Abstract
Synthesis of phosphatidylcholine, the most abundant brain membrane phosphatide, requires three circulating precursors: choline; a pyrimidine (e.g., uridine); and a polyunsaturated fatty acid. Supplementing a choline-containing diet with the uridine source uridine-5′-monophosphate (UMP) or, especially, with UMP plus the omega-3 fatty acid docosahexaenoic acid (given by gavage), produces substantial increases in membrane phosphatide and synaptic protein levels within gerbil brain. We now compare the effects of various polyunsaturated fatty acids, given alone or with UMP, on these synaptic membrane constituents. Gerbils received, daily for 4 weeks, a diet containing choline chloride with or without UMP and/or, by gavage, an omega-3 (docosahexaenoic or eicosapentaenoic acid) or omega-6 (arachidonic acid) fatty acid. Both of the omega-3 fatty acids elevated major brain phosphatide levels (by 18-28%, and 21-27%) and giving UMP along with them enhanced their effects significantly. Arachidonic acid, given alone or with UMP, was without effect. After UMP plus docosahexaenoic acid treatment, total brain phospholipids levels and those of each individual phosphatide increased significantly in all brain regions examined (cortex, striatum, hippocampus, brain stem, and cerebellum). The increases in brain phosphatides in gerbils receiving an omega-3 (but not omega-6) fatty acid, with or without UMP, were accompanied by parallel elevations in levels of pre- and post-synaptic proteins (syntaxin-3, PSD-95 and Synapsin-1) but not in those of a ubiquitous structural protein, β-tubulin. Hence administering omega-3 polyunsaturated fatty acids can enhance synaptic membrane levels in gerbils, and may do so in patients with neurodegenerative diseases, especially when given with a uridine source, while the omega-6 polyunsaturated fatty acid arachidonic acid is ineffective.
Keywords: Docosahexaenoic acid, Eicosapentaenoic acid, Arachidonic acid, membrane phosphatide, synapse, uridine
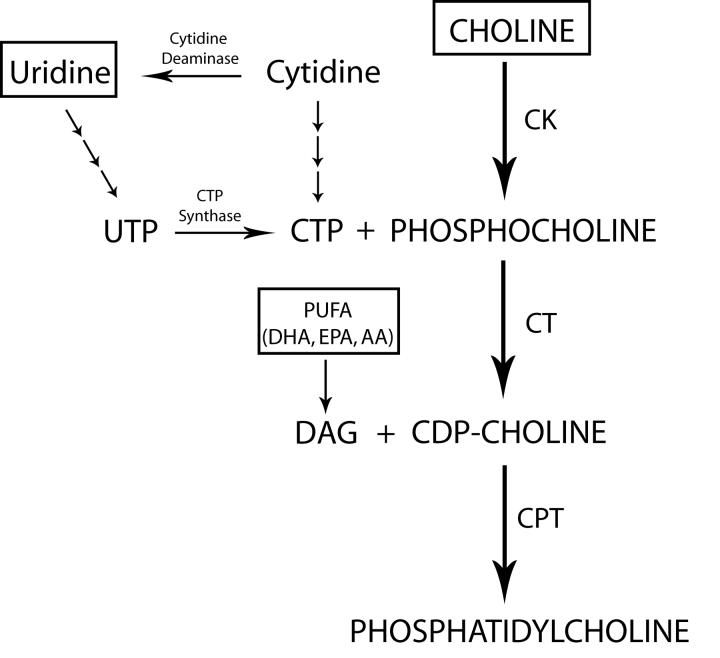
Brains of patients with Alzheimer’s disease contain fewer and smaller synapses (Terry et al., 1991; Selkoe, 2002), and reduced levels of membrane phosphatides (Nitsch et al., 1992) and synaptic proteins (Coleman et al., 2004), while levels of phosphatide breakdown products like glycerophosphocholine are increased (Nitsch et al., 1992). The synthesis of PC, the most abundant brain phosphatide, via the Kennedy pathway (Kennedy and Weiss, 1956) can utilize three precursors obtained from the circulation (Figure 1): choline; a pyrimidine (e.g., uridine, converted to UTP and CTP); and a polyunsaturated fatty acid (PUFA), for example the omega-3 fatty acids docosahexaenoic acid (DHA; 22:6n-3) or eicosapentaenoic acid (EPA; 20:5n-3), or the omega-6 fatty acid arachidonic acid (AA; 20:4n-6). Brain levels of choline (Nitsch et al., 1992), DHA, and AA (Soderberg et al., 1991) are reportedly reduced in Alzheimer’s Disease, while administration of DHA improved cognitive functions in patients with mild disease (Freund-Levi et al., 2006).
Figure 1.
Phosphatidylcholine (PC) biosynthesis via the Kennedy cycle. In rats, plasma cytidine is the major circulating pyrimidine; in gerbils and humans the primary circulating pyrimidine is uridine. Only small amounts of circulating cytidine are converted to brain CTP, since the blood-brain barrier (BBB) high-affinity transporter for pyrimidines (CNT2) has a very low affinity for cytidine; uridine, in contrast, readily enters the brain via CNT2, yielding UTP which can then be converted to CTP by CTP synthase (Cansev, 2006). CTP then reacts with phosphocholine to form endogenous CDP-choline, which combines with diacylglycerol (DAG), preferentially species containing PUFAs like DHA, EPA or AA, (Holub, 1978; Morisaki et al., 1983) to form PC. Boxes indicate the compounds that are obtained from the circulation.
We previously showed that a single dose of the uridine source uridine-5′-monophosphate (UMP) increases brain uridine, UTP, CTP, and cytidine-5′-diphosphocholine (CDP-choline) levels (Cansev et al., 2005), suggesting that it also accelerates PC synthesis (Lopez-Coviella et al., 1995). Moreover, supplementing a choline-containing diet with UMP or DHA, or with both compounds, for 3 or 4 weeks produced substantial increases in membrane phosphatide and synaptic protein levels in gerbil brain (Wurtman et al., 2006). These increases were accompanied by enhanced number of dendritic spines in gerbil brains in vivo (Sakamoto and Wurtman, 2006).
In this study, we tested the effects on synaptic membrane constituents of administering various PUFAs, alone or in combination with a UMP-supplemented diet. The omega-3 PUFAs DHA or EPA were effective; the omega-6 PUFA AA was not. Administration of DHA plus UMP increased phosphatide levels in all brain regions examined (cortex, striatum, hippocampus, brain stem, and cerebellum).
EXPERIMENTAL PROCEDURES
Animals
We used gerbils for our studies, because pyrimidine metabolism in this species more closely resembles that in humans than does pyrimidine metabolism in rats (Traut, 1994; Cansev and Wurtman, 2005): Plasma levels of uridine are higher than those of cytidine in humans and gerbils, but not rats, both basally and after giving a uridine (Cansev et al., 2005) or cytidine (Wurtman et al., 2000; Cansev and Wurtman, 2005) source. Adult male gerbils (M. unguiculatus) weighing 60-80 g (4-6 months old) were exposed to light between 7 AM and 7 PM and given access to food (as described below) and water ad libitum. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. Formal approval to conduct the experiments described was obtained from the Committee on Animal Care at MIT. All efforts were made to minimize the number of animals used and their suffering.
