Summary
Hemolysis, long discounted as a critical measure of sickle cell disease severity when compared with sickle vaso-occlusion, may be the proximate cause of some disease complications. New mechanistic information about hemolysis and its effects on nitric oxide (NO) biology and further examination of the subphenotypes of disease requires a reappraisal and deconstruction of the clinical features of sickle cell disease. The biology underlying clinical phenotypes linked to hemolysis may increase our understanding of the pathogenesis of other chronic hemolytic diseases while providing new insights into treating sickle cell disease.
The pathophysiological roles of dysregulated NO homeostasis and sickle reticulocyte adherence have linked hemolysis and hemolytic rate to sickle vasculopathy. Nitric oxide binds soluble guanylate cyclase which converts GTP to cGMP, relaxing vascular smooth muscle and causing vasodilatation. When plasma hemoglobin liberated from intravascularly hemolyzed sickle erythrocytes consumes NO, the normal balance of vasoconstriction:vasodilation is skewed toward vasoconstriction. Pulmonary hypertension, priapism, leg ulceration and stroke, all subphenotypes of sickle cell disease, can be linked to the intensity of hemolysis. Hemolysis plays less of a role in the vaso-occlusive-viscosity complications of disease like the acute painful episode, osteonecrosis of bone and the acute chest syndrome.
Agents that decrease hemolysis or restore NO bioavailability or responsiveness may have potential to reduce the incidence and severity of the hemolytic subphenotypes of sickle cell disease. Some of these drugs are now being studied in clinical trials.
Keywords: Sickle cell, Pulmonary hypertension, Priapism, Ulcer, Hemolysis, Nitric oxide
Introduction
Sickle cell disease, a systemic disorder whose proximate cause is a mutation in the β-globin chain of hemoglobin, has as its major clinical features acute episodes of pain, stroke, priapism and acute chest syndrome and chronic organ damage, like osteonecrosis, renal failure and chronic hemolytic anemia.1 Dysregulated NO homeostasis, a consequence of hemolytic anemia, may be responsible for some of the complications of sickle cell disease and other chronic forms of hemolytic anemia. This article will review the evidence for a pathophysiological model of sickle cell disease that relates certain clinical complications primarily to blood viscosity and vaso-occlusion, and other complications mainly to hemolysis-linked endothelial dysfunction.
Nitric oxide and the hemolysis phenotype
Hemolytic anemia varies in intensity among the genotypes of sickle cell disease. It is most severe in patients with sickle cell anemia who are homozygous for the sickle hemoglobin gene mutation (HBB; glu6val), less severe in individuals with sickle cell anemia and concurrent α thalassemia (homozygous or heterozygous for a single α-globin gene (HbA1, HBA2) deletion, a genotype found in a third of individuals with sickle cell anemia, and least severe in patients with HbSC disease (compound heterozygosity for HbS and HbC (HBB; glu6lys). Even within a single genotype, the hemoglobin concentration is variable. For example, in sickle cell anemia,51Cr red cell survival ranges between two and 21 days and this is reflected in similarly wide variations of total hemoglobin concentration, reticulocyte count, bilirubin level and lactic dehydrogenase (LDH) levels, all clinical markers of hemolysis.2,3 Hemolytic anemia may be the driving force behind some complications of sickle cell disease because of its effects on NO bioavailability.4 Nitric oxide binds soluble guanylate cyclase, which converts GTP to cGMP, relaxing vascular smooth muscle and causing vasodilatation (Fig. 1). Plasma hemoglobin liberated from intravascularly destroyed sickle erythrocytes consumes NO, producing methemoglobin and bio-inactive nitrate. A state of reduced endothelial NO bioavailability in sickle cell disease impairs downstream homeostatic vascular functions of NO, like inhibition of platelet activation and aggregation and transcriptional repression of the cell adhesion molecules, VCAM-1, ICAM-1 (vascular cell adhesion molecule-1, intercellular adhesion molecule-1), P-selectin and E-selectin.5 Hemoglobin, heme and heme iron catalyze the production of oxygen radicals, further limiting NO bioavailability and activating endothelium.6 Lysed erythrocytes also liberate arginase that destroys L-arginine, the substrate for NO production, providing another mechanism for endothelial NO deficiency.7 Although not linked directly to hemolysis, reactive oxygen species, generated at high rates in patients with sickle cell disease, also consume NO.8,9 The normal balance of vasoconstriction to vasodilation is therefore skewed toward vasoconstriction, endothelial activation and proliferation. Both hemolytic rate and splenectomy (surgical and functional) are associated with red cell membrane damage, phosphatidylserine exposure at the red cell membrane surface, activation of tissue factor and thrombosis.10–18 Speculatively, chronic anemia and tissue ischemia might also contribute to a proliferative vasculopathy via activation of HIF-1-(hypoxia inducible factor) dependent factors such as iNOS (inducible nitric oxide synthase), erythropoietin, and VEGF (vascular endothelial growth factor), which has been seen in an animal model of pulmonary hypertension.19,20
Figure 1.
