Abstract
It is unknown whether nutritional deficiencies affect the morphology and function of structural cells, such as epithelial cells, and modify the susceptibility to viral infections. We developed an in vitro system of differentiated human bronchial epithelial cells (BEC) grown either under selenium adequate (Se+) or selenium deficient (Se-) conditions, to determine whether selenium deficiency impairs host defense responses at the level of the epithelium. Se- BECs had normal SOD activity, but decreased activity of the selenium-dependent enzyme GPX1. Interestingly, catalase activity was also decreased in Se- BECs. Both Se- and Se+ BECs differentiated into a mucociliary epithelium; however, Se- BEC demonstrated increased mucus production and increased Muc5AC mRNA levels. This effect was also seen in Se+ BEC treated with 3-aminotriazole, and inhibitor of catalase activity, suggesting an association between catalase activity and mucus production. Both Se- and Se+ were infected with influenza A/Bangkok/1/79 and examined 24 hours post-infection. Influenza-induced IL-6 production was greater while influenza-induced IP-10 production was lower in Se- BECs. In addition, influenza-induced apoptosis was greater in Se- BEC as compared to the Se+ BECs. These data demonstrate that selenium deficiency has a significant impact on the morphology and influenza-induced host defense responses in human airway epithelial cells.
Keywords: Influenza, selenium, bronchial epithelial cells, in vitro
Introduction
The trace element selenium (Se) is an essential nutrient for all mammalian species and is of fundamental importance for human biology. Se functions primarily through selenoproteins, which contain selenocysteine, the 21st amino acid and all selenoproteins with enzymatic activities contain selenocysteine at their active site. Selenocysteine is specifically incorporated into selenoproteins through a cotranslational event directed by the UGA codon [1]. Of the selenoproteins that have been identified and characterized thus far, the groups of glutathione peroxidases and thioredoxin reductases maintain cellular redox homeostasis and the group of iodothyronine deiodinases maintains thyroid hormone metabolism [2], indicating that adequate Se status is of utmost importance.
In the lung, Se levels may be associated with lung function parameters. For example, higher serum Se levels are positively associated with higher FEV1 and are protective for lung function [3,4]. In addition, several epidemiological studies have observed lowered serum Se levels in asthma patients [5-8], yet the level of Se deficiency that is associated with asthma is not clearly established. In a review of several randomized controlled trials studying the efficiency of Se supplementation treatment in chronic asthma [9], one trial found that Se supplementation produced improvement in subjective symptoms for patients with chronic asthma. However, these improvements could not be validated by significant changes in separate clinical parameters of lung function [10]. Thus, the role and importance of Se in asthma is not yet clear.
Several studies have shown that nutritional deficiencies in certain trace elements, such as Se, can significantly impair immune defense parameters. For example, low serum Se levels were associated with low percentage of NK cells, especially in women [11]. In addition, deprivation of serum Se in HIV-infection is associated with increased levels of markers of disease progression and inflammatory response [12]. Furthermore, addition of Se enhances the phagocytic and bacteriacidal functions of human neutrophils in vitro [13] and Se deficiency increases macrophage PGE2 and TGF-ß production in rats [14], suggesting that adequate Se status is crucial for proper functioning of these cell types.
With regards to host defense against invading pathogens, numerous studies have shown that nutritional deficiency in vitamins or trace elements enhances the susceptibility to infections [15-17] including respiratory virus infections [18,19]. Generally, it is thought that this is a result of the effects of the nutritional deficiency on the immune system and the ability to fight the infection. However, recent studies have demonstrated that nutritional deficiencies of the host can also affect viral pathogens themselves [18] resulting in viral species that are more virulent than the parent species. For example, a strain of influenza virus that results in a mild infection when given to mice, influenza A/Bangkok/1/79, exhibited increased virulence when given to Se deficient mice [20]. Furthermore, virus subsequently isolated from such Se deficient mice had significant changes in the viral genome and exhibited greater virulence when given to mice fed a normal diet [20]. Thus, besides affecting the immune system and the ability to fight an infection, deficiencies in trace elements such as Se can also modify the virulence of the virus.
Although, as indicated above, numerous studies have examined the effects of nutritional deficiencies on the immune system, there are few studies investigating the effects of nutritional deficiencies on histological and functional changes of structural cells, such as airway epithelial cells. More importantly, since influenza virus predominantly infects and replicates in epithelial cells lining the respiratory tract, changes in airway epithelial cell morphology and function could potentially alter the ability of host cells to respond to an influenza virus infection. Therefore, we developed an in vitro model of Se-deficient human bronchial epithelial cells and determined the effects of Se deficiency on mucociliary differentiation, antioxidant enzyme balance, and influenza-induced cytokine production. Our data show that Se deficiency enhances mucus production, modifies influenza-induced IL-6 and IP-10 production, and increases influenza-induced apoptosis in bronchial epithelial cells, suggesting that effects of Se deficiency on structural cells could modify the immune response to influenza infections.
Materials and Methods
Cell Culture
Primary human bronchial epithelial cells were obtained from healthy nonsmoking adult volunteers by cytologic brushing at bronchoscopy as described before [21]. Human bronchial epithelial cells were expanded to passage 2 in bronchial epithelial growth medium (BEGM, Cambrex Bioscience Walkersville, Inc., Walkersville, MD ) and then plated on collagen-coated filter supports with a 0.4 μM pore size (Trans-CLR; Costar, Cambridge, MA) and cultured in a 1:1 mixture of bronchial epithelial cell basic medium (BEBM) and DMEM-H with SingleQuot supplements (Cambrex), bovine pituitary extracts (13mg/ml), bovine serum albumin (BSA, 1.5 μg/ml), and nystatin (20 units). Upon confluency, all-trans retinoic acid was added to the medium and air liquid interface (ALI) culture conditions (removal of the apical medium) were created to promote differentiation. From this point on, half of the samples were cultured in a BEGM : DMEM-H mixture that contained specially formulated Se-deficient BEBM (Se- BEBM, Cambrex Bioscience Walkersville, Inc.) (see schematic in Figure 1). In some experiments, differentiated bronchial epithelial cells were treated with 3-Aminotriazole (50mM; Sigma).
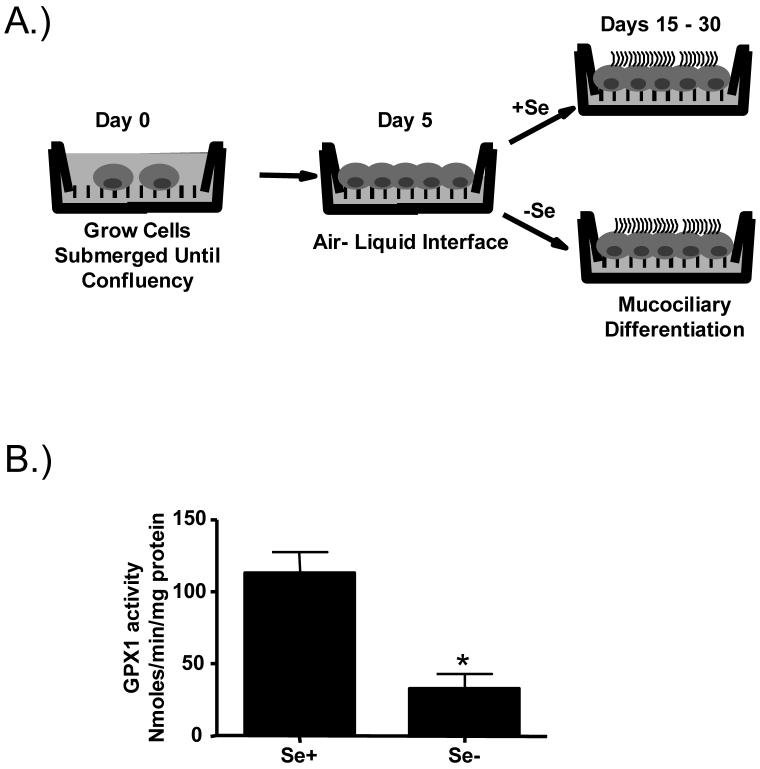
Figure 1.