Treatments
Control gerbils consumed a standard choline-containing (0.1%) diet lacking DHA, EPA, or AA but containing the AA and DHA precursors C18:2n6 linoleic acid (LA; 23 mg/g) and C18:3n-3 α-linolenic acid (ALA; 1.5 mg/g) (Table 1); experimental groups were given 1) this diet supplemented with UMP (0.5%); 2) the unsupplemented diet plus DHA, EPA, or AA (each 300 mg/kg, by gavage); or 3) both the UMP-supplemented diet and the gavaged DHA, EPA or AA. Animals not receiving PUFAs were gavaged daily with its vehicle, 5% gum Arabic solution (referred to as “Vehicle”). DHA was purchased from Nu-Chek Prep, Inc. (Elysian, MN, USA). UMP was kindly provided by Numico Research (Wageningen, Netherlands). Control and UMP-containing diets were prepared by Harlan-Teklad (Madison, WI, USA). Each of the three PUFAs was divided into daily doses; pipetted into light-protecting glass vials under a nitrogen gas flow intended to diminish their auto-oxidation; and kept at -80°C until given by gavage. Animals each consumed an average of 5 g of food per day, so that their average intakes of added uridine, choline, and PUFAs were 240, 80, and 300 mg/kg/day, respectively (among those having access to these compounds). None of the groups exhibited significant changes in body weight during the course of the experiment (data not shown). All experiments were carried out in accordance with 1996 Guide for the Care and Use of Laboratory Animals (National Institutes of Health) and Massachusetts Institute of Technology policies.
Table 1.
Composition of control diet
| Proximate Analysis (%) | Fatty Acids (g/kg) | ||
|---|---|---|---|
| Protein | 16.7 | Saturated | 7.34 |
| Carbohydrate | 60.9 | Unsaturated | |
| Oil, Fiber, Ash | 13.7 | C18:1n-9 Oleic Acid | 8.96 |
| Choline | 0.1 | C18:2n-6 Linoleic Acid | 23.12 |
| C18:3n-3 Linolenic Acid | 1.53 |
Treatments were given for 28 days, and on the morning of the 29th day animals were sacrificed under Telazol (80 mg/kg; i.m.) anesthesia by decapitating them using a guillotine. To study the effects of various PUFAs on synaptic membranes, whole brains were quickly removed and divided into two halves, each of which was kept on dry ice; subsequently they were homogenized in deionized water, and aliquots of whole homogenates were used for the assays described below. To study the effects of the UMP-supplemented diet plus DHA on phosphatide levels in various brain regions, samples of cortex, hippocampus, striatum, brain stem and cerebellum were removed; placed on dry ice; and then homogenized; aliquots were assayed as described above for whole brain samples. Bloods were placed in a chilled water bath and centrifuged; plasmas were saved for future assays. Gastric contents were examined, and found in all cases to contain undigested food, indicating that uridine was still being absorbed at the time of sacrifice. Experimental groups consisted of 6-8 gerbils, except as noted.
Phospholipid Assay
Brain phosphatides were extracted according to the method of Folch et al. (1957), and measured as described previously (Ulus et al., 1989; Lopez-Coviella et al., 1995). Briefly, frozen brain hemispheres were weighed and homogenized in 50 volumes of ice-cold deionized water using a tissue degrader (Polytron PT 10-35, Kinematica AG, Switzerland); frozen brain regions were homogenized in 100 volumes of ice-cold deionized water with 10 up-and-down strokes, using a teflon-glass homogenizer (Wheaton, Milville, NJ). One ml aliquots were then mixed with 3 ml of a chloroform plus methanol mixture (2:1 v/v) and vortexed vigorously for 30 seconds. After cooling for about 1 h on ice, each such mixture was mixed sequentially with 3 ml of the chloroform plus methanol mixture, and 1 ml of ice-cold deionized water; it was then vortexed vigorously and allowed to stand overnight in the cold (18-20 h). The organic (lower) and aqueous (upper) phases were separated by centrifugation (10 min at 4°C; 1000 g). Aliquots (0.1-0.4 ml) of the lower (organic) phase were dried under vacuum for phospholipid analysis. Residues of 0.1 ml aliquots of the lower phase were assayed for total phospholipid content by measuring phosphorus (Svanborg and Svennerholm, 1961). Residues of 0.4 ml aliquots of the lower phase were reconstituted in 40 μl methanol and subjected to thin-layer chromatography using silica G plates (Adsorbosil Plus-1, Alltech), and a solvent system consisting of chloroform/ethanol/triethylamine/water (30:34:30:8) as the mobile phase. Phospholipid standards (purchased from Sigma Chemicals, St. Louis, MO, USA) were used to identify the corresponding bands under UV light after the plates were sprayed with 0.1% diphenylhexatriene in petroleum ether. Bands for individual phospholipid classes (PC, PE, PS and PI) were scraped off the plates and extracted into1 ml of methanol; dried under vacuum; and assayed for phosphorus content (Svanborg and Svennerholm, 1961).
Aliquots of whole brain homogenates were assayed for protein using a bicinchoninic acid reagent (Perkin Elmer, Norwalk, CT, USA), and for DNA by a fluorometric method (Labarca and Paigen, 1980). Phosphatide levels were calculated as nmol/mg protein.
Western Blot Analysis
Synaptic proteins were assayed by Western Blot as described previously (Wurtman et al., 2006). Briefly, aliquots of brain homogenates were mixed with equal volumes of KFL loading buffer and boiled for 5 minutes prior to gel electrophoresis. Equal amounts of protein were loaded and separated using SDS-PAGE (4-20%; Bio-Rad, Hercules, CA, USA). Proteins were then transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P, Millipore, Billerica, MA, USA). The remaining binding sites were blocked with 4% non-fat dry milk (Varnation, Glendale, CA, USA) for 30 min in TBST. Membranes were then rinsed five times in TBST buffer and immersed in TBST solution containing the antibody of interest (rabbit anti-syntaxin-3 [Abcam, Cambridge, MA, USA], mouse anti-PSD-95 [Upstate, Lake Placid, NY, USA], mouse anti-synapsin-1 [Calbiochem, San Diego, CA, USA], and mouse anti-β-tubulin [Chemicon, Temecula, CA, USA]). Following overnight incubation and five rinses in TBST buffer, blots were incubated for 1 h with the appropriate peroxidase-linked secondary antibody. Blots were then rinsed in TBST buffer five times, and protein-antibody complexes were detected and visualized using the enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA) and Kodak X-AR film. Films were digitized using a Supervista S-12 scanner with a transparency adapter (UMAX Technologies, Freemont, CA, USA). Immunoreactive bands were compared densitometrically using the Public Domain NIH Image program available on the internet at http://rsb.info.NIH.gov/nih-image/. Areas under the absorbance curve were expressed as arbitrary units and normalized as percents of those generated in the same blot using samples from brains of control animals. Membranes were rinsed using a stripping buffer (Pierce, Rockford, IL, USA) and then incubated with β-tubulin antibody. β-tubulin was used as the loading control for proteins since its levels were unaffected by treatment with the phosphatide precursors.
Data Analysis
Data were expressed as means ± SEM. The effects on brain phospholipid and synaptic protein levels of daily PUFA (e.g., DHA, EPA, or AA) administration were investigated, first in gerbils receiving the control diet, and then in animals receiving the UMP-supplemented diet. Statistical comparisons of the means observed in each treatment group were made using One-Way ANOVA followed by Tukey test. Comparisons of the effects of the UMP-supplemented and control diets were made using Student’s t test. The statistical significance of the effects on brain phosphatides of giving the UMP diet, the various gavaged PUFAs, and their interactions was determined using two-way ANOVA followed by Tukey test. P values less than 0.05 were considered significant.
RESULTS
Effects of DHA, EPA or AA alone or in combination with a UMP-supplemented diet on brain phosphatide levels
Daily administration of DHA (300 mg/kg) alone to animals consuming the control diet increased brain PC, PI, PE, and PS levels significantly, by 18%, 20%, 22%, and 28% respectively (Table 2A). Likewise, administration of EPA to gerbils consuming the control diet increased brain PE, PS, and PI levels significantly, by 21%, 24% and 27%, respectively. (Brain PC rose by 11%, but this effect did not attain significance [P=0.14]) (Table 2A). In contrast, AA administration failed to affect brain levels of any phosphatide (Table 2A).
Table 2.