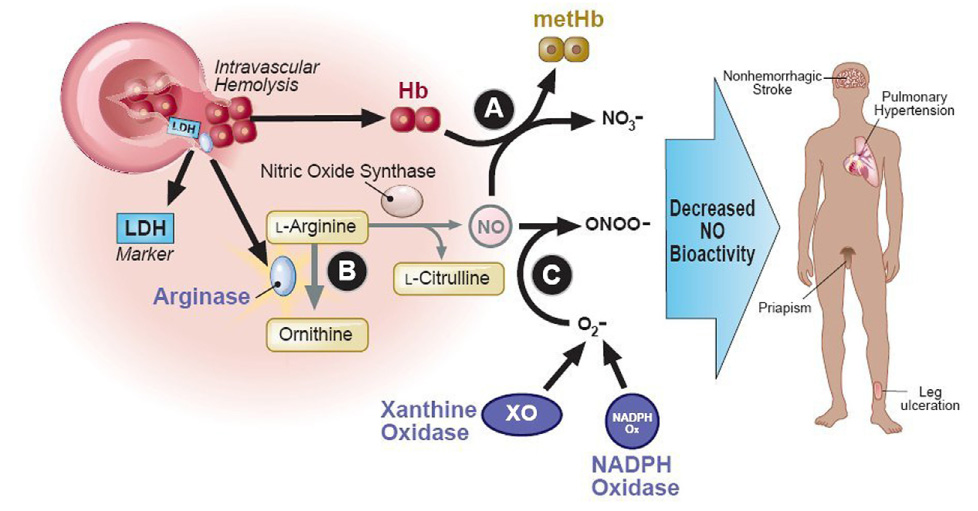
Intravascular hemolysis reduces nitric oxide bioactivity. Nitric oxide is produced by isoforms of nitric oxide (NO) synthase, using the substrate L-arginine. Intravascular hemolysis simultaneously releases hemoglobin, arginase, and lactate dehydrogenase (LDH) from red cells into blood plasma. Cell-free plasma hemoglobin stochiometrically inactivates NO, generating methemoglobin and inert nitrate (A). Plasma arginase consumes plasma L-arginine to ornithine, depleting its availability for NO production (B). LDH also released from the red cell into blood serum serves as a surrogate marker for the magnitude of hemoglobin and arginase release. NO is also consumed by reactions with reactive oxygen species produced by the high levels of xanthine oxidase activity and NADPH oxidase activity seen in sickle cell disease, producing oxygen radicals like peroxynitrite (ONOO-)(C). The resulting decreased NO bioactivity in sickle cell disease is associated with pulmonary hypertension, priapism, leg ulceration, and possibly with non-hemorrhagic stroke. A similar pathobiology is seen in other chronic intravascular hemolytic anemias.