In vitro model of Se-deficient human bronchial epithelial cells. A) Schematic of in vitro differentiation of bronchial epithelial cells under Se-deficient and adequate conditions. B.) GPX1 activity in lysates from differentiated Se adequate (Se+) and Se deficient (Se-) bronchial epithelial cells. * significantly different from Se+ controls; p<0.05
Analysis of selenium levels
Selenium content in the ALI medium was determined as described before [22] by flow injection hydride generation- /in-situ/ trapping in graphite furnace- atomic absorption spectrometry. An Analyst 800 spectrometer (Perkin-Elmer, Norwalk, Mass, U.S.A.) equipped with FIAS 400 flow injection accessory was employed. Spectrometer parameters were set as recommended by manufacturer, Se System II Electrodeless discharge lamp (Perkin-Elmer) as the radiation source with End-capped graphite tubes permanently modified with 40 μg of iridium were used for trapping and atomization. Conditions for hydride generation were: carrier gas flow rate 50 ml/min, HCl (1M) flow rate 4.5 ml/min, NaBH4 (0.5%) in KOH (0.4%) with flow rate 1.7 ml/min, waste flow rate from separator15 ml/min. Sample coil volume was 500 μl. Samples were diluted 1:1 by HCl (1 M) before analysis. Calibration was performed by the method of standard additions. Limit of detection of the method was 22 pg of Se, corresponding to 0.044 ng/ml or 0.56nM.
Analysis of antioxidant enzyme activities
Cellular activity levels for glutathione peroxidase 1 (GPX1), catalase, and superoxide dismutase (SOD) were measured by the NIH funded Biochemistry Core of the UNC Clinical Nutrition Research Unit (DK56350) according to previously published methods [23-25].
Immunohistochemistry
For the analysis of mucus production, paraformaldehyde-fixed cultures of differentiated nasal and bronchial epithelial cells were embedded in paraffin and 0.4 micron sections were used for histochemical analysis of mucous glycoconjugates using alcian blue/periodic acid-Schiff (AB/PAS) staining. For immunohistochemical analysis of the presence of cilia, paraformaldehyde-fixed whole mounts of differentiated bronchial epithelial cells were incubated with antibodies against acetylated α-tubulin (Invitrogen) at 4°C overnight and fluorescently-labeled secondary antibodies (Alexa-488 conjugated anti-mouse antibody; Invitrogen). Immunofluorescence was visualized by epifluorescence using a Nikon Microphot-SA fluorescent microscope as described before [21]. Differentiated epithelial cells were examined for the presence of fragmented DNA in apoptotic cells by the terminal deoxynucleotidyl transferase UTP nick end labelling (TUNEL) technique, using the Roche TUNEL staining kit (Roche; Indianapolis, IN). For the immunohistochemical analysis of influenza-infected cells and localization of apoptotic cells, acetone-fixed whole mounts of differentiated bronchial epithelial cells were incubated with antibodies against influenza A (Argene, North Massapequa, NY) and activated Caspase 3 (Cell Signaling), respectively, followed by incubation with either Alexa-488 conjugated anti-mouse antibodies (for influenza) or Alexa-594 conjugated anti-rabbit antibodies (for Caspase 3). Cells were washed with TBS and coverslipped using VectaShield with DAPI (Vector Labs, Burlingame, CA), to stain the nuclei. Immunofluorescence was visualized by use of a Zeiss 510 laser scanning microscope at the Michael Hooker Microscopy Core Facility at the University of North Carolina at Chapel Hill as described before [26].
Western blotting
Whole cell lysates were prepared by lysing the cells in RIPA buffer containing 1% Nonidet P (NP)-40, 0.5% deoxycholate, 0.1% SDS, and protease inhibitors (Cocktail Set III; Calbiochem, San Diego, CA). One hundred micrograms of whole cell lysate was separated by SDS-PAGE as described before [21]. This was followed by immunoblotting using specific antibodies to acetylated α-tubulin (1:2000, Invitrogen), or cytokeratin 13 (1:1000, Novacastra Laboratories Ltd., Newcastle upon Tyne, UK). Antigen-antibody complexes were stained with anti-rabbit or anti-mouse, horseradish peroxidase-conjugated antibody (1:4000, Santa Cruz Biotechnology) and SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). The chemiluminescent signals were acquired using a 16-bit CCD camera (GeneGnome system; Syngene, Frederick, MD) and visualized using the GeneSnap software (Syngene).
Infection with influenza
Throughout this study we used influenza A/Bangkok/1/79 (H3N2 serotype) which was propagated in 10-day-old embryonated hen’s eggs. The virus was collected in the allantoic fluid and titered by 50% tissue culture infectious dose in Madin-Darby canine kidney cells and hemagglutination as described before [27]. Stock virus was aliquoted and stored at -80°C until use. For infection of differentiated bronchial epithelial cells approximately 3 × 105 cells were incubated with 320 HAU of influenza A Bangkok 1/79 from the apical side for 1 hour, after which the remaining unattached virus was removed. This infection protocol results in a mild infection in which about 10-20% of the cells become infected with influenza A. Throughout the study, effects of selenium deficiency on influenza virus infections were assessed 24 hours post-infection.
RT-PCR
Total RNA was extracted using TRizol (Invitrogen) as per the supplier’s instruction. First-strand cDNA synthesis and real-time RT-PCR was performed as described previously [21;26;28]. The sequences for the Taqman primers and probes used in this study are as following: HA: probe, 5′-FAM-TGATGGGAAAAACTGCACACTGATAGATGC-TAMRA-3′; sense, 5′-CGACAGTCCTCACCGAATCC-3′; antisense, 5′-TCACAATGAGGGTCTCCCAATAG-3′; Muc5AC: probe, 5′ -FAM-CATACAGCCATGCCCAGGATGG-TAMRA-3′; sense, 5′ -GAGTGTTGGCCGGAGGAA-5′; antisense, 5′ -GGGCAGGGTGGTGCTTGTA-3′; GAPDH: probe, 5′-JOE-CAAGCTTCCCGTTCTCAGCC-TAMRA-3′; sense, 5′-GAAGGTGAAGGTCGGAGTC-3′; antisense, 5′-GAAGATGGTGATGGGATTTC-3′. For the analysis of catalase mRNA levels, real-time PCR was conducted using commercially available primer and probe sets for human catalase and normalized to β-actin mRNA levels (both from Applied Biosystems) according to the supplier’s instructions. For all PCR analyses, relative quantitation of mRNA levels was done using a standard curve obtained through serial dilutions of a reference sample known to express high levels of the respective target mRNA.
Analysis of cytotoxicity and cytokine production
Cell culture supernatants from the basolateral compartment were collected 24 hours post-infection and stored at -20°C until analysis. For analysis of cytotoxicity, supernatants were analyzed for the presence of lactate dehydrogenase (LDH), a marker of necrotic cell death, using a commercially available kit (TaKaRa, Shiga, Japan ) according the supplier’s instructions. IL-6 and IP-10 levels were determined using commercially available ELISA kits (BD™ OptEIA ELISA kits, BD Bioscience, San Jose, CA) according to the manufacturer’s instructions.
Statistical Analysis
Data are expressed as means ± S.E.M. of at least three separate experiments. The ELISA and LDH data in figures 6 and 7 were expressed as fold change of Se-deficient over Se-adequate cells and analyzed using the Wilcoxon Signed Rank Test, assuming a theoretical mean of 1. All other data were analyzed using paired t-tests. A value of P <0.05 was considered to be significant.
Figure 6.