Effects of various PUFA, given with a control diet (Table 2A) or a UMP-supplemented diet (Table 2B), on gerbil brain phosphatide levels. Data are presented as nmol/mg protein.
| Table 2A | Total PL | PC | PE | PS | PI |
|---|---|---|---|---|---|
| Control diet + Vehicle | 322 ± 3 | 113 ± 3 | 63 ± 3 | 25 ± 1 | 15 ± 1 |
| Control diet + AA | 326 ± 5 | 114 ± 1 | 65 ± 2 | 28 ± 1 | 16 ± 1 |
| Control diet + DHA | 344 ± 13 | 133 ± 6* | 77 ± 4* | 32 ± 2*** | 18 ± 1* |
| Control diet + EPA | 347 ± 6 | 125 ± 8 | 76 ± 4* | 31 ± 1** | 19 ± 1**, a |
| #UMP diet + Vehicle | 332 ± 7 | 131 ± 2* | 70 ± 1 | 29 ± 1* | 16 ± 1 |
| Table 2B | Total PL | PC | PE | PS | PI |
| UMP diet + Vehicle | 332 ± 7 | 131 ± 2 | 70 ± 1 | 29 ± 1 | 16 ± 1 |
| UMP diet + AA | 379 ± 21 | 132 ± 3 | 81 ± 6 | 31 ± 3 | 20 ± 2 |
| UMP diet + DHA | 384 ± 7* | 147 ± 3**, y | 88 ± 2** | 39 ± 2** | 22 ± 1** |
| UMP diet + EPA | 407 ± 7*** | 148 ± 2**, y | 91 ± 1*** | 41 ± 2**, x | 25 ± 1*** |
Groups of gerbils were given a control diet, and received by gavage AA, DHA, or EPA (each 300 mg/kg; in a vehicle of 5% gum Arabic solution) or just its vehicle for 28 days.
Groups of gerbils were given a UMP-containing (0.5%) diet, and received by gavage AA, DHA, or EPA (each 300 mg/kg; in a vehicle of 5% gum Arabic solution) or just its vehicle for 28 days.
On the 29th day their brains were harvested and assayed for phosphatides as described in the text. Data are given as means ± SEM.
P<0.05
P<0.01
P<0.001 compared to Control diet + Vehicle group
P<0.05 compared to Control diet + AA group by One Way ANOVA.
Data from gerbils receiving the UMP diet but no PUFA are included in Table 2A to illustrate that uridine alone also affects phosphatide levels.
P<0.05
P<0.01 compared to UMP diet + AA group by One Way ANOVA.
Consuming the UMP-supplemented diet alone increased brain PS and PC levels significantly (by 15% and 16%, respectively) compared with those in control gerbils. Among animals receiving both UMP and DHA, brain PC, PE, PS, and PI levels rose significantly by 12%, 26%, 34%, and 38%, respectively (Table 2B). Similarly, among gerbils receiving both UMP and EPA, brain PC, PE, PS, and PI levels rose significantly by 13%, 30%, 41% and 56%, respectively (Table 2B). In contrast, giving UMP with AA failed to increase levels of any brain phosphatide above those found in gerbils receiving UMP alone (Table 2B). Total brain phospholipid levels were also elevated significantly, by 16% and 23%, following treatment with UMP plus DHA, or with UMP plus EPA, respectively (Table 2B), but not by treatment with UMP plus AA (Table 2B). Two-way ANOVA revealed a significant effect of dietary UMP or gavaged DHA or EPA on brain PC, PE, PS, and PI levels (all P<0.001). Two-way ANOVA also revealed significant interactions between dietary UMP and gavaged DHA or EPA on brain PC, PE, PS, and PI levels (all P<0.05).
Essentially similar results were obtained when data were expressed per μg DNA, instead of per mg protein (data not shown).
Effects of UMP-supplemented diet and DHA on phosphatide levels in various brain regions
To determine whether the effects of UMP plus an omega-3 fatty acid were present throughout the gerbil brain, we examined phosphatide levels in five specific brain regions: cortex, striatum, hippocampus, brain stem, and cerebellum. Among control animals phosphatide levels (nmol/mg protein) in these brain regions were similar, except for the brain stem, in which total phosphatide levels were 68-70% higher than in other regions (i.e., 450 nmol/mg protein in brain stem vs 264-270 nmol/mg protein in the other regions investigated) (Table 3). This difference was due mainly to PE, since PE levels in brain stem were about double those in any other region (i.e., 117 nmol/mg protein in brain stem vs 58-64 nmol/mg protein in other regions) (Table 3). PC and PS levels in brain stem were 12-21% and 25% higher than in other regions (Table 3). Treatment with UMP and DHA increased phosphatide levels significantly in all of the regions examined, by approximately the same proportion, i.e., by 18-38%, 21-57%, 15-33%, 16-33%, and 11-33%, in cortex, striatum, hippocampus, brain stem, and cerebellum, respectively (Table 3).
Table 3.
Effects of giving UMP-supplemented diet (0.5%) and DHA (300 mg/kg) on phosphatide levels in different gerbil brain regions.
| Cortex | Striatum | Hippocampus | Brain Stem | Cerebellum | |
|---|---|---|---|---|---|
| Total PL | |||||
| Control diet + Vehicle | 267 ± 7 | 265 ± 8 | 264 ± 11 | 450 ± 10 | 270 ± 9 |
| UMP diet + DHA | 316 ± 9** | 339 ± 9*** | 314 ± 6** | 521 ± 15** | 317 ± 15** |
| PC | |||||
| Control diet + Vehicle | 94 ± 3 | 100 ± 2 | 102 ± 1 | 114 ± 2 | 98 ± 2 |
| UMP diet + DHA | 122 ± 2*** | 126 ± 9* | 117 ± 2*** | 139 ± 2*** | 111 ± 1*** |
| PE | |||||
| Control diet + Vehicle | 58 ± 1 | 60 ± 3 | 61 ± 2 | 117 ± 2 | 64 ± 3 |
| UMP diet + DHA | 80 ± 4** | 85 ± 1*** | 81 ± 4*** | 156 ± 3*** | 85 ± 2*** |
| PS | |||||
| Control diet + Vehicle | 24 ± 1 | 24 ± 1 | 24 ± 1 | 30 ± 1 | 24 ± 1 |
| UMP diet + DHA | 30 ± 1*** | 29 ± 1* | 28 ± 1*** | 35 ± 1*** | 29 ± 1** |
| PI | |||||
| Control diet + Vehicle | 10.6 ± 0.2 | 7.6 ± 0.4 | 8.8 ± 0.2 | 9.3 ± 0.6 | 10.4 ± 0.3 |
| UMP diet + DHA | 13.2 ± 0.4** | 11.9 ± 0.4*** | 11 ± 0.3*** | 11.8 ± 0.6* | 11.5 ± 0.2* |
Groups of gerbils were given a UMP-containing (0.5%) diet and, received by gavage, DHA (300 mg/kg; in a vehicle of 5% gum Arabic solution) or just the vehicle, for 28 days. On the 29th day various brain regions were harvested and assayed for phosphatides as described in the text. Data are presented as nmol/mg protein.
P<0.05
P<0.01
P<0.001 compared to Control diet + Vehicle group using Student’s t test.