Pulmonary hypertension and hemolysis
Pulmonary hypertension affects about 30% of patients with sickle cell anemia and is a major risk factor for near-term death.21–24 Other varieties of hemolytic anemia have also been linked to pulmonary hypertension, particularly in splenectomized patients, including beta-thalassemia intermedia and major, pyruvate kinase deficiency, and hereditary spherocytosis.11,12,25–36 Pulmonary thrombosis, a common complication of pulmonary hypertension, is seen frequently in these hemolytic disorders, especially following splenectomy.12,15,27,35,37,38 Splenectomy has been previously suspected to play a role in the development of vasculopathy in otherwise healthy patients, although this point remains controversial.39,40 In patients with thalassemia, plasma hemoglobin levels preliminarily have been reported to be higher in splenectomized patients than non-splenectomized patients.41 This remains to be confirmed and further investigated, but splenectomy removes a large portion of the reticuloendothelial system, lowering the overall hemolytic rate, possibly shifting some of the hemolysis from extravascular to intravascular compartment. This hypothetically would liberate more plasma hemoglobin, which would lead to more intense scavenging of nitric oxide, with vasoconstrictive and prothrombotic consequences. Surgical asplenia in thalassemia and functional asplenia in sickle cell disease might also lead to prolonged circulation of abnormal red cells with cell surface phosphatidylserine, implicated in hemostatic activation in both diseases.10,11,17,42
In patients with sickle cell disease or thalassemia, markers of hemolysis are associated with indicators of reduced NO availability, endothelial dysfunction and pulmonary hypertension. Markers of hemolysis such as plasma hemoglobin and serum lactate dehydrogenase (LDH) correlate closely with nitric oxide consumption and dysregulated metabolism of arginine, the substrate of nitric oxide synthase.4,43 These markers also correlate with endothelial dysfunction manifested as abnormal vascular reactivity in forearm blood flow studies, or as elevated plasma levels of soluble endothelial adhesion molecules.43–45 Finally, serum LDH, high bilirubin and low total hemoglobin are linked to the prevalence and severity of pulmonary hypertension.22,43 Some of these same observations have been made in thalassemia intermedia and major.46
Hemolysis and the priapism paradox
Priapism has a distinctive relationship to hemolysis and pulmonary hypertension. It is one of the only complications of sickle cell disease found to be associated with pulmonary hypertension in sickle cell disease.22,43 Priapism is associated with reduced hemoglobin level and the hemolytic markers, reticulocyte count, bilirubin, LDH and aspartate aminotransferase (AST).43,47 Patients with a history of priapism have a fivefold greater risk of developing pulmonary hypertension.22 Consistent with an epidemiological link between pulmonary hypertension, priapism, and hemolytic pathobiology, priapism and pulmonary hypertension are more common in individuals with sickle cell anemia than patients with sickle cell anemia-α thalassemia or HbSC disease.7,47 Similar to pulmonary hypertension, priapism may also complicate unstable hemoglobinopathy, β thalassemia intermedia, paroxysmal nocturnal hemoglobinuria and other types of hemolytic anemia where NO scavenging by plasma hemoglobin is likely.48–55
Since NO is generally believed to play a role in normal penile erection, it is paradoxical that chronically impaired NO bioavailability is associated with priapism. For example, it is well known that increasing NO-dependent cGMP levels by inhibition of phosphodiesterase 5 (PDE5) with drugs like sildenafil increases erectile responses. However, consistent with this association, increased priapic activity is seen in the severely NO deficient double nos3/nos1 knockout mouse.56 This unexpected priapism was attributed to severe down-regulation of PDE5 due to chronic NO deficiency resulting in episodic uncontrolled cGMP-dependent vasodilation of the penile erectile tissue, presumably by activation of soluble guanylate cyclase by other non-NO dependent mediators such as CO or voltage gated signaling. Such a paradox suggests a potential beneficial effect of PDE5 inhibitors, such as sildenafil, on priapic activity.57,58 Perhaps more importantly, this pathobiology suggests that efforts to control hemolytic rate may reduce priapic activity.
