the potential loss of factors secreted from the different types of extrinsic nerve fibers is not responsible for the initiation of an increased peptide expression in cultured cardiac ganglia. In addition, cardiac neuron inactivity probably did not stimulate PACAP expression as chronic exposure to veratridine did not suppress the increase in PACAP-IR neurons.
It is well established that members of the glial-derived family of trophic factors, which includes neurturin, are key regulatory molecules determining the migration and development of many parasympathetic ganglia, including the intrinsic cardiac ganglia (Enomoto et al, 2000; Hiltunen et al, 2000; Hashino et al, 2001; Airaksinen and Saarma, 2002). In our initial studies, we showed that the addition of neurturin suppressed the increase in PACAP-IR neurons during culture (Girard et al, 2006a). However, when we assayed the effect of neurturin on PACAP transcript levels after 72 hours in culture, there was no significant effect on PACAP transcript levels (Girard et al, 2006b). The 72 hour time point was chosen in our initial experiments because we had determined previously that neurturin significantly suppressed CARTp transcript levels in 72 hour cultures (Girard et al, 2006a). In the present study, we tested the effect of neurturin on PACAP transcript levels over a range of times in culture. We confirmed that neurturin did not significantly decrease PACAP transcript levels after 72 hours in culture and found that no suppression was evident after 24 hours. However, neurturin did significantly suppress PACAP transcript levels in 48 hour cultures. Based on these observations, we postulate that the loss of neurturin very likely was only one of multiple mechanisms potentially contributing to the stimulation of PACAP expression during culture. This hypothesis was consistent with the fact that a population of cardiac neurons (7 ± 1%) continued to exhibit PACAP immunoreactivity even in preparations cultured for 72 hours with neurturin present. In contrast, much fewer CARTp-IR neurons (1 ± 0.4 %) are evident when ganglia are cultured with neurturin for 72 hours (Girard et al, 2006a). In addition, neurturin significantly suppressed CARTp transcript levels at all time points (24, 72 and 72 hours) (Girard and Parsons, unpublished observations).
Neurturin activates intracellular signaling cascades through a membrane GRFα2 receptor coupled to Ret tyrosine kinase (Airaksinen and Saarma, 2002). Previously, we determined that neurturin message is present in adult guinea pig atria and that guinea pig cardiac neurons express GFRα2 receptors (Girard et al, 2006a). Multiple signaling cascades including PI-3-kinase and MAPK pathways are downstream mediators of neurturin (Airaksinen and Saarma, 2002). In the present study, co-treatment with wortmannin and PD 98059, inhibitors of PI-3 kinase and MEK kinase, reduced the neurturin-induced suppression of the percentage of PACAP-IR neurons. In the absence of neurturin, neither inhibitor by itself affected PACAP expression. These observations indicated that the suppression of PACAP expression by neurturin was mediated through these two signaling cascades.
When rat SCG are axotomized in vivo or maintained in explant culture, expression of VIP, a member of the same family of peptides as PACAP, increases (Hyatt-Sachs et al, 1993; Sun et al, 1994; Sun and Zigmond, 1996). However, in the case of guinea pig cardiac neurons, VIP expression does not increase during culture of cardiac ganglia explants. This difference in result indicates that the type of peptide and mechanisms regulating peptide expression may differ between neurons in parasympathetic and sympathetic ganglia or between autonomic neurons in different species.
PACAP peptides are potent trophic molecules and it is commonly proposed that an increased expression of PACAP peptides following injury may support neuronal survival and regeneration (Moller et al, 1997a,b; Zhou et al, 1999; Boeshore et al, 2004; Pettersson et al, 2004). PACAP can protect cardiomyocytes against oxidative stress-induced apoptosis (Gasz et al, 2006). Thus, PACAP production and secretion by regenerating cardiac neurons in vivo could be beneficial to both the cardiac neurons and cardiac muscle following injury. However, an increased production and secretion of PACAP from injured cardiac neurons also might have an unwanted effect on cardiac function through actions on myocardial pacemaker tissues. PACAP facilitates the parasympathetic inhibitory drive to cardiac tissues by increasing cardiac neuron excitability and enhancing acetylcholine release (Seebeck et al, 1996; Chang et al, 2005). In addition, PACAP can also act directly on cardiac tissues to induce tachycardia and arrhythmias (Chang et al, 2005). If under in vivo situations, injured cardiac neurons produce and secrete PACAP onto cardiac tissues, then this could potentially change the normal inhibitory output of the cardiac neurons to a mixed inhibitory/excitatory influence on pacemaker tissue promoting aberrant electrical activity.
In conclusion, we show that the chemical pheonotype of cardiac neurons can change in explant culture, a preparation we have used to test PACAP expression in response to injury. We postulate that in explant culture, the increase in PACAP expression in cardiac neurons that occurs in response to injury is facilitated by the loss of a target-derived factor, very likely neurturin, which normally suppresses PACAP production in vivo. Thus, during culture, PACAP production is released from a neurturin-induced suppression. Also, although many common regulatory factors did not enhance PACAP production under the conditions of these experiments, we do not exclude the possibility that an as yet unidentified paracrine factor also might contribute to the enhanced PACAP expression.