Effects of DHA, EPA or AA alone or in combination with a UMP-supplemented diet on synaptic protein levels
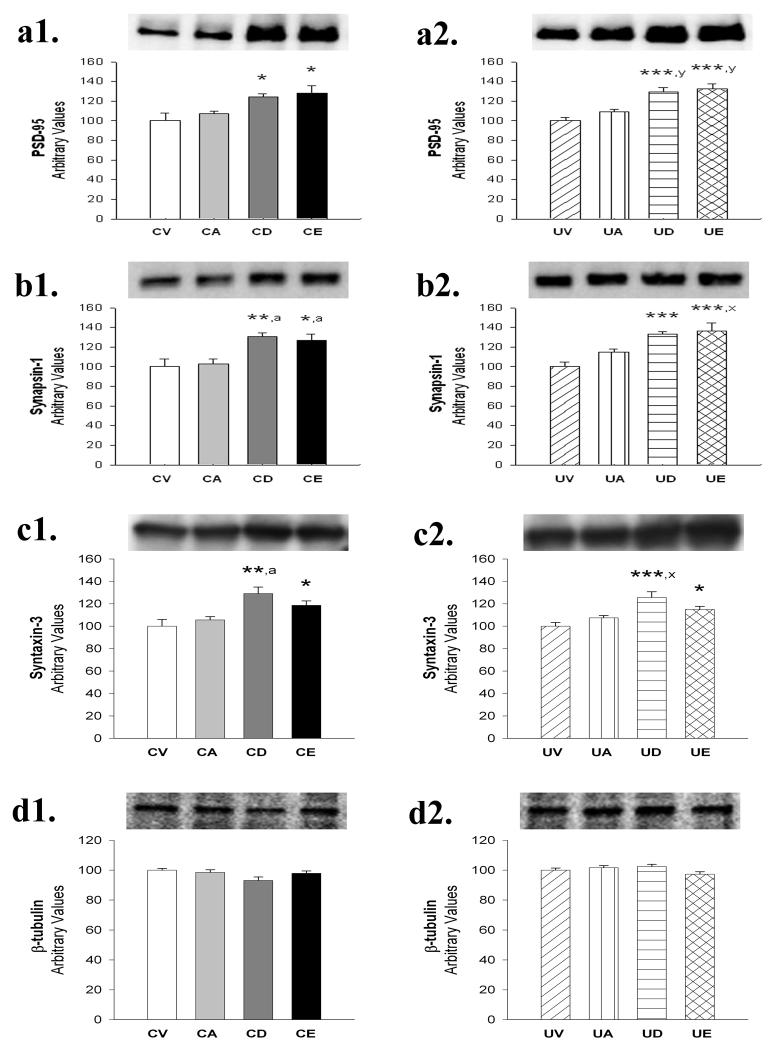
DHA or EPA treatment alone significantly increased brain levels of the post-synaptic density protein PSD-95, by 24% or 28% (Figure 2a1). When combined with a UMP-supplemented diet, DHA or EPA increased brain PSD-95 levels by 29% or 33% over those found after UMP-supplementation alone (Figure 2a2). AA failed to affect brain PSD-95 levels both when given alone and in combination with the UMP-supplemented diet (Figure 2a).
Figure 2.
Effects of AA, DHA or EPA, alone or in combination with a UMP-supplemented diet, on levels of the presynaptic or postsynaptic proteins PSD-95 (a1, a2); Synapsin-1 (b1, b2) and Syntaxin-3 (c1, c2). CV, control diet + vehicle; CA, control diet + AA; CD, control diet + DHA; CE, control diet + EPA; UV, UMP-supplemented diet + vehicle; UA, UMP-supplemented diet + AA; UD, UMP-supplemented diet + DHA; UE, UMP-supplemented diet + EPA. *P<0.05; **P<0.01; and ***P<0.001 compared with CV, and aP<0.05 compared with CA on the left-sided columns (a1, b1, and c1) using One Way ANOVA. *P<0.05; **P<0.01; and ***P<0.001 compared with UV, and xP<0.05; and yP<0.01 compared with UA on the right-sided columns (a2, b2, and c2) using One Way ANOVA.
Levels of Synapsin-1, a presynaptic vesicular protein, were significantly increased, by 31% or 27% respectively, by giving DHA or EPA treatment alone (Figure 2b1). Giving DHA or EPA with UMP increased brain Synapsin-1 by 33% or 36% over levels observed in gerbils receiving only UMP, respectively (Figure 2b2). AA failed to affect brain Synapsin-1 levels when given alone or in combination with a UMP-supplemented diet (Figure 2b).
DHA or EPA administration significantly increased brain levels of Syntaxin-3, a plasma membrane SNARE (soluble N-ethylmaleimidesensitive-factor attachment protein receptor) protein which reportedly mediates the stimulation by PUFAs of neurite outgrowth (Darios and Davletov, 2006), and exocytosis (Teng et al., 2001), by 29% or 19%, respectively (Figure 2c1); again, AA was without effect (Figure 2c1). In combination with UMP, DHA or EPA increased Syntaxin-3 by 26% or 15% compared with the effects of UMP alone (Figure 2c2), and again, Syntaxin-3 levels were unaffected by treatment with AA in combination with UMP (Figure 2c). Giving the UMP-supplemented diet alone elevated brain PSD-95, Synapsin-1 and Syntaxin-3 levels by 8-11% compared with those observed after consumption of the control diet; however these increases were not statistically significant (data not shown).
None of the PUFA changed brain levels of the structural protein, β-tubulin, whether given alone or in combination with UMP. Hence, β-tubulin was used as the loading control for assays of synaptic proteins by Western Blot (Figure 2d).
DISCUSSION
These data show that administration of the omega-3 PUFAs DHA or EPA to gerbils consuming a standard, choline-containing diet can increase brain membrane phosphatide levels, and that, in contrast, the omega-6 PUFA AA is without effect (Table 2A). A UMP-supplemented diet alone also increases brain PC and PS levels, and, when administered in combination with a PUFA, amplifies the effects of DHA or EPA, but does not cause AA to become effective (Table 2B). In all cases the changes in brain phosphatide levels are associated with parallel increases in levels of such pre- and post-synaptic proteins as Synapsin-1, Syntaxin-3 and PSD-95 (Figure 2), but not in a ubiquitous structural protein, β-tubulin (Figure 2d). As shown in other studies, DHA but not AA also increases hippocampal dendritic spines (Sakamoto and Wurtman, 2006).
Synthesis of brain PC, the most abundant membrane phosphatide, via the predominant Kennedy pathway (Kennedy and Weiss, 1956) utilizes three circulating compounds; choline, a pyrimidine (e.g., uridine) and a PUFA (Figure 1). Circulating choline is transported into the brain’s extracellular fluid (ECF) principally via a facilitated blood-brain barrier diffusion process catalyzed by a sodium-independent, hemicholinium-3-sensitive, transport protein (Cornford et al., 1978). Choline’s flux can be bidirectional; net flux into brain occurs when plasma levels (normally 7-10 μM in the fasting state and up to 30 μM after meals [Cohen and Wurtman, 1976]) are above 15 μM (Klein et al., 1990). Once in the brain’s ECF, choline can be taken up into all brain cells by an unsaturated low-affinity transport protein (Km=30-100 μM), or into cholinergic nerve terminals by a high-affinity uptake protein (Km=0.1-10 μM) (Haga and Noda, 1973; Yamamura and Snyder, 1973; Blusztajn and Wurtman, 1983).
The principal mechanism by which circulating uridine is taken up into the brain utilizes the CNT2 transporter (reviewed in Cansev, 2006) located at the BBB (Li et al., 2001); small amounts (Spector, 1985) also enter via the equilibrative transport proteins ENT1 and ENT2, and the concentrative transporter CNT3, located at the CP epithelium (Redzic et al., 2005). Once in the brain’s extracellular space uridine is taken up into brain cells via equilibrative and concentrative nucleoside transport proteins (Redzic et al., 2005).
Although the processes by which circulating PUFAs cross the BBB and, subsequently, enter brain cells await full characterization, they are thought to include both simple diffusion (also termed “flip-flop”; Kamp et al., 1993) and protein-mediated transport (Abumrad et al., 1984); one such transport protein (B-FATP) (Chmurzynska, 2006) has been cloned (Shimizu et al., 1997). DHA, EPA and AA are then transported from the brain’s ECF into cells, and can be activated to their corresponding CoA species (e.g., docosahexaenoyl-CoA; eicosapentaenoyl-CoA; arachidonoyl-CoA) and acylated to the sn-2 position of DAG (reviewed in Robinson et al., 1992) to form DAG species rich in each of the PUFAs (Bazan, 1990; Thies et al., 1994). The acylation of DHA is catalyzed by a specific acyl-CoA synthetase, Acsl6 (Marszalek et al., 2005) with a low affinity for DHA (Km=26 μM; Reddy et al, 1984) relative to usual brain DHA levels (1.3-1.5 μM; Contreras et al., 2000), hence treatments that raise blood DHA levels rapidly increase its uptake into, and retention by brain cells.