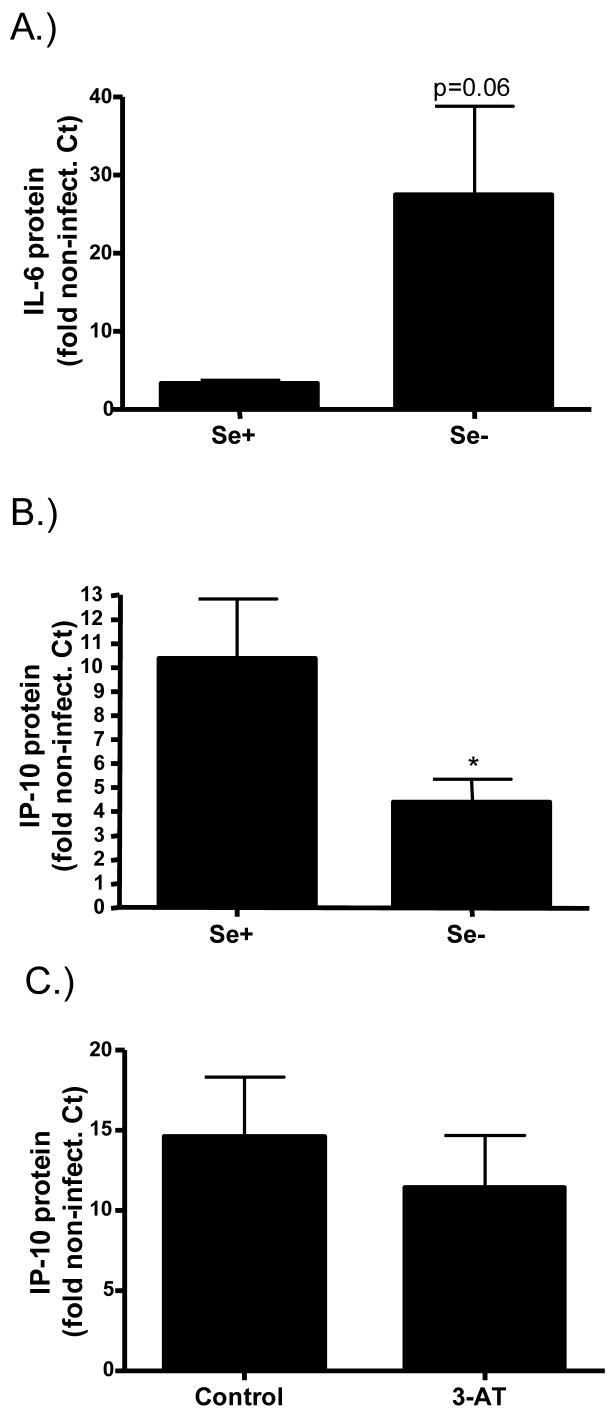
Effects of Se deficiency on influenza-induced cytokine production. Basolateral supernatants from influenza-infected Se+ and Se- bronchial epithelial cells were analyzed for A.) IL-6 and B.) IP-10 levels 24 hours post-infection and expressed as fold change over the levels present in non-infected control cells. *significantly different from Se+ cells; p<0.05 C.) Differentiated bronchial epithelial cells were treated with 50mM 3-Aminotriazole (3-AT) and infected with influenza. IP-10 levels were analyzed in basolateral supernatants collected 24 hours post-infection and expressed as fold change over the levels present in non-infected control cells.
Figure 7.
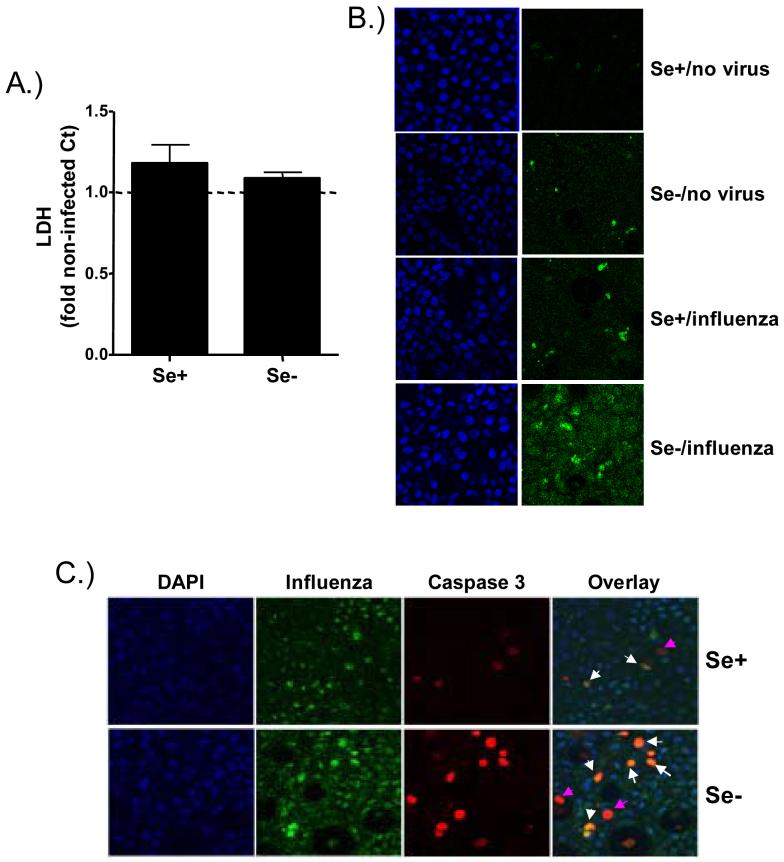
Effects of Se deficiency on influenza-induced cell death. A.) Basolateral supernatants from influenza-infected Se+ and Se- bronchial epithelial cells were analyzed for LDH levels and expressed as fold change over the levels present in non-infected control cells. B.) and C.) Cultures of differentiated Se+ and Se- bronchial epithelial cells were fixed in acetone and examined B.) for detection of apoptotic cells using TUNEL assay (FITC) and nuclear staining using DAPI (blue) or C.) for co-localization of apoptotic and influenza infected cells using anti-influenza A antibodies (green) and anti-activated caspase 3 antibodies (red) and visualized using confocal microscopy.
Results
In Vitro Model of Se-deficient human respiratory epithelial cells
Previous studies have shown that primary human bronchial epithelial cells can undergo mucociliary differentiation in vitro when grown under defined culture conditions [21;29;30]. We have expanded this cell culture model and developed an in vitro model of Se-deficient bronchial epithelial cells. Figure 1A is a schematic of this in vitro model, which is described in more detail in the Materials and Method section. Briefly, human bronchial epithelial cells are grown submerged on collagen-coated membranes. Upon reaching confluency, the media in the apical compartment is removed to establish air-liquid interface condition. At this point the cell culture samples are divided into two groups: 1.) Se-adequate group (Se+), which receives normal media with about 4nM of Se and 2.) Se-deficient group (Se-), which receives media that is deficient (i.e. <0.56 nM) in Se. Mucociliary differentiation is achieved within 15-30 days, upon which the cell culture samples are ready to be used. To assure that Se-deficiency was achieved by culturing and differentiating bronchial epithelial cells in Se-deficient media, we analyzed the activity of the Se-dependent antioxidant enzyme glutathione peroxidase-1 (GPX1). Figure 1B shows that differentiation in Se-deficient media significantly decreased GPX1 activity, indicating that cellular Se-deficiency was achieved.