Acknowledgments
We thank Dr. Victor May for helpful discussions during the course of this study and Drs. Rae Nishi and Cynthia Forehand for constructive review of an earlier draft of this manuscript. We also thank Drs. Jan Fahrenkrug and Jens Hannibal for the generous gift of the anti-PACAP antibody and Dr. Ethan Lerner for the generous gift of M65. The work was supported by NIH grants HL -65481 and NCRR P20 RR-16435.
Abbreviations
- ANOVA
Analysis of variance
- bFGF
Fibroblast growth factor basic
- CARTp
Cocaine- and amphetamine-regulated transcript peptide
- ChAT
Choline acetyl transferase
- CNTF
Ciliary neurotrophic factor
- CT
Threshold cycle
- Cy3
Indocarbocyanine
- FITC
Fluorescein isothiocyanate
- GDNF
Glial-derived neurotrophic factor
- GFRα2
Receptor for neurturin
- Il-1β
Interleukin-1 beta
- IR
Immunoreactive
- LIF
Leukemia inhibitory factor
- M65
PAC1 receptor antagonist
- MAPK
Mitogen activated protein kinase
- NGF
Nerve growth factor
- PAC1
PACAP selective receptor
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- PCR
Polymerase chain reaction
- PI3-kinase
Phosphatidylinositol 3-kinase
- SCG
Superior cervical ganglion
- TGFβ
Transforming growth factor- Beta
- TNFα
Tumor necrosis factor- Alpha
- VIP
Vasoactive intestinal polypeptide
Footnotes
Section Editor: Dr. Constantino Sotelo
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol. 2004;59:216–235. doi: 10.1002/neu.10308. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Bio chem. 1999;274(39):27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, Rossignol TM, Girard BM, May V, Parsons RL. Pituitary adenylate cyclase activating polypeptide (PACAP) decreases neuronal somatostatin immunoreactivity in cultured guinea-pig parasympathetic cardiac ganglia. Neuroscience. 2004;126:335–346. doi: 10.1016/j.neuroscience.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol. 2000;423:26–39. [PubMed] [Google Scholar]
- Chang Y, Lawson LJ, Hancock JC, Hoover DB. Pituitary adenylate cyclase-activating polypeptide: localization and differential influence on isolated hearts from rats and guinea pigs. Regul Pept. 2005;129:139–146. doi: 10.1016/j.regpep.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Cheng JG, Pennica D, Patterson PH. Cardiotrophin-1 induces the same neuropeptides in sympathetic neurons as do neuropoietic cytokines. J Neurochem. 1997;69:2278–2284. doi: 10.1046/j.1471-4159.1997.69062278.x. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Hotchkiss AJ. Cyclic adenosine monophosphate regulates vasoactive intestinal polypeptide and enkephalin biosynthesis in cultured bovine chromaffin cells. Neuropeptides. 1983;4(1):1–9. doi: 10.1016/0143-4179(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Heuckeroth RO, Golden JP, Johnson EM, Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development. 2000;127:4877–4889. doi: 10.1242/dev.127.22.4877. [DOI] [PubMed] [Google Scholar]
- Fink JS, Verhave M, Walton K, Mandel G, Goodman RH. Cyclic AMP- and phorbol ester-induced transcriptional activation are mediated by the same enhancer element in the human vasoactive intestinal peptide gene. J Biol Chem. 1991;266(6):3882–3887. [PubMed] [Google Scholar]
- Fukuchi M, Tabuchi A, Tsuda M. Activity-dependent transcriptional activation and mRNA stabilization for cumulative expression of pituitary adenylate cyclase-activating polypeptide mRNA controlled by calcium and cAMP signals in neurons. J Biol Chem. 2004;279(46):47856–47865. doi: 10.1074/jbc.M409090200. [DOI] [PubMed] [Google Scholar]
- Gasz B, Racz B, Roth E, Borsiczky B, Ferencz A, Tamas A, Cserepes B, Lubics A, Gallyas F, Jr, Toth G, Lengvari I, Reglodi D. Pituitary adenylate cyclase activating polypeptide protects cardiomyocytes against oxidative stress-induced apoptosis. Peptides. 2006;27:87–94. doi: 10.1016/j.peptides.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept. 2002a;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PAC1 receptor signaling pathway regulation of sympathetic neuropeptide expression. Program No 637.5; 2002 Abstract Viewer/Itinerary Planner; Washington, DC. 2002b. Online. [Google Scholar]
- Girard BM, Young BA, Buttolph TR, Locknar SA, White SL, Parsons RL. Trophic factor modulation of cocaine- and amphetamine-regulated transcript peptide (CARTp) expression in explant cultured guinea pig cardiac neurons. Neuroscience. 2006a;139:1329–1341. doi: 10.1016/j.neuroscience.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Girard BM, Young B, Buttolph T, White S, Parsons R. Modulation of PACAP expression in explant cultured guinea pig cardiac ganglia neurons. Ann NY Acad Sci. 2006b;1070:298–302. doi: 10.1196/annals.1317.030. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, Mori W, Koyama Y, Matsuda T, Baba A. Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem. 2000;74:501–507. doi: 10.1046/j.1471-4159.2000.740501.x. [DOI] [PubMed] [Google Scholar]