Each step in the incorporation of choline, uridine, or DHA into brain phosphatides is catalyzed by a relatively low-affinity enzyme; this characteristic allows the administration of each precursor to affect the rate of phosphatide synthesis. Choline kinase (CK), which phosphorylates choline to form phosphocholine, has a Km for choline of 2.6 mM (Spanner and Ansell, 1979), which is substantially higher than usual brain choline concentrations (35-100 μM; Cohen and Wurtman, 1976); hence choline administration increases brain phosphocholine levels in rats (Millington and Wurtman, 1982) and humans (Babb et al., 2004). Similarly, uridine-cytidine kinase (UCK), which phosphorylates uridine to form UTP (Canellakis, 1957) has a Km of 270 μM (Orengo, 1969) while brain uridine levels are approximately 20-25 μM (Cansev et al., 2005), and the CTP synthase which converts UTP to CTP (Lieberman, 1956) has a Km for UTP of 600 μM (Kizaki et al., 1980) while brain UTP levels are 250-300 μM (Cansev et al., 2005). Finally, the Km’s of CTP:phosphocholine cytidylyltransferase (CT), which combines CTP and phosphocholine to form CDP-choline, for these substrates are 1-1.3 mM and 0.30-0.31 mM (Mages et al., 1988; Ross et al., 1997), respectively, whereas their brain levels are only 70-110 μM (Mandel and Edel-Harth, 1966; Abe et al., 1987; Cansev et al., 2005) and 0.32-0.69 mM (Millington and Wurtman, 1982; Nitsch et al., 1992; Klein et al., 1993), respectively. Hence this enzyme also is unsaturated with both of its substrates. Providing neurons with choline and uridine increases its saturation, thus producing substantial increases in brain CDP-choline synthesis and levels (Cansev et al., 2005).
The enzyme cholinephosphotransferase (CPT), which catalyzes the combination of CDP-choline with DAG (Figure 1), also exhibits low affinities for its substrates (Km=200 μM and Km=150 μM) respectively (Cornell, 1992), relative to brain levels of these compounds (∼10 μM [Cansev et al., 2005] and ∼75 μM [Abe et al., 1987]). Unsaturation with their substrates apparently characterizes the Kennedy cycle enzymes throughout the brain, inasmuch as supplementation with UMP, choline, and DHA was found to increase phosphatide levels in all of the brain regions examined (Table 3). The affinities of CPT for DAG and CDP-choline are decreased if the DAG contains a polyunsaturated fatty acid; this, in turn, would decrease CPT’s substrate saturation (Mantel et al., 1993) and allow DAGs rich in PUFAs to be preferentially utilized for phosphatide synthesis, as has been shown to be the case (Holub, 1978; Morisaki et al., 1983). That supplementation with uridine and/or the omega-3 PUFA DHA increases brain phosphatide levels (Wurtman et al., 2006) is confirmed in the present study which also shows that EPA, another omega-3 PUFA, shares this effect. In contrast the omega-6 PUFA AA, a known constituent of brain phosphatides, failed to do likewise (Table 2).
The control diet contained no DHA, EPA or AA (Table 1), however it did contain the AA precursor LA and the DHA precursor ALA, hence it is possible that DHA or AA formed in vivo from these precursors might have contributed significantly to the total dosages received by the experimental groups. LA and ALA are, respectively, converted to AA and DHA, principally in the liver (Marangoni et al, 1992) but also in brain astrocytes (Moore et al, 1991). Animals receiving exogenous LA for 4 days (4 g/kg; Sinclair and Collins, 1970) or 3 months (1 g/kg; Marangoni et al, 1992) or, as corn oil, for 14 days (150 mg/kg; Jenkins and Kramer, 1990) exhibited no increases in plasma AA, even though plasma LA levels were more than doubled compared with those in control rats. Moreover, in a recent study in which healthy men received low or high levels of LA for 4-week periods, plasma AA levels exhibited no changes, and those of EPA fell, possibly because of inhibition by LA of the conversion of ALA to EPA (Liou et al, 2007). LA and ALA are converted to AA or DHA, by astrocytic but not neuronal cultures (Moore et al, 1991). The precursors can also be converted to AA and EPA by cultured brain endothelium, and released into the culture medium (Moore et al, 1990). Moreover, giving [1-14C]linoleic acid (18:2n-6, LA) intracranially caused accumulation of labeled AA in brain, raising the possibility that some of this conversion occurred in brain (Green and Yavin, 1993). Administration of LA (approximately 300 mg/kg) elevated brain AA levels by about 15% (Mohrhauer and Holman, 1963), however this required that it be given by gavage, which probably causes much greater increases in plasma LA levels than those following its consumption in a chow diet, and probably reflects AA synthesis in the liver. When rats received isotopically-labeled LA orally, 0.06% of the administered dose was present in brain after 22 hours as AA; in contrast, among animals receiving oral AA, approximately 30 times as much was present in brain as unchanged AA (Hassam and Crawford, 1976).
It is theoretically possible that the AA formed in the livers and brains of our gerbils resulting from the daily consumption of approximately 1640 mg/kg LA might have provided their brains with sufficient AA to saturate brain enzymes that convert AA to phosphatides, causing the animals not to respond to the gavaged AA (300 mg/kg). However, this seems highly unlikely since basal brain AA levels (2.5-3.5 μM [Deutsch et al., 1997; Rabin et al., 1997]) are an order of magnitude below the concentrations that would be needed even to half-saturate arachidonoyl-CoA synthetase (Km for AA: 36 μM [Reddy and Bazan, 1983]), the enzyme that acylates AA leading to formation of AA-containing DAG, and then to phosphatides. Moreover, oral administration of LA in daily doses up to 4 g/kg did not significantly increase plasma AA concentrations (Sinclair and Collins, 1970; Jenkins and Kramer, 1990), and, AA levels of brain phospholipids can be significantly increased in animals already consuming about 3680 mg/kg daily LA if they are supplemented with AA (about 920 mg/kg over a 24-hour period [Ward et al., 1998]). Hence, the LA normally present in rodent chow probably will not give rise to sufficient brain AA to inhibit possible effects of gavaged AA on brain phosphatide levels.
Confirming prior studies on DHA (Wurtman et al., 2006), we found that the increases in membrane phosphatide levels following EPA or DHA administration were accompanied by increased levels of specific synaptic proteins (Figure 2), including Synapsin-1, a presynaptic vesicular protein (Ferreira and Rapoport, 2002); PSD-95, the postsynaptic density protein (Fujita and Kurachi, 2000), which is known to be involved in maturation of excitatory synapses (El-Husseini et al., 2000); and Syntaxin-3, a protein in plasma membranes (Figure 2). In contrast, levels of the ubiquitous structural protein β-tubulin were unaffected by any of the treatments (Figure 2d). Our data thus suggest that the omega-3 PUFAs specifically but not necessarily exclusively increase the quantities of synaptic membranes in brain. This hypothesis is supported by the observation that DHA supplementation increases the number of dendritic spines in hippocampal neurons (Sakamoto and Wurtman, 2006), particularly when animals also receive UMP. The increase in synaptic proteins could reflect decreases in their metabolism, perhaps resulting from protection against enzymatic degradation conferred by the increased amounts of membrane phosphatide, or from increases in their synthesis, perhaps mediated by activation of a growth-related receptor by a precursor or product of one of the administered compounds, or by activation of genes encoding their synthesis (Kothapalli et al., 2007). UTP formed from uridine activates P2Y receptors, and this activation is necessary in order for the uridine to promote neurite outgrowth from PC12 cells (Pooler et al., 2005). Similarly, DHA can activate the Syntaxin-3 protein (Darios and Davletov, 2006), and this activation may be required in order for the DHA to promote growth cones in PC12 cells.