Leg ulcers and hemolysis
Patients with leg ulcers had lower hemoglobin levels and higher levels of lactate dehydrogenase, bilirubin, aspartate aminotransferase and reticulocytes than did age and sex matched control patients with sickle cell anemia but without leg ulcers.59 Age-adjusted comparisons showed that sickle cell anemia-α thalassemia and HbSC disease were more frequent among controls than leg ulcer cases. These results strongly suggested that the likelihood of having leg ulcers was related to the intensity of hemolysis.43,47 Similar to pulmonary hypertension and priapism, cutaneous leg ulcers are also seen in other forms of hemolytic anemia.60–69 The incidence of leg ulcers does not appear to be linked to frequency of vaso-occlusive crisis.59,70
Stroke and hemolysis
The evidence linking stroke to hemolysis is more circumstantial and less definitive. In several studies of stroke in sickle cell disease, stroke was associated with lower hemoglobin concentration.71 Coexistent α thalassemia protects patients with sickle cell anemia from stroke.72 Likewise, the prevalence of α thalassemia was significantly higher in children with normal transcranial Doppler (TCD) flow rates than in patients with high flow velocity, a risk factor for stroke.73 Whether this is due to the known effect of α thalassemia to reduce hemolysis and increase hemoglobin, or to some other effect of α thalassemia on the sickle erythrocyte is unknown. Supporting a link between hemolytic risk and stroke risk, in children with sickle cell disease and abnormally high TCD velocities, chronic transfusion simultaneously reduces the hemolytic rate, plasma hemoglobin level and the risk for stroke.74,75 In another study, silent cerebral infarct also was associated with lower hemoglobin, although there was no association of α thalassemia, reticulocyte count, AST or bilirubin with silent infarct.76
A self-reported history of stroke is associated with pulmonary hypertension in patients with sickle cell disease.22 In addition, we have reported a case series of six adult patients with sickle cell disease and cerebrovascular disease, with overt or sub-clinical cerebral infarcts, all of whom had pulmonary hypertension.77 All six patients shared a characteristic profile of particularly severe hemolysis seen in most sickle cell patients with pulmonary hypertension. There are many similarities in the epidemiological, physiological and histopathological features of these two complications of sickle cell disease, and it is intriguing to hypothesize that, like pulmonary hypertension, part of the pathophysiology of cerebrovascular disease might involve impaired NO bioavailability.
Fetal hemoglobin (HbF), α thalassemia and the viscosity-vaso-occlusive phenotype
High HbF levels reduce the incidence of some subphenotypes of sickle cell disease, like osteonecrosis,78 acute chest syndrome79,80 and acute painful episodes (Table 1).80,81 HbF level has not been associated with protection from pulmonary hypertension, stroke or priapism.22,47,71 This is paradoxical, since HbF expression in patients with sickle cell disease is well known to be associated with decreased overall hemolysis. The solution to the paradox may lie in the remarkably high rate of intense hemolysis in the fraction of red cells that fail to express HbF.82 In addition, potential associations of HbF with these subphenotypes may also be obscured by analytical approaches that fail to account for the interactions of many other genetic modifiers with HbF.83 The principal observed clinical benefits of HbF are on the viscosity-vaso-occlusive phenotype, potentially due to the anti-sickling effect of HbF.81
Table 1.