Effects of Se-deficiency on mucociliary differentiation
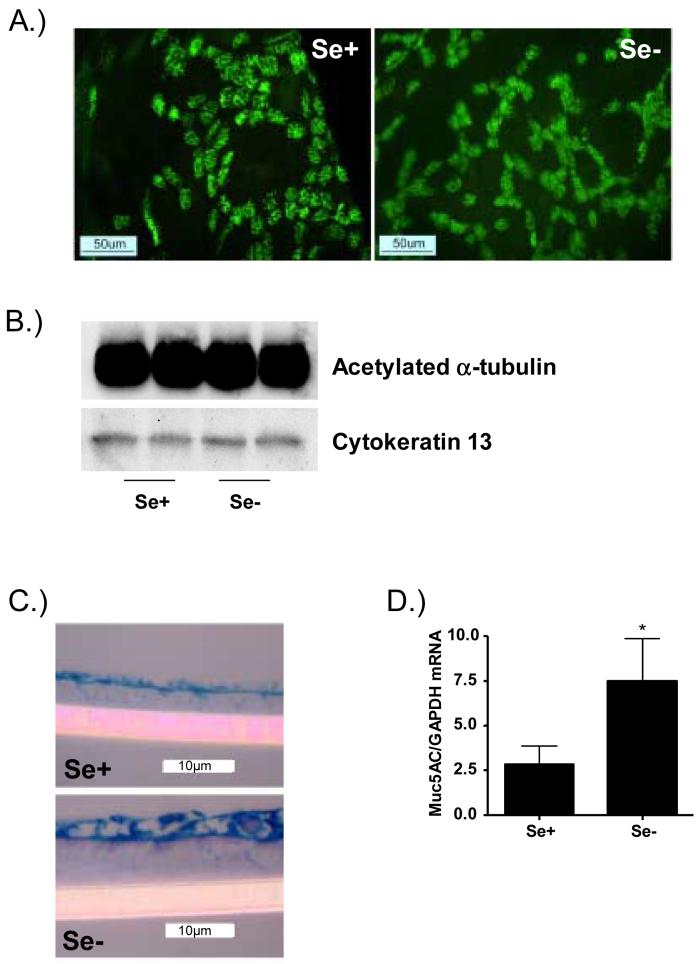
Previous studies have demonstrated that nutritional deficiencies can lead to ultrastructural changes in the cell [31;32]. To determine whether Se-deficiency modified mucociliary differentiation and epithelial cell morphology, we analyzed the level of ciliation in these cells by immunohistochemistry using anti-acetlyated α-tubulin antibodies, since acetylated α-tubulin is a major component of cilia. Differentiated Se+ and Se- bronchial epithelial cells were fixed in paraformaldehyde, stained for acetylated α-tubulin, and examined using an epifluorescence microscope. Figure 2A shows that anti-acetylated α-tubulin antibody strongly reacts with cilia and that Se+ and Se- bronchial epithelial cells contain large beds of ciliated cells. In addition, Se-deficiency did not significantly modify the level of ciliation in bronchial epithelial cells. We also confirmed these observations by analyzing the expression of acetylated α-tubulin in whole cell lysates from both Se- and Se+ bronchial epithelial cells, which would yield a more quantitiative analysis of ciliation throughout the entire sample. Figure 2B confirms the findings shown in figure 2A in that there was no observable difference in markers of ciliation between Se- and Se+ bronchial epithelial cells. In addition, we also analyzed the level of cytokeratin 13, a marker of squamous cells. Figure 2B demonstrates that Se deficiency had no significant effect on the level of cytokeratin 13. To determine whether Se-deficiency modifies mucus production in differentiated bronchial epithelial cells, paraffin-embedded sections were stained for mucus glycoproteins using AB/PAS. Figure 2C shows that Se+ bronchial epithelial cells are covered by a layer of mucus and that Se-deficiency increased the layer of mucus lining these cells. Although the mucus layer covering the bronchial epithelial cells appeared greater in Se- cells, there was no apparent increase in the number of goblet cells. To confirm the observations on increased mucus layer, we analyzed mRNA levels for Muc5AC, one of the predominant mucins produced by human respiratory epithelial cells [33]. Figure 2D shows that in bronchial epithelial cells Se-deficiency significantly increases Muc5AC mRNA levels. Taken together these data indicate that Se-deficiency increases mucus production in bronchial epithelial cells.
Figure 2.
Effect of Se deficiency on the morphology of differentiated bronchial epithelial cells. A.) Ciliated cells in cultures of differentiated Se-adequate (Se+) and Se-deficient (Se-) bronchial epithelial cells were visualized using indirect immunofluorescent localization of acetylated α-tubulin. B.) Whole cell lysates from Se+ and Se- bronchial epithelial cells were examined for acetylated α-tubulin and cytokeratin 13 levels Western blotting. Representative immunoblots for acetylated α-tubulin and cytokeratin 13 from two separate samples are shown. C.) Sections of paraffin-embedded samples of differentiated Se adequate (Se+) and Se deficient (Se-) bronchial epithelial cells were stained for mucus glycoproteins using AB/PAS. D.) Total RNA isolated from Se+ and Se- bronchial epithelial cells was analyzed for Muc5AC mRNA levels. Data were normalized for the expression of GAPDH and expressed as mean ± S.E.M. *significantly different from Se+ cells; p<0.05
Effects of Se-deficiency on antioxidant enzyme activity
Se is essential for the production and activity of antioxidant enzymes such as glutathione peroxidase-1 (GPX1), which we have shown was suppressed by Se-deficiency in bronchial epithelial cells (figure 1B). To examine whether Se-deficiency also modifies the activity of Se-independent antioxidant enzymes, we also determined catalase and Cu,Zn-SOD activity in these cells. Interestingly, while Cu,Zn-SOD activity did not change in Se-deficient bronchial epithelial cells (figure 3A), catalase activity was significantly lower in Se-deficient bronchial cells (figure 3B). To determine whether the decrease in catalase activity was caused by a suppression of catalase synthesis in Se-deficient cells, we analyzed catalase mRNA levels in Se+ and Se- cells. Figure 3C shows that the decrease in catalase activity was not caused by a decrease in catalase mRNA levels. These data suggest that Se-deficiency also affects the activity, but not the transcription, of Se-independent antioxidant enzyme activities in bronchial epithelial cells, and that the suppression of catalase activity in Se-deficient bronchial epithelial cells may render bronchial epithelial cells even more oxidatively stressed.
Figure 3.
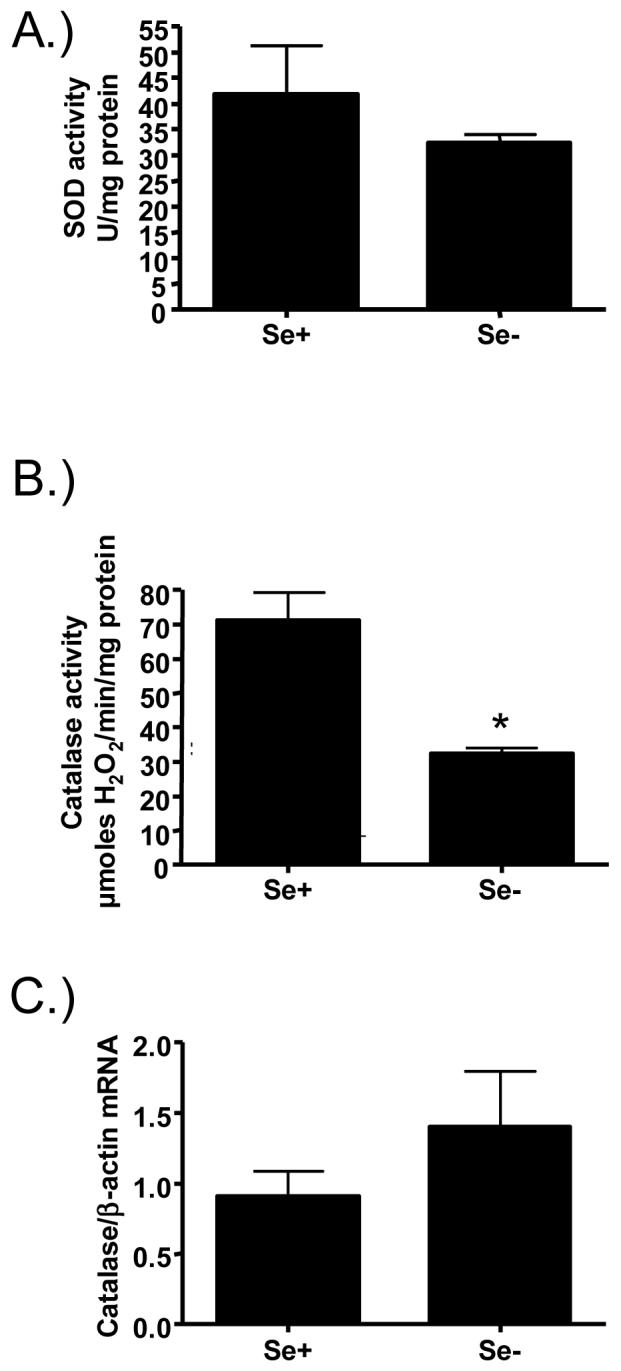
Effects of Se deficiency on antioxidant enzyme activities. Lysates from differentiated Se adequate (Se+) and Se deficient (Se-) bronchial epithelial cells were analyzed for A.) total superoxide dismutase (SOD) activity or B.) catalase activity. C.) Total RNA isolated from of Se+ and Se- bronchial epithelial cells was analyzed for catalase mRNA levels. Data were normalized for the expression of ß-actin. *significantly different from Se+ cells; p<0.05
Previous studies have demonstrated that oxidative stress can enhance mucus production in bronchial epithelial cells and that catalase can mitigate this effect [34]. To determine whether the increased mucus production seen in Se-deficient bronchial epithelial cells (Figures 2B and 2C) was caused by the effects of Se-deficiency on catalase activity, we treated normally differentiated bronchial epithelial with 3-aminotriazole (3-AT), an inhibitor of catalase activity, and subsequently analyzed Muc5AC mRNA levels. Figure 4 demonstrates that treatment with 3-AT significantly enhances Muc5AC mRNA levels in bronchial epithelial cells, suggesting that the effect of Se-deficiency on mucus production in bronchial epithelial cells may have been caused by the reduced catalase activity observed in these cells.