In vitro, uridine in concentrations of 50 μM or greater, or UTP in concentrations of 10 μM, increases the size of neurites (Chorna et al., 2004; Pooler et al., 2005) generated by NGF-differentiated PC12 cells (Araki and Wurtman, 1997). The uridine effect, which is associated with accelerated phosphatide synthesis (Richardson et al., 2003) and increased levels of the neurofilament proteins NF-M and NF-70 (Pooler et al., 2005), is blocked by drugs like suramin, reactive blue 2 and PPADS, which inhibit P2Y receptors (P2Y2, P2Y4 and P2Y6) involved in neuronal differentiation (Arthur et al., 2005; Cavaliere et al., 2005). Likewise, omega-3 and omega-6 PUFAs promote cell membrane expansion and the genesis of neuronal growth cones by binding to Syntaxin-3 (Darios and Davletov, 2006), a plasma membrane SNARE protein that is involved in exocytosis (Teng et al., 2001), and levels of syntaxin-3 are increased in brains of animals given DHA and UMP (Figure 2). Direct links between P2Y receptor stimulation or Syntaxin-3 activation and the syntheses of special synaptic proteins have not yet been established.
The mechanism that allows DHA and EPA, omega-3 fatty acids, but not AA, an omega-6 fatty acid, to increase the quantity of synaptic membrane is unclear. EPA apparently can be converted to DHA by brain astrocytes (Moore et al., 1991), hence its effects on brain phosphatides and synaptic proteins could involve mediation by DHA itself. Exogenously administered AA, like DHA, is preferentially incorporated into brain phosphatides (DeGeorge et al., 1991; Sarda et al., 1991), as well as into other lipids, e.g. the plasmalogens (Farooqui and Horrocks, 2001; Nagan and Zoeller, 2001) and AA also shares with DHA the ability to activate syntaxin-3 (Darios and Davletov, 2006). Mechanisms underlying the differential effects of omega-3 and omega-6 PUFAs on membrane synthesis might include, among others, different efficiencies in their uptakes into brain or acylation; different affinities for enzymes that control their incorporation into DAG and phosphatides; differences in the rates at which the PUFAs are removed from phosphatides by deacylation; the differential activation of genes encoding proteins needed for membrane synthesis (Kothapalli et al., 2007); or the tendency of AA to be incorporated into phospholipids by the acylation of 1-acyl-2-lyso-sn-glycerophospholipids, not via the Kennedy cycle (Lands et al., 1982).
DHA and AA are major components of brain membrane phospholipids (O’Brien and Sampson, 1965). While AA is widespread through the brain and is abundant in PI and PC, DHA is concentrated in synaptic regions of gray matter (Breckenridge et al., 1972) and is especially abundant in PE and PS (Svennerholm, 1968). In contrast, EPA is found only in trace amounts in brain phosphatides, mostly in PI (Hicks et al., 2006). No significant differences have been described between the proportions of ingested omega-3 and omega-6 PUFAs that enter the blood stream (Carlier et al., 1991; Bezard et al., 1994). Moreover, the rates at which radioactively-labeled DHA and AA are taken up into brain and incorporated into phospholipids following systemic injections also are similar (DeGeorge et al., 1991; Rapoport et al., 2001). [To our knowledge, no study has compared the brain uptake of EPA with that of another PUFA in rodents or humans, however exogenously administered EPA does increase brain EPA levels in vivo (Philbrick et al., 1987)]. On the other hand, the half-lives of the omega-3 PUFAs in the blood (20 ± 5.2 hours for DHA and 67 ± 14 hours for EPA [Pawlosky et al., 2001]) are substantially higher than that for AA (3.8 seconds [Zhou et al., 2002]). Similarly, the half-life of DHA in brain PC (22.4 ± 2.9 hours), but not in PI or PE, is much longer than that of AA (3.79 ± 0.12 hours) (Rapoport, 2005). Thus, a considerable proportion of AA may be cleared from plasma or oxidized before it is utilized for PC synthesis, or, once incorporated into phosphatides, it may be liberated by hydrolysis mediated by phospholipase A2 (Strokin et al., 2003), and then oxidized.
Our data suggest that, if a physiological or behavioral effect of DHA is reproduced by EPA, but not by AA, that effect might be mediated by an increase in the levels of synaptic membranes or perhaps synapses. Apparently, no studies have compared the in vivo effects of DHA, EPA and AA on the metabolism of brain membranes or the relative involvement of the three fatty acids in particular brain diseases. Alzheimer’s disease is known to be associated with reduced levels of membrane phosphatides (Nitsch et al., 1992) and synaptic proteins (Hatanpaa et al., 1999; Coleman et al., 2004), and increased levels of phosphatide breakdown products (Nitsch et al., 1992), but it has not been determined whether phosphatides containing a particular omega-3 or omega-6 fatty acid are particularly vulnerable in this disorder.
In conclusion, these data show that administration of DHA or EPA to adult animals, alone or in combination with a UMP-supplemented diet, enhances levels of brain membranes, particularly, those of synaptic regions. AA, however, is without effect. This difference may be significant in formulating fatty acid mixtures for preventing or treating diseases, particularly those, like Alzheimer’s disease associated with a reduction in the number of synapses. A small study (174 subjects) on patients with very mild Alzheimer’s disease found significant improvement in cognitive functions following the chronic administration of DHA (Freund-Levi et al., 2006).
Acknowledgements
This work was supported by grants from the National Institutions of Health (Grant MH-28783), the Center for Brain Sciences and Metabolism Charitable Trust. The authors thank Mark Vangel for useful discussions.