Patterns of association of known prognostic factors with specific complications of sickle cell disease
| Prognostic Factor | Viscosity-Vaso-occlusive Subphenotype |
Hemolysis-Endothelial Dysfunction Syndrome |
|||||
|---|---|---|---|---|---|---|---|
| VOC | ACS | AVN | Ulcers | PHT | Priapism | Stroke | |
| High LDH | - | - | - | ↑ | ↑ | ↑ | ? |
| High hemoglobin | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ |
| α-thalassemia | ↑a | ↑/↓ | ↑a | ↓ | ? | ↓ | ↓ |
| High HbF | ↓ | ↓ | ↓ | ↓ | - | - | - |
| Hydroxyurea | ↓ | ↓ | ? | ? | - | ? | ↓ |
Abbreviations: VOC, vaso-occlusive pain crisis; ACS, acute chest syndrome; AVN, avascular necrosis of bone; PHT, pulmonary hypertension; LDH, serum lactate dehydrogenase; HbF, fetal hemoglobin; ↑, increased risk; ↓, decreased risk; -, no change in risk observed; ?, effect on risk has not been well evaluated. The information in this table is derived from recent reviews and other publications.43,47,81,125 More detailed information is presented in the text.
Permissive in patients with HbSS, protective in patients with HbSC.
While concurrent α thalassemia also decreases hemolysis,2,84 it also is protective for the putatively hemolysis-associated phenotypes of leg ulcers,85–87 priapism47 and stroke,72,73,88 and it is associated with increased risk of the viscosity-vaso-occlusive phenotypes of acute painful episodes89–92 and osteonecrosis.85,93,94 Similarly, α thalassemia has also been associated with increased incidence of another viscosity-vaso-occlusive complication, namely the acute chest syndrome,85 although the opposite result has been seen in smaller studies or in children.87,89 In general, the phenotypic associations of α thalassemia closely mirror the associations of high hemoglobin (Table 1).
Hemolysis and the sickle reticulocyte, initiators of vaso-occlusion
Hemolysis promotes adhesive properties of circulating cells and the vessel wall. Sickle reticulocytes, increased in number in response to hemolysis, display receptors and ligands responsible for their adherence to endothelium and leukocytes. Under flow conditions, reticulocytes were the most adherent of the heterogeneous population of sickle erythrocytes.95 Reticulocyte adherence provides an additional link between hemolytic anemia and sickle vaso-occlusion. Sickle erythrocytes adhere to cultured endothelial cells and the tenacity of adherence reflects the severity of disease.96,97 Receptors and ligands for this interaction have been characterized.98,99 Sickle erythrocyte adherence also varies according to the hemoglobin genotype being most manifest in sickle cell anemia. Even among patients with this genotype, adherence varies about twenty-fold.96 Since most of the endothelial cell adhesion molecules that bind sickle reticulocytes are normally suppressed by NO, decreased NO bioavailability resulting from intravascular hemolysis might also contribute to sickle erythrocyte adherence.44,100–105
Hemolysis in geographic subgroups
The prevalence of priapism and leg ulcers is reported to be much higher in patients with sickle cell disease in Jamaica, who manifest severe hemolysis, than those in India or Greece, in whom less severe hemolysis is seen.106–108 However, the rates of vaso-occlusive crisis and acute chest syndrome are comparable between these geographic subgroups. Although one might suspect the geographic difference in priapism and leg ulcer rates to be due to differences in fetal hemoglobin levels, in the Cooperative Study of Sickle Cell Disease conducted in the U.S.A., priapism and leg ulcers were associated with severity of hemolysis and not with fetal hemoglobin levels.47,59 This further supports the concept of a hemolysis-endothelial dysfunction subphenotype distinct from the viscosity-vaso-occlusion subphenotype.
Hemolysis and desaturation
Several groups have found an association of low transcutaneous oxygen saturation of hemoglobin with elevated serum lactate dehydrogenase, severity of anemia and reticulocytosis, suggesting a link between hemolysis and hypoxemia.43,109–112 Speculatively, this link might involve hemolysis-associated pulmonary hypertension and consequent ventilation-perfusion mismatch, although other factors may also play a role.113 Further investigation is required to understand this observation.