Figure 4.
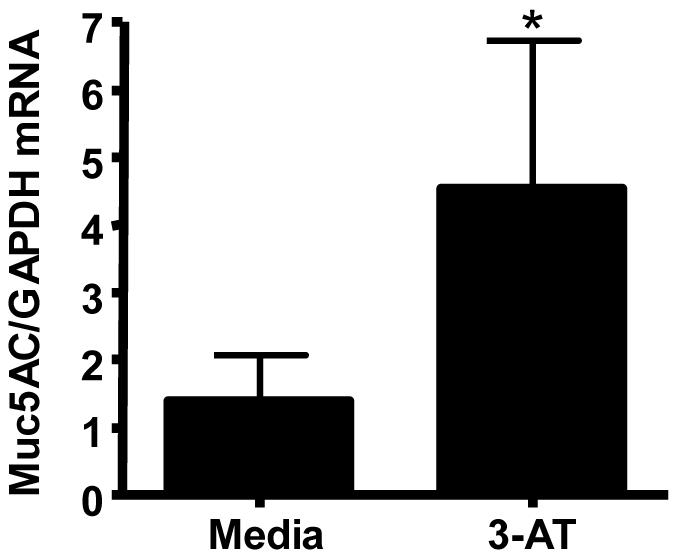
Effects of 3-Aminotriazole on Muc5AC expression. Differentiated bronchial epithelial cells were treated with 50mM 3-Aminotriazole (3-AT) for 24 hrs and analyzed for Muc5AC mRNA levels. Data were normalized to GAPDH mRNA. *significantly different from control cells; p<0.05
Effects of Se-deficiency on influenza virus replication in human bronchial epithelial cells
Because we demonstrated that Se-deficient bronchial epithelial cells had increased mucus production and decreased GPX1 and catalase activity, we wanted to determine if viral replication would be affected by Se status. The respiratory epithelium is the major site for influenza virus replication and it is reasonable to hypothesize that morphological changes of the respiratory epithelium could modify its susceptibility to influenza virus infections. Se+ and Se- bronchial epithelial cells were infected with influenza A/Bangkok/1/79 and examined for viral replication by influenza hemagglutinin (HA) RNA levels and immunohistochemistry 24 hours post-infection. Figure 5A shows that in bronchial epithelial cells, Se-deficiency had no significant effect on influenza virus HA RNA levels. Visualization of influenza-infected cells as shown in figure 5B demonstrated that both ciliated and non-ciliated epithelial cells are infected with influenza and that Se-deficiency had no significant effect on the number of influenza infected bronchial epithelial cells.
Figure 5.
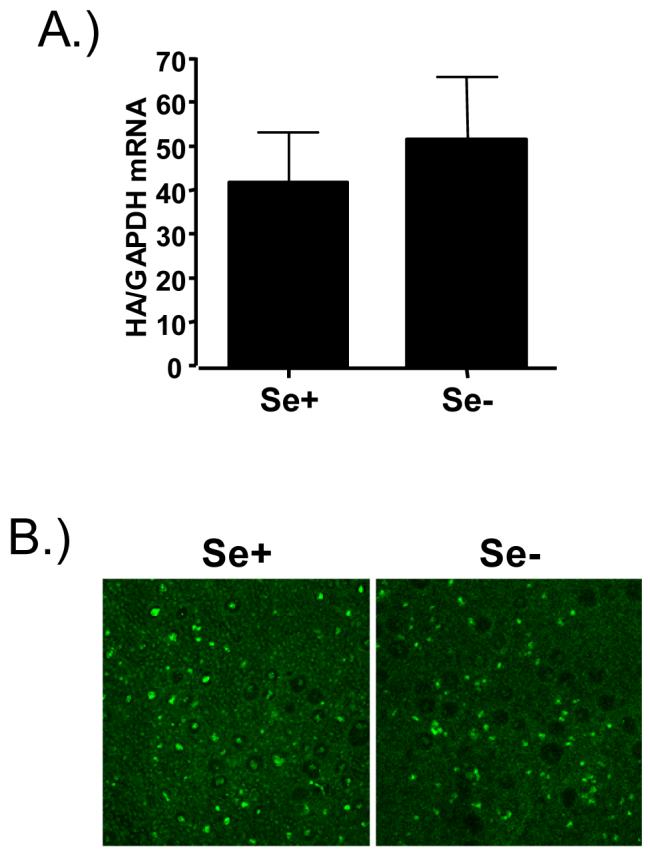
Effects of Se deficiency on influenza virus infections. A.) Total RNA isolated from of Se+ and Se- bronchial epithelial cells was analyzed for HA mRNA levels 24 hours post-infection. Data were normalized for the expression of GAPDH. B.) Acetone-fixed cultures of differentiated Se+ and Se- bronchial epithelial cells were labeled with anti-influenza A antibodies and visualized using confocal microscopy.
Effects of Se-deficiency on influenza-induced cytokine production in bronchial epithelial cells
Cytokines produced by influenza-infected cells are essential in recruiting and activating immune cells, whose goal it is to ultimately clear the infection. However, too much inflammation can be damaging to the surrounding tissue. Interleukin-6 (IL-6) is a pleiotropic pro-inflammatory cytokine released by epithelial cells in response to infection and has been shown to play a key role during infection. The expression of interferon-inducible protein 10 (IP-10) or CXCL10 is also upregulated by influenza infection. IP-10 recruits and activates T cells to the site of injury and is also a potent inducer of NK cell activation [35;36]. Thus, both IL-6 and IP-10 play important roles during influenza infections, with overlapping yet different roles. To determine the effects of Se-deficiency on influenza-induced cytokine production, we measured IL-6 and IP-10 protein levels in cell culture supernatants. Figure 6 shows that Se-deficiency enhanced influenza-induced IL-6 levels (figure 6A) but decreased influenza-induced IP-10 levels (figure 6B) in bronchial epithelial cells. Interestingly, treatment with 3-AT had no effect on influenza-induced IP-10 levels (figure 6C), suggesting that the effects of Se-deficiency on influenza-induced cytokine levels are not mediated by its effects on catalase activity.