List of Abbreviations
- PUFA
polyunsaturated fatty acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- LA
linoleic acid
- ALA
α-linolenic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- AA
arachiconic acid
- UMP
uridine-5′ -monophosphate
- UTP
uridine-5′ -triphosphate
- CTP
cytidine-5′ -triphosphate
- CDP-choline
cytidine-5′ -diphosphocholine
- DAG
diacylglycerol
- CK
choline kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Kogure K, Yamamoto H, Imazawa M, Miyamoto K. Mechanism of arachidonic acid liberation during ischemia in gerbil cerebral cortex. J Neurochem. 1987;48:503–509. doi: 10.1111/j.1471-4159.1987.tb04121.x. [DOI] [PubMed] [Google Scholar]
- Abumrad NA, Park JH, Park CR. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984;259:8945–8953. [PubMed] [Google Scholar]
- Araki W, Wurtman RJ. Control of membrane phosphatidylcholine synthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc Natl Acad Sci USA. 1997;94:11946–11950. doi: 10.1073/pnas.94.22.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb SM, Ke Y, Lange N, Kaufman MJ, Renshaw PF, Cohen BM. Oral choline increases choline metabolites in human brain. Psychiatry Res. 2004;130:1–9. doi: 10.1016/S0925-4927(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. Vol. 8. Raven Press; New York, NY: 1990. pp. 1–24. [Google Scholar]
- Bezard J, Blond JP, Bernard A, Clouet P. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod Nutr Dev. 1994;34:539–568. doi: 10.1051/rnd:19940603. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- Breckenridge WC, Gombos G, Morgan IG. The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim Biophys Acta. 1972;266:695–707. doi: 10.1016/0006-3002(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Canellakis ES. Pyrimidine metabolism. II. Enzymatic pathways of uracil anabolism. J Biol Chem. 1957;227:329–338. [PubMed] [Google Scholar]
- Cansev M. Uridine and cytidine in the brain: Their transport and utilization. Brain Res Brain Res Rev. 2006;52:389–397. doi: 10.1016/j.brainresrev.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Cansev M, Watkins CJ, van der Beek EM, Wurtman RJ. Oral Uridine 5′ monophosphate (UMP) increases brain CDP-choline levels in gerbils. Brain Res. 2005;1058:101–108. doi: 10.1016/j.brainres.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Cansev M, Wurtman RJ.Exogenous cytidine-5′-diphosphocholine increases brain cytidine-5′-diphosphocholine levels in gerbils 200520th Biennial Meeting of the ISN-ESN AbstractsInnsbruck, Austria21-26 August 2005 J Neurochem 94 (Suppl. 2):105-106 [Google Scholar]
- Carlier H, Bernard A, Caselli C. Digestion and absorption of polyunsaturated fatty acids. Reprod Nutr Dev. 1991;31:475–500. doi: 10.1051/rnd:19910501. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Nestola V, Amadio S, D’Ambrosi N, Angelini DF, Sancesario G, Bernardi G, Volonte C. The metabotropic P2Y4 receptor participates in the commitment to differentiation and cell death of human neuroblastoma SH-SY5Y cells. Neurobiol Dis. 2005;18:100–109. doi: 10.1016/j.nbd.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Chmurzynska A. The multigene family of fatty acid-binding proteins: Function, structure and polymorphism. J Appl Genet. 2006;47:39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y2 receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Cohen EL, Wurtman RJ. Brain acetylcholine: control by dietary choline. Science. 1976;191:561–562. doi: 10.1126/science.1251187. [DOI] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Contreras MA, Greiner RS, Chang MC, Myers CS, Salem N, Jr, Rapoport SI. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J Neurochem. 2000;75:2392–2400. doi: 10.1046/j.1471-4159.2000.0752392.x. [DOI] [PubMed] [Google Scholar]
- Cornell RB. Cholinephosphotransferase from mammalian sources. Methods Enzymol. 1992;209:267–272. doi: 10.1016/0076-6879(92)09033-y. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Braun LD, Oldendorf WH. Carrier mediated blood-brain barrier transport of choline and certain choline analogs. J Neurochem. 1978;30:299–308. doi: 10.1111/j.1471-4159.1978.tb06530.x. [DOI] [PubMed] [Google Scholar]
- Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. J Neurochem. 1991;56:352–355. doi: 10.1111/j.1471-4159.1991.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue. J Mol Neurosci. 2001;16:263–272. doi: 10.1385/jmn:16:2-3:263. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Rapoport M. The synapsins: beyond the regulation of neurotransmitter release. Cell Mol Life Sci. 2002;59:589–595. doi: 10.1007/s00018-002-8451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Freund-Levi Y, Eriksdotter-Johnhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- Fujita A, Kurachi Y. SAP family proteins. Biochem Biophys Res Commun. 2000;269:1–6. doi: 10.1006/bbrc.1999.1893. [DOI] [PubMed] [Google Scholar]
- Green P, Yavin E. Elongation, desaturation, and esterification of essential fatty acids by fetal rat brain in vivo. J Lipid Res. 1993;34:2099–2107. [PubMed] [Google Scholar]
- Haga T, Noda H. Choline uptake systems of rat brain synaptosomes. Biochim Biophys Acta. 1973;291:564–575. doi: 10.1016/0005-2736(73)90508-7. [DOI] [PubMed] [Google Scholar]
- Hassam AG, Crawford MA. The differential incorporation of labeled linoleic, γ-linolenic, dihomo-γ-linolenic and arachidonic acids into the developing rat brain. J Neurochem. 1976;27:967–968. doi: 10.1111/j.1471-4159.1976.tb05163.x. [DOI] [PubMed] [Google Scholar]
- Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer’s disease. J Neuropathol Exp Neurol. 1999;58:637–643. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Hicks AM, DeLong CJ, Thomas MJ, Samuel M, Cui Z. Unique molecular signatures of glycerophospholipid species in different rat tissues analyzed by tandem mass spectrometry. Biochim Biophys Acta. 2006;1761:1022–1029. doi: 10.1016/j.bbalip.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Holub BJ. Differential utilization of 1-palmitoyl and 1-stearoyl homologues of various unsaturated 1,2-Diacyl-sn-glycerols for phosphatidylcholine and phosphatidylethanolamine synthesis in rat liver microsomes. J Biol Chem. 1978;253:691–696. [PubMed] [Google Scholar]
- Jenkins KJ, Kramer JKG. Effects of dietary corn oil and fish oil concentrate on lipid composition of calf tissues. J Diary Sci. 1990;73:2940–2951. doi: 10.3168/jds.S0022-0302(90)78983-7. [DOI] [PubMed] [Google Scholar]
- Kamp F, Westerhoff HV, Hamilton JA. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 1993;32:11074–11086. doi: 10.1021/bi00092a017. [DOI] [PubMed] [Google Scholar]
- Kennedy EM, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipids. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- Kizaki H, Williams JC, Morris HP, Weber G. Increased cytidine-5′-triphosphate synthetase activity in rat and human tumors. Cancer Res. 1980;40:3921–3927. [PubMed] [Google Scholar]
- Klein J, Koppen A, Loffelholz K. Small rises in plasma choline reverse the negative arteriovenous difference of brain choline. J Neurochem. 1990;55:1231–1236. doi: 10.1111/j.1471-4159.1990.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Klein J, Gonzales R, Koppen A, Loffelholz K. Free choline and choline metabolites in rat brain and body fluids: sensitive determination and implications for choline supply to the brain. Neurochem Int. 1993;22:293–300. doi: 10.1016/0197-0186(93)90058-d. [DOI] [PubMed] [Google Scholar]
- Kothapalli KSD, Anthony JC, Pan BS, Hsieh AT, Nathanielsz PW, Brenna JT. Differential cerebral cortex transcriptomes of baboon neonates consuming moderate and high docosahexaenoic acid formulas. PLoS ONE. 2007;2:e370. doi: 10.1371/journal.pone.0000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lands WEM, Inoue M, Sugiura Y, Okuyama H. Selective incorporation of polyunsaturated fatty acids into phosphatidylcholine by rat liver microsomes. J Biol Chem. 1982;257:14968–14972. [PubMed] [Google Scholar]
- Li JY, Boado RJ, Pardridge WM. Cloned blood-brain barrier adenosine transporter is identical to the rat concentrative Na+ nucleoside cotransporter CNT2. J Cereb Blood Flow Metab. 2001;21:929–936. doi: 10.1097/00004647-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Lieberman I. Enzymatic amination of uridine triphosphate to cytidine triphosphate. J Biol Chem. 1956;222:765–775. [PubMed] [Google Scholar]
- Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant a-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- Lopez-Coviella I, Agut J, Savci V, Ortiz JA, Wurtman RJ. Evidence that 5′-Cytidinediphosphocholine can affect brain phospholipid composition by increasing choline and cytidine plasma levels. J Neurochem. 1995;65:889–894. doi: 10.1046/j.1471-4159.1995.65020889.x. [DOI] [PubMed] [Google Scholar]