Hemolysis and animal models
More definitive scientific evidence has been obtained in animals showing that hemolysis causes vasomotor instability, supporting the extensive epidemiological evidence in humans. Experimentally induced hemolysis in dogs provokes systemic and pulmonary hypertension, renal dysfunction, and diminished vascular response to NO donors.114 These findings are directly associated with biochemical evidence of stoichiometric oxidation of nitric oxide by cell-free plasma hemoglobin. Furthermore, they are attenuated by administration of inhaled nitric oxide, which oxidizes cell-free plasma hemoglobin to methemoglobin, preventing its scavenging of endogenous NO. These biochemical and physiological data in a large animal model provide the strongest mechanistic evidence to date for a human syndrome of hemolysis-associated dys-egulation of NO bioactivity.
Augmenting the NO pathway in sickle cell disease
Therapies directed at restoring NO homeostasis have shown promise in preliminary studies in patients with sickle cell disease. In children with sickle cell disease presenting to the emergency department with vaso-occlusive pain crisis, inhaled nitric oxide therapy was associated with trends toward lower pain scores, decreased analgesic requirements, and shorter hospital stay.115 A larger scale study is currently under way. Oral administration of the NO synthase substrate L-arginine has been effective in a pilot study of sickle cell pulmonary hypertension.116 Inhibition of phosphodiesterase-5 by sildenafil promotes accumulation of cGMP, amplifying the effect of NO in pulmonary vascular smooth muscle, improving hemolysis-associated pulmonary hypertension in two preliminary human trials.117–119 These therapies cannot yet be recommended until larger scale clinical trials are completed, but further research is clearly warranted in this area.120
Conclusions: A new perspective on sickle subphenotypes and goals for treatment
Hemolytic and viscosity-vaso-occlusive phenotypes must have substantial areas of overlap. Nevertheless, this dichotomization helps place subphenotypes of sickle cell disease into a new context (Fig. 2).121 Hemolytic anemia and increased NO scavenging play a major role in the propensity to acquire the subphenotypes of pulmonary hypertension, stroke, leg ulcer and priapism. At least the latter three are ameliorated by α thalassemia that reduces hemolysis and improves anemia. HbF, while it should not be disregarded as a modulator of these subphenotypes, has little direct protective effect against these vasculopathic complications. Distinct from these phenotypes are ones associated with increased blood viscosity, like osteonecrosis, acute chest syndrome and painful episodes. Adversely affected by α thalassemia, their prevalence is directly associated with higher hemoglobin concentration and HbF has a protective effect. These data serve to support a concept of subphenotypes in sickle cell disease, as previously proposed by others.70,121
Figure 2.
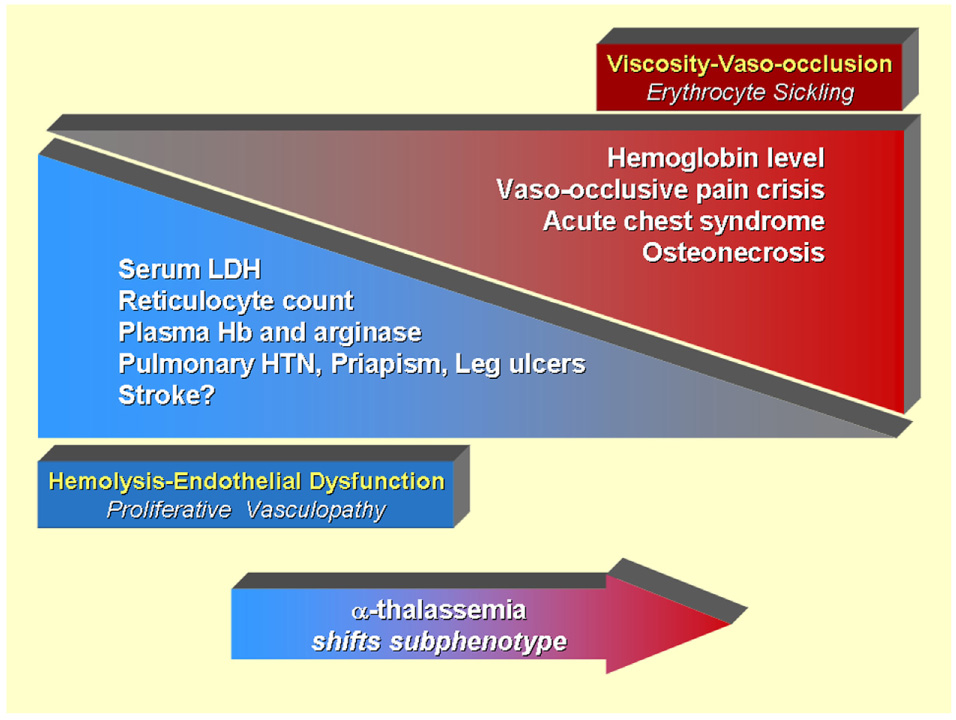