Effects of Se-deficiency on influenza-induced cell death in bronchial epithelial cells
Infection with influenza can cause cell death and has been demonstrated to occur via predominantly apoptotic pathways [37]. To examine whether Se-deficiency modifies influenza-induced cell death, we analyzed markers of necrotic as well as apoptotic cell death in Se+ and Se- cells at 24 hours post influenza infection. Figure 7A demonstrates that influenza infection does not significantly increase the release of LDH, a marker of cell necrosis, in either Se+ or Se- cells. In addition, we examined the levels of apoptosis using TUNEL assay and immunohistochemical staining of cleaved and thus activated caspase 3, in both influenza-infected Se+ and Se- cells. Figure 7B demonstrates that non-infected Se+ and Se- cells had little to no appearance of TUNEL-positive apoptotic cells, which was increased by influenza infections in both Se+ and Se- cells. In addition, Influenza-infected Se+ and Se- cells were stained for both influenza and activated caspase 3 and examined using confocal microscopy to determine the level of influenza-induced apoptosis using another marker of apoptosis and whether infected or non-infected neighboring cells undergo apoptosis. Figure 7C demonstrates that Se-deficiency enhances the number of apoptotic cells and that while some apoptotic cells were non-infected cells and did not positively stain for influenza (pink arrows), the majority of apoptotic cells were also infected with influenza (white arrows). These data suggest that Se-deficiency enhances influenza-induced apoptosis.
Discussion
Numerous studies have demonstrated that nutritional deficiencies can significantly affect the susceptibility to viral infections, and many of these studies point to an impaired immune response as the main cause for these effects [15;38;39]. However, the susceptibility to respiratory virus infections could also depend on morphology and function of structural cells, such as respiratory epithelial cells, since these cells are the predominant cell type for respiratory virus replication. Very little is known whether nutritional deficiencies could induce functional and/or morphological changes of the respiratory epithelium, which could potentially alter the susceptibility to viral infections. Using a cell culture model of differentiated human airway epithelial cells our data presented here demonstrate that deficiency in the essential nutrient Se induces significant changes in epithelial morphology, influenza-induced cytokine production, as well as influenza-induced apoptosis, suggesting that effects of nutritional deficiencies on structural cells can significantly modify the susceptibility to invading pathogens.
Cellular GPX1 is one of the most abundant selenoproteins in mammals and is often used as a biomarker for Se status [40]. GPX1 is a tetrameric enzyme with each one of the four subunits containing one active site of selenocysteine residue. Therefore, a deficiency in Se leads to decreased GPX1 activity, as seen in our deficient bronchial epithelial cells and shown by others [41;42]. GPX1 and catalase overlap in their ability to detoxify hydrogen peroxide, with GPX1 also detoxifying lipid hydroperoxides. However, an unexpected finding was that catalase activity, a Se-independent enzyme, was also decreased under conditions of Se deficiency. Similar to GPX1, catalase is a tetramer. Within the tetramer are four porphyrin heme (iron) groups, which are necessary for catalase to interact with its substrate hydrogen peroxide and therefore are essential for catalase activity. There is evidence that Se affects iron metabolism, iron uptake, and the activity of other heme-containing enzymes. For example, rats fed Se-deficient diets showed decreased ferrochelatase activity, the enzyme required for heme synthesis, as well as heme levels in the intestine, which also led to the decreased activity of heme-containing enzyme cytochrome P-450 [43]. In addition, in splenic mononuclear cells isolated from rats, Se deficiency impaired transferrin receptor internalization [44], which is the major mechanism of nutritional iron uptake. Thus, Se deficiency could decrease catalase activity in bronchial epithelial cells by modifying iron uptake and/or heme synthesis.
In our in vitro model, differentiation of human bronchial epithelial cells into ciliated cells was not significantly affected by Se-deficiency. However, Se deficient bronchial epithelial cells demonstrated enhanced mucus production. Specifically, Se deficiency significantly enhanced Muc5AC expression and mucus glycoprotein levels covering bronchial epithelial cells. Interestingly, treatment with the catalase inhibitor 3-aminotriazole (3-AT) had similar effects on Muc5AC mRNA levels as did Se-deficiency, suggesting that the decreased catalase activity in Se-deficient cells is at least partially responsible for this effect. This is supported by previous studies, which have shown that hydrogen peroxide and other forms of oxidative stress can enhance mucus production in airway epithelial cells and that catalase can mitigate this effect [34;45-47]. Thus, enhanced levels of oxidative stress in Se-deficient cells caused by decreased GPX1 and catalase activity is likely the basis for the enhanced mucus production in Se-deficient bronchial epithelial cells. More recent work has started to delineate the mechanisms by which oxidative stress enhances mucus production and mucus cells metaplasia. Specifically, work by Casalino-Matsuda et al. demonstrated that in differentiated bronchial epithelial cells, reactive-oxygen intermediates (ROI) activated tissue kallikrein, a serine protease related to the formation of kinins, which in turn induced EGFR activation and enhanced expression of MUC5AC [45]. In addition, recombinant tissue kallikrein was able to mimic the effects of ROI with regards to MUC5AC expression. Furthermore, catalase was an inhibitor of tissue kallikrein [45], suggesting that decreased catalase activity could enhance tissue kallikrein activity and therefore be a potential mechanism by which Se-deficiency enhanced MUC5AC expression and mucus levels in bronchial epithelial cells. Interestingly, preliminary analysis of gene array data comparing Se-deficient and Se-adequate bronchial epithelial cells indicate that kallikrein expression may be enhanced in Se-deficient epithelial cells (data not shown), which we are currently examining further.
Although Se-deficient cells had increased mucus production and decreased GPX1 and catalase activities, Se deficiency did not have a significant effect on the ability of influenza virus to infect bronchial epithelial cells. However, despite the lack of significant effects on influenza virus replication, Se deficiency did modify influenza-induced cytokine release. Specifically, influenza-induced IL-6 release was enhanced, while IP-10 release was decreased in Se-deficient bronchial epithelial cells. This response was not due to differences in viral load, which were equivalent between infected Se-adequate and deficient cells. IL-6 is a pleiotropic pro-inflammatory cytokine, which is also a mediator of fever and the acute phase response. In addition, IL-6 induces the differentiation of B cells into antibody producing plasma cells and the activation of T cells. Thus, while IL-6 is essential for the resolution of the infection, an increased IL-6 release is likely to contribute to enhance the overall inflammatory response and symptoms associated with the infection. IP-10 is a chemoattractant for T lymphocytes and activator of NK cells, thus playing an important role in orchestrating the innate and adapative immune defense against the invading virus [35;36;48]. Therefore, a decreased release of IP-10 by influenza-infected epithelial cells would impair recruitment and activation of immune cells that are essential for the innate defense response in the lung as well as subsequent T cell infiltration. Previous studies have demonstrated that in an elderly population, decreased serum Se levels are associated with lower percent NK cells [11]. In addition, healthy human volunteers supplemented with Se demonstrated greater NK cell cytotoxicity [49], further strengthening the notion that decreased IP-10 released by Se-deficient epithelial cells could impair immune cell parameters. Taken together, the opposite effects of Se deficiency on influenza-induced IL-6 and IP-10 release by bronchial epithelial cells may enhance the overall inflammatory response to the virus and decrease the ability to recruit and activate immune cells to the site of infection, thus contributing to an overall impaired defense response against the infection.
The induction of apoptotic cell death in a variety of cell types is commonly observed following influenza infections both in vitro and in vivo [37;50-52]. Apoptotic cell death is often regarded as part of the host defense response, because it shuts down virus-replicating cells without inducing an inflammatory response. In addition, many viruses, including influenza virus, have developed strategies to prevent apoptosis of the host cell [53], which is another indication that apoptotic death of the host cell is unfavorable for the virus. Our data indicate that Se deficiency enhanced influenza-induced apoptosis and that the majority of cells undergoing apoptosis were infected and not non-infected neighboring cells. Specifically, we demonstrate here that in Se deficient cells influenza infections cause a greater level of activated caspase 3. Assuming that apoptosis is beneficial to the host, these data would suggest that Se deficiency primes epithelial cells to limit the infection. However, the role of apoptosis and the consequences of this process for influenza virus replication or host cell defense have been questioned. For example, recent data demonstrated that influenza virus replication was strongly impaired by caspase inhibitors and ectopic expression of caspase 3 significantly increased virus replication [54]. The mechanism of this effect appears to be mediated by enhanced export of viral RNP complexes through caspase 3 activation [54]. These data suggest that for its own propagation, influenza virus may have the ability to use components of the apoptotic process, which is supposed to shut down viral replication. Therefore, enhanced caspase 3 activation in Se-deficient influenza-infected cells may have a dual role and ultimately enhance propagation of the infection. However, in our in vitro cultures, viral load was not changed under conditions of Se deficiency, suggesting that apoptosis is not a major mechanism for enhancing or decreasing viral titers.