- Hashino E, Shero M, Junghans D, Rohrer H, Milbrandt J, Johnson EM., Jr GDNF and neurturin are target-derived factors essential for cranial parasympathetic neuron development. Development. 2001;128:3773–3782. doi: 10.1242/dev.128.19.3773. [DOI] [PubMed] [Google Scholar]
- Heller S, Huber J, Finn TP, Nishi R, Rohrer H. GPA and CNTF produce similar effects in sympathetic neurones but differ in receptor binding. Neuroreport. 1993;5:357–360. [PubMed] [Google Scholar]
- Hiltunen JO, Laurikainen A, Airaksinen MS, Saarma M. GDNF family receptors in the embryonic and postnatal rat heart and reduced cholinergic innervation in mice hearts lacking ret or GFRalpha2. Dev Dyn. 2000;219:28–39. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1031>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hyatt-Sachs H, Schreiber RC, Bennett TA, Zigmond RE. Phenotypic plasticity in adult sympathetic ganglia in vivo: effects of deafferentation and axotomy on the expression of vasoactive intestinal peptide. J Neurosci. 1993;13:1642–1653. doi: 10.1523/JNEUROSCI.13-04-01642.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AL, Harakall SA, Lynch SW, Braas KM, Hardwick JC, Mawe GM, Parsons RL. Expression and physiological actions of neuropeptide Y in guinea pig parasympathetic cardiac ganglia. J Auton Nerv Syst. 1998;71:190–195. doi: 10.1016/s0165-1838(98)00072-1. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Rao MS, Symes AJ, Dauer WT, Fink JS, Landis SC, Hyman SE. Coordinate regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- Lynch SW, Braas KM, Harakall SA, Kennedy AL, Mawe GM, Parsons RL. Neuropeptide Y (NPY) expression is increased in explanted guinea pig parasympathetic cardiac ganglia neurons. Brain Res. 1999;827:70–78. doi: 10.1016/s0006-8993(99)01308-6. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Talmage EK, Lee KP, Parsons RL. Expression of choline acetyltransferase immunoreactivity in guinea pig cardiac ganglia. Cell Tissue Res. 1996;285:281–286. doi: 10.1007/s004410050645. [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, Sundler F. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997a;775:166–182. doi: 10.1016/s0006-8993(97)00923-2. [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M. Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res. 1997b;775:156–165. doi: 10.1016/s0006-8993(97)00937-2. [DOI] [PubMed] [Google Scholar]
- Moro O, Wakita K, Ohnuma M, Denda S, Lerner EA, Tajima M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxidilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- Parsons RL, Locknar SA, Young BA, Hoard JL, Hoover DB. Presence and co-localization of vasoactive intestinal polypeptide with neuronal nitric oxide synthase in cells and nerve fibers within guinea pig intrinsic cardiac ganglia and cardiac tissue. Cell Tissue Res. 2006;323:197–209. doi: 10.1007/s00441-005-0074-3. [DOI] [PubMed] [Google Scholar]
- Pettersson LME, Heine T, Verge VMK, Sundler F, Danielsen N. PACAP mRNA Is Expressed in Rat Spinal Cord Neurons. J Comp Neurol. 2004;471:85–96. doi: 10.1002/cne.20015. [DOI] [PubMed] [Google Scholar]
- Seebeck J, Schmidt WE, Kilbinger H, Neumann J, Zimmermann N, Herzig S. PACAP induces bradycardia in guinea-pig heart by stimulation of atrial cholinergic neurones. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:424–430. doi: 10.1007/BF00168432. [DOI] [PubMed] [Google Scholar]
- Suarez V, Guntinas-Lichius O, Streppel M, Ingorokva S, Grosheva M, Neiss WF, Angelov DN, Klimaschewski L. The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur J Neurosci. 2006;24:1555–1564. doi: 10.1111/j.1460-9568.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996;67:1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Rao MS, Zigmond RE, Landis SC. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J Neurobiol. 1994;25:415–430. doi: 10.1002/neu.480250407. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Fink JS, Mandel G, Goodman RH. Identification of a region in the human vasoactive intestinal polypeptide gene responsible for regulation by cyclic AMP. J Biol Chem. 1987;262(18):8743–8747. [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vaudry D, Taupenot L. Fast-breaking results on the PACAP/VIP/Secretin peptide family in chromaffin cells. Ann NY Acad Sci. 2002;971:460–466. doi: 10.1111/j.1749-6632.2002.tb04509.x. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, Sundler F. Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience. 1996;74:1099–1110. doi: 10.1016/0306-4522(96)00168-6. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, Fahrenkrug J, Hokfelt T. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–158. doi: 10.1016/0006-8993(95)01150-1. [DOI] [PubMed] [Google Scholar]
- Zhou X, Rodriguez WI, Casillas RA, Ma V, Tam J, Hu Z, Lelievre V, Chao A, Waschek JA. Axotomy-induced changes in pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J Neurosci Res. 1999;57:953–961. [PubMed] [Google Scholar]