- Mandel P, Edel-Harth S. Free nucleotides in the rat brain during post-natal development. J Neurochem. 1966;13:591–595. doi: 10.1111/j.1471-4159.1966.tb11955.x. [DOI] [PubMed] [Google Scholar]
- Mages F, Rey C, Fonlupt P, Pacheco H. Kinetic and biochemical properties of CTP:choline-phosphate cytidylyltransferase from the rat brain. Eur J Biochem. 1988;178:367–372. doi: 10.1111/j.1432-1033.1988.tb14459.x. [DOI] [PubMed] [Google Scholar]
- Mantel CR, Schulz AR, Miyazawa K, Broxmeyer HE. Kinetic selectivity of cholinephosphotransferase in mouse liver: the Km for CDP-choline depends on diacylglycerol structure. Biochem J. 1993;289:815–820. doi: 10.1042/bj2890815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni F, Mosconi C, Galella G, Galli C. Increments of dietary linoleate raise liver arachidonate, but markedly reduce heart n-6 and n-3 fatty acids in the rat. Lipids. 1992;27:624–628. doi: 10.1007/BF02536121. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Kitidis C, DiRusso CC, Lodish HF. Long-chain acyl-CoA synthetase 6 preferentially promotes DHA metabolism. J Biol Chem. 2005;280:10817–10826. doi: 10.1074/jbc.M411750200. [DOI] [PubMed] [Google Scholar]
- Millington WR, Wurtman RJ. Choline administration elevates brain phosphorylcholine levels. J Neurochem. 1982;38:1748–1752. doi: 10.1111/j.1471-4159.1982.tb06658.x. [DOI] [PubMed] [Google Scholar]
- Mohrhauer H, Holman RT. Alteration of the fatty acid composition of brain lipids by varying levels of dietary essential fatty acids. J Neurochem. 1963;10:523–530. doi: 10.1111/j.1471-4159.1963.tb09855.x. [DOI] [PubMed] [Google Scholar]
- Moore SA, Yoder A, Murphy S, Spector AA. Role of the blood-brain barrier in the formation of long-chain w-3 and w-6 fatty acids from essential fatty acid precursors. J Neurochem. 1990;55:391–402. doi: 10.1111/j.1471-4159.1990.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Moore SA, Yoder A, Murphy S, Dutton GR, Spector AA. Astrocytes, not neurons, produce docosahexaenoic acid (22:6w-3) and arachidonic acid (20:4w-6) J Neurochem. 1991;56:518–524. doi: 10.1111/j.1471-4159.1991.tb08180.x. [DOI] [PubMed] [Google Scholar]
- Morisaki N, Saito Y, Kumagai A. Synthesis and metabolism of arachidonyl- and eicosapentaenoyl-CoA in rat aorta. Biochem Biophys Acta. 1983;752:301–306. [PubMed] [Google Scholar]
- Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci USA. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JS, Sampson EL. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J Lipid Res. 1965;6:545–551. [PubMed] [Google Scholar]
- Orengo A. Regulation of enzymic activity by metabolites: I. Uridine-cytidine kinase of Novikoff ascites rat tumor. J Biol Chem. 1969;244:2204–2209. [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of a-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–1265. [PubMed] [Google Scholar]
- Philbrick DJ, Mahadevappa VG, Ackman RG, Holub BJ. Ingestion of fish oil or a derived n-3 fatty acid concentrate containing eicosapentaenoic acid (EPA) affects fatty acid compositions of individual phospholipids of rat brain, sciatic nerve and retina. J Nutr. 1987;117:1663–1670. doi: 10.1093/jn/117.10.1663. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in nerve growth factor-differentiated pheochromocytoma cells. Neuroscience. 2005;134:207–214. doi: 10.1016/j.neuroscience.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Rabin O, Deutsch J, Grange E, Pettigrew KD, Chang MCJ, Rapoport SI, Purdon AD. Changes in cerebral acyl-CoA concentrations following ischemia-reperfusion in awake gerbils. J Neurochem. 1997;68:2111–2118. doi: 10.1046/j.1471-4159.1997.68052111.x. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Chang MCJ, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- Rapoport SI. In vivo approaches and rationale for quantifying kinetics and imaging brain lipid metabolic pathways. Prostaglandins Other Lipid Mediat. 2005;77:185–196. doi: 10.1016/j.prostaglandins.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Reddy TS, Bazan NG. Kinetic properties of arachidonoyl-coenzyme A synthetase in rat brain microsomes. Arch Biochem Biophys. 1983;226:125–133. doi: 10.1016/0003-9861(83)90277-1. [DOI] [PubMed] [Google Scholar]
- Reddy TS, Sprecher P, Bazan NG. Long-chain acyl-coenzyme A synthetase from rat brain microsomes. Kinetic studies using [1-14C]docosahexaenoic acid substrate. Eur J Biochem. 1984;145:21–29. doi: 10.1111/j.1432-1033.1984.tb08517.x. [DOI] [PubMed] [Google Scholar]
- Redzic ZB, Biringer J, Barnes K, Baldwin SA, Al-Sarraf H, Nicola PA, Young JD, Cass CE, Barrand MA, Hlandky SB. Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells. J Neurochem. 2005;94:1420–1426. doi: 10.1111/j.1471-4159.2005.03312.x. [DOI] [PubMed] [Google Scholar]
- Richardson UI, Watkins CJ, Pierre C, Ulus IH, Wurtman RJ. Stimulation of CDP-choline synthesis by uridine or cytidine in PC12 rat pheochromocytoma cells. Brain Res. 2003;971:161–167. doi: 10.1016/s0006-8993(03)02333-3. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Blusztajn JK, Sherwin A, Lozano A, Kish SJ. Phospholipid biosynthetic enzymes in human brain. Lipids. 1997;32:351–358. doi: 10.1007/s11745-997-0044-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Wurtman RJ. Increased dendritic spine density in adult gerbil hippocampus following oral UMP and DHA supplementation; 10th International Conference on Alzheimer’ Disease and Related Disorders; Madrid, Spain. 2006. [Google Scholar]
- Sarda N, Gharib A, Moliere P, Grange E, Bobillier P, Lagarde M. Docosahexaenoic acid (cervonic acid) incorporation into different brain regions in the awake rat. Neurosci Lett. 1991;123:57–60. doi: 10.1016/0304-3940(91)90157-o. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shimizu F, Watanabe TK, Shinomiya H, Nakamura Y, Fujiwara T. Isolation and expression of a cDNA for human brain fatty acid-binding protein (B-FABP) Biochim Biophys Acta. 1997;1354:24–28. doi: 10.1016/s0167-4781(97)00115-2. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Collins FD. The effect of dietary essential fatty acids on the concentration of serum and liver lipids in the rat. Br J Nutr. 1970;24:971–982. doi: 10.1079/bjn19700100. [DOI] [PubMed] [Google Scholar]
- Soderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phosholipids in aging and in Alzheimer’s disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- Spanner S, Ansell GB. Choline kinase and ethanolamine kinase activity in the cytosol of nerve endings from rat forebrain. Biochem J. 1979;178:753–760. doi: 10.1042/bj1780753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R. Uridine transport and metabolism in the central nervous system. J Neurochem. 1985;45:1411–1418. doi: 10.1111/j.1471-4159.1985.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ British J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg A, Svennerholm L. Plasma total lipids, cholesterol, triglycerides, phospholipids and free fatty acids in a healthy Scandinavian population. Acta Med Scand. 1961;169:43–49. [Google Scholar]
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9:570–579. [PubMed] [Google Scholar]
- Teng FYH, Wang Y, Tang BL.The syntaxins Genome Biol 20012 Reviews 3012–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am J Physiol. 1994;267:R1273–R1279. doi: 10.1152/ajpregu.1994.267.5.R1273. [DOI] [PubMed] [Google Scholar]
- Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Ward GR, Huang YS, Bobik E, Xing H-C, Mutsaers L, Auestad N, Montalto M, Wainwright P. Long-chain polyunsaturated fatty acid levels in formulae influence deposition of docosahexaenoic acid and arachidonic acid in brain and red blood cells of artificially reared neonatal rats. J Nutr. 1998;128:2473–2487. doi: 10.1093/jn/128.12.2473. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000;60:989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Ulus IH, Cansev M, Watkins CJ, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006;1088:83–92. doi: 10.1016/j.brainres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Yamamura HI, Snyder SH. High affinity transport of choline into synaptosomes of rat brain. J Neurochem. 1973;21:1355–1374. doi: 10.1111/j.1471-4159.1973.tb06022.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Vessby B, Nilsson A. Quantitative role of plasma free fatty acids in the supply of arachidonic acid to extrahepatic tissues in rats. J Nutr. 2002;132:2626–2631. doi: 10.1093/jn/132.9.2626. [DOI] [PubMed] [Google Scholar]