How Se deficiency enhances influenza-induced apoptosis is still unclear. Previous studies have shown that oxidative stress can induce apoptosis in airway epithelial cells [55;56], suggesting that the cellular oxidative stress caused by the suppressed GPX-1 and catalase activity seen in our Se-deficient bronchial epithelial cells could have been responsible for the enhanced apoptosis seen in these cells. This notion is further supported by studies demonstrating GPX1 and catalase activities influence the sensitivity to oxidative stress-induced apoptosis [57-59]. Thus, the suppressed antioxidant enzyme activities may have rendered Se-deficient epithelial cells more susceptible to influenza-induced apoptosis.
Taken together, the results presented here strongly suggest that nutritional deficiencies can modify the morphology of the respiratory epithelium and that these effects are associated with an altered response to influenza infection. While several studies have demonstrated effects of nutritional deficiencies on immune functions [15;38;39;60], our data demonstrates that nutritional deficiencies can have a significant impact on structural cells and their response to a viral infection. Interestingly, chronic lung diseases such as asthma, COPD, and cystic fibrosis are also associated with lower serum Se levels [4;61-63] and supplementation with Se has been shown to improve lung function in these patients [49]. All of these lung diseases are also marked by enhanced mucus production and exacerbation of disease symptoms by respiratory virus infections [64;65]. How decreased serum Se levels impact the pathogenesis of these chronic lung diseases is not well understood. However, our data suggest that the increased mucus production and an altered response to viral infection in Se-deficient bronchial epithelial cells could potentially contribute to the phenotype and enhanced susceptibility to viral infections in these lung diseases, as well as in normal individuals with marginal to deficient Se status.
Acknowledgements
We thank Lisa Dailey for her technical assistance and Dr Vlasta Korunova for her assistance with the analysis of media selenium levels. This work was supported in part by NIH grant AI055050 (MAB) and by the U.S. Environmental Protection Agency grant CR829522 (IJ) as well as support from the NIH funded Clinical Nutrition Research Unit (DK56350). Its content are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the U.S. EPA. The views expressed in this document are solely those of the authors. Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency through cooperative agreement CR829522 with the Center for Environmental Medicine, Asthma, and Lung Biology, it has not been subjected to the Agency’s required peer and policy review, and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
List of Abbreviations
- BEC
Bronchial Epithelial Cells
- GPX1
Glutathione Peroxidase 1
- SOD
Superoxide Dismutase
- Se
Selenium
- IL-6
Interleukin 6
- IP-10
Interferon inducible protein 10
- FEV1
Forced expiratory volume1
- NK cell
Natural Killer cell
- PGE2
Prostaglandin E2
- TGF-ß
Transforming Growth Factor ß
- 3-AT
3-aminotriazole
- HA
Hemagglutinin
- LDH
Lactate Dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heider J, Baron C, Bock A. Coding from a distance: dissection of the mRNA determinants required for the incorporation of selenocysteine into protein. EMBO J. 1992;11:3759–66. doi: 10.1002/j.1460-2075.1992.tb05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenzie RC, Arthur JR, Beckett GJ. Selenium and the regulation of cell signaling, growth, and survival: molecular and mechanistic aspects. Antioxid Redox Signal. 2002;4:339–51. doi: 10.1089/152308602753666398. [DOI] [PubMed] [Google Scholar]
- 3.Pearson P, Britton J, McKeever T, Lewis SA, Weiss S, Pavord I, Fogarty A. Lung function and blood levels of copper, selenium, vitamin C and vitamin E in the general population. Eur J Clin Nutr. 2005;59:1043–8. doi: 10.1038/sj.ejcn.1602209. [DOI] [PubMed] [Google Scholar]
- 4.Hu G, Cassano PA. Antioxidant nutrients and pulmonary function: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Epidemiol. 2000;151:975–81. doi: 10.1093/oxfordjournals.aje.a010141. [DOI] [PubMed] [Google Scholar]
- 5.Stone J, Hinks LJ, Beasley R, Holgate ST, Clayton BA. Reduced selenium status of patients with asthma. Clin Sci (Lond) 1989;77:495–500. doi: 10.1042/cs0770495. [DOI] [PubMed] [Google Scholar]
- 6.Flatt A, Pearce N, Thomson CD, Sears MR, Robinson MF, Beasley R. Reduced selenium in asthmatic subjects in New Zealand. Thorax. 1990;45:95–9. doi: 10.1136/thx.45.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw R, Woodman K, Crane J, Moyes C, Kennedy J, Pearce N. Risk factors for asthma symptoms in Kawerau children. N Z Med J. 1994;107:387–91. [PubMed] [Google Scholar]
- 8.Kadrabova J, Mad’aric A, Kovacikova Z, Podivinsky F, Ginter E, Gazdik F. Selenium status is decreased in patients with intrinsic asthma. Biol Trace Elem Res. 1996;52:241–8. doi: 10.1007/BF02789165. [DOI] [PubMed] [Google Scholar]
- 9.Allam MF, Lucane RA. Selenium supplementation for asthma. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003538.pub2. CD003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselmark L, Malmgren R, Zetterstrom O, Unge G. Selenium supplementation in intrinsic asthma. Allergy. 1993;48:30–6. [PubMed] [Google Scholar]
- 11.Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani E, Savarino L, Sassi S, Cucinotta D, Lenaz G. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged >/=90 y. Am J Clin Nutr. 2000;71:590–8. doi: 10.1093/ajcn/71.2.590. [DOI] [PubMed] [Google Scholar]
- 12.Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Spengler U, Sauerbruch T. Serum selenium versus lymphocyte subsets and markers of disease progression and inflammatory response in human immunodeficiency virus-1 infection. Biol Trace Elem Res. 1997;56:31–41. doi: 10.1007/BF02778982. [DOI] [PubMed] [Google Scholar]
- 13.Urban T, Jarstrand C. Selenium effects on human neutrophilic granulocyte function in vitro. Immunopharmacology. 1986;12:167–72. doi: 10.1016/0162-3109(86)90042-1. [DOI] [PubMed] [Google Scholar]
- 14.Eskew ML, Zarkower A, Scheuchenzuber WJ, Burgess JR, Scholz RW, Hildenbrandt G, Reddy CC. Effects of inadequate vitamin E and/or selenium nutrition on the release of arachidonic acid metabolites in rat alveolar macrophages. Prostaglandins. 1989;38:79–89. doi: 10.1016/0090-6980(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 15.Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr. 2001;85(Suppl 2):S75–80. doi: 10.1079/bjn2000297. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–28. doi: 10.1016/j.jaci.2005.04.036. quiz 1129. [DOI] [PubMed] [Google Scholar]
- 17.Ferencik M, Ebringer L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol (Praha) 2003;48:417–26. doi: 10.1007/BF02931378. [DOI] [PubMed] [Google Scholar]
- 18.Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–23. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephensen CB, Blount SR, Schoeb TR, Park JY. Vitamin A deficiency impairs some aspects of the host response to influenza A virus infection in BALB/c mice. J Nutr. 1993;123:823–33. doi: 10.1093/jn/123.5.823. [DOI] [PubMed] [Google Scholar]
- 20.Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA, Beck MA. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1846–8. [PubMed] [Google Scholar]
- 21.Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, Madden MC. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005;85:990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- 22.Korunova V, Skodova Z, Dedina J, Valenta Z, Parizek J, Pisa Z, Styblo M. Serum selenium in adult Czechoslovak (central Bohemia) population. Biol Trace Elem Res. 1993;37:91–9. doi: 10.1007/BF02783784. [DOI] [PubMed] [Google Scholar]
- 23.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 26.Ciencewicki J, Brighton L, Wu WD, Madden M, Jaspers I. Diesel exhaust enhances virus- and poly(I:C)-induced Toll-like receptor 3 expression and signaling in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1154–63. doi: 10.1152/ajplung.00318.2005. [DOI] [PubMed] [Google Scholar]
- 27.Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–3. [PubMed] [Google Scholar]
- 28.Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and proteasomal degradation of IkBa in airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:769–777. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- 29.Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–12. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 30.Clark CR, Henderson TR, Royer RE, Brooks AL, McClellan RO, Marshall WF, Naman TM. Mutagenicity of diesel exhaust particle extracts: influence of fuel composition in two diesel engines. Fundam Appl Toxicol. 1982;2:38–43. doi: 10.1016/s0272-0590(82)80062-6. [DOI] [PubMed] [Google Scholar]
- 31.Ruz M, Codoceo J, Galgani J, Munoz L, Gras N, Muzzo S, Leiva L, Bosco C. Single and multiple selenium-zinc-iodine deficiencies affect rat thyroid metabolism and ultrastructure. J Nutr. 1999;129:174–80. doi: 10.1093/jn/129.1.174. [DOI] [PubMed] [Google Scholar]
- 32.Van Vleet JF, Ruth G, Ferrans VJ. Ultrastructural alterations in skeletal muscle of pigs with selenium-vitamin E deficiency. Am J Vet Res. 1976;37:911–22. [PubMed] [Google Scholar]
- 33.Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol. 1999;20:595–604. doi: 10.1165/ajrcmb.20.4.3442. [DOI] [PubMed] [Google Scholar]
- 34.Adler KB, Holden-Stauffer WJ, Repine JE. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism. J Clin Invest. 1990;85:75–85. doi: 10.1172/JCI114436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 36.Trifilo MJ, Montalto-Morrison C, Stiles LN, Hurst KR, Hardison JL, Manning JE, Masters PS, Lane TE. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J Virol. 2004;78:585–94. doi: 10.1128/JVI.78.2.585-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76(Pt 11):2869–73. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 38.Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80:154–62. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 39.Bogden JD, Kemp FW, Han S, Li W, Bruening K, Denny T, Oleske JM, Lloyd J, Baker H, Perez G, Kloser P, Skurnick J, Louria DB. Status of selected nutrients and progression of human immunodeficiency virus type 1 infection. Am J Clin Nutr. 2000;72:809–15. doi: 10.1093/ajcn/72.3.809. [DOI] [PubMed] [Google Scholar]
- 40.Ullrey DE. Biochemical and physiological indicators of selenium status in animals. J Anim Sci. 1987;65:1712–26. doi: 10.2527/jas1987.6561712x. [DOI] [PubMed] [Google Scholar]
- 41.Bettger WJ. Zinc and selenium, site-specific versus general antioxidation. Can J Physiol Pharmacol. 1993;71:721–4. doi: 10.1139/y93-108. [DOI] [PubMed] [Google Scholar]
- 42.Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004;58:391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- 43.Pascoe GA, Sakai-Wong J, Soliven E, Correia MA. Regulation of intestinal cytochrome P-450 and heme by dietary nutrients. Critical role of selenium. Biochem Pharmacol. 1983;32:3027–35. doi: 10.1016/0006-2952(83)90245-9. [DOI] [PubMed] [Google Scholar]
- 44.Pighetti GM, Eskew ML, Reddy CC, Sordillo LM. Selenium and vitamin E deficiency impair transferrin receptor internalization but not IL-2, IL-2 receptor, or transferrin receptor expression. J Leukoc Biol. 1998;63:131–7. doi: 10.1002/jlb.63.1.131. [DOI] [PubMed] [Google Scholar]
- 45.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581–91. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:447–52. doi: 10.1165/ajrcmb.26.4.4473. [DOI] [PubMed] [Google Scholar]
- 47.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–52. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- 48.Taima K, Imaizumi T, Yamashita K, Ishikawa A, Fujita T, Yoshida H, Takanashi S, Okumura K, Satoh K. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells. Respiration. 2006;73:360–4. doi: 10.1159/000091646. [DOI] [PubMed] [Google Scholar]
- 49.Wood SM, Beckham C, 1, Yosioka A, 2, Darban H, 3, Watson RR. beta-Carotene and selenium supplementation enhances immune response in aged humans. Integr. Med. 2000;2:85–92. doi: 10.1016/s1096-2190(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 50.Takizawa T, Tatematsu C, Ohashi K, Nakanishi Y. Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol Immunol. 1999;43:245–52. doi: 10.1111/j.1348-0421.1999.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 51.Fesq H, Bacher M, Nain M, Gemsa D. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 1994;190:175–82. doi: 10.1016/S0171-2985(11)80292-5. [DOI] [PubMed] [Google Scholar]
- 52.Hinshaw VS, Olsen CW, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–73. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhirnov OP, Konakova TE, Wolff T, Klenk HD. NS1 protein of influenza A virus down-regulates apoptosis. J Virol. 2002;76:1617–25. doi: 10.1128/JVI.76.4.1617-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wurzer WJ, Planz O, Ehrhardt C, Giner M, Silberzahn T, Pleschka S, Ludwig S. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22:2717–28. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okada M, Sugita K, Inukai T, Goi K, Kagami K, Kawasaki K, Nakazawa S. Hepatocyte growth factor protects small airway epithelial cells from apoptosis induced by tumor necrosis factor-alpha or oxidative stress. Pediatr Res. 2004;56:336–44. doi: 10.1203/01.PDR.0000134255.58638.59. [DOI] [PubMed] [Google Scholar]
- 56.Comhair SA, Xu W, Ghosh S, Thunnissen FB, Almasan A, Calhoun WJ, Janocha AJ, Zheng L, Hazen SL, Erzurum SC. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol. 2005;166:663–74. doi: 10.1016/S0002-9440(10)62288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho FY, Tsang WP, Kong SK, Kwok TT. The critical role of caspases activation in hypoxia/reoxygenation induced apoptosis. Biochem Biophys Res Commun. 2006;345:1131–7. doi: 10.1016/j.bbrc.2006.04.178. [DOI] [PubMed] [Google Scholar]
- 58.Rezvani HR, Mazurier F, Cario-Andre M, Pain C, Ged C, Taieb A, de Verneuil H. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J Biol Chem. 2006;281:17999–8007. doi: 10.1074/jbc.M600536200. [DOI] [PubMed] [Google Scholar]
- 59.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–61. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritz BW, Gardner EM. Malnutrition and energy restriction differentially affect viral immunity. J Nutr. 2006;136:1141–4. doi: 10.1093/jn/136.5.1141. [DOI] [PubMed] [Google Scholar]
- 61.Rubin RN, Navon L, Cassano PA. Relationship of serum antioxidants to asthma prevalence in youth. Am J Respir Crit Care Med. 2004;169:393–8. doi: 10.1164/rccm.200301-055OC. [DOI] [PubMed] [Google Scholar]
- 62.Michalke B. Selenium speciation in human serum of cystic fibrosis patients compared to serum from healthy persons. J Chromatogr A. 2004;1058:203–8. [PubMed] [Google Scholar]
- 63.Santos MC, Oliveira AL, Viegas-Crespo AM, Vicente L, Barreiros A, Monteiro P, Pinheiro T, Bugalho De Almeida A. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers. 2004;9:461–9. doi: 10.1080/13547500400024768. [DOI] [PubMed] [Google Scholar]
- 64.Caramori G, Ito K, Contoli M, Di Stefano A, Johnston SL, Adcock IM, Papi A. Molecular mechanisms of respiratory virus-induced asthma and COPD exacerbations and pneumonia. Curr Med Chem. 2006;13:2267–90. doi: 10.2174/092986706777935159. [DOI] [PubMed] [Google Scholar]
- 65.van Ewijk BE, van der Zalm MM, Wolfs TF, van der Ent CK. Viral respiratory infections in cystic fibrosis. J Cyst Fibros. 2005;4(Suppl 2):31–6. doi: 10.1016/j.jcf.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]