Model of overlapping subphenotypes of sickle cell disease. Published data suggest that patients with sickle cell disease with higher hemoglobin levels have a higher frequency of viscosity-vaso-occlusive complications closely related to polymerization of sickle hemoglobin, resulting in erythrocyte sickling and adhesion. Such complications include vaso-occlusive pain crisis, acute chest syndrome, and osteonecrosis. In contrast, a distinct set of hemolysis-endothelial dysfunction complications involving a proliferative vasculopathy and dysregulated vasomotor function, including leg ulcers, priapism, pulmonary hypertension, and possibly non-hemorrhagic stroke, is associated with low hemoglobin levels, and high levels of hemolytic markers such as reticulocyte counts, serum lactate dehydrogenase, plasma hemoglobin and arginase, producing a state of impaired nitric oxide bioavailability. The spectrum of prevalence and severity of each of these subphenotypes overlap with each other. Patients with α-thalassemia trait tend to have less hemolysis and higher hemoglobin levels, tending to decrease the prevalence of hemolysis-endothelial dysfunction, and tending to increase the prevalence of viscosity-vaso-occlusion. The effect of fetal hemoglobin expression or chronic red cell transfusion is more complex, simultaneously increasing hemoglobin level, but reducing sickling and hemolysis.
Hemolytic anemia-induced phenotypes are likely to be improved by transfusion and agents that increase NO bioavailability or dramatically reduce hemolysis.122 HbF induced by hydroxyurea appears to have an anti-sickling effect that reduces the severity of viscosity-vaso-occlusive complications. However, the heterocellular HbF distribution induced by hydroxyurea may not reduce hemolysis sufficiently to correct the hemolysis-endothelial dysfunction complications.82 In order to significantly impact the hemolytic complications, HbF-inducing agents might have to achieve a pancellular distribution of HbF.
An increased fraction of dense cells is present in most patients with sickle cell disease and HbS polymerization is critically dependent on cell density. Drugs capable of reducing cell density are accompanied by decreased hemolysis and clinical trials of some of these agents have started.123,124 Agents that decrease hemolysis or restore NO bioavailability or the responsiveness of the vasculature to NO may have potential to reduce the incidence and severity of the hemolytic subphenotypes of sickle cell disease.
Practice Points
Adults with sickle cell disease and thalassemia intermedia or major should be screened by echocardiogram for a tricuspid regurgitant jet velocity (TRV) ⩾2.5 m/sec, suggestive of pulmonary hypertension. Patients with elevated TRV should be referred to a pulmonologist or cardiologist knowledgeable in hemoglobinopathy-associated pulmonary hypertension.
Clinicians should be alert to a syndrome of hemolysis-endothelial dysfunction, including high serum lactate dehydrogenase levels, pulmonary hypertension, leg ulcers and priapism.
Research Agenda
Role of oxidant stress in reducing nitric oxide bioactivity in sickle cell disease.
Efficacy of the endothelin receptor antagonist bosentan or phosphodiesterase-5 inhibitors in hemolysis-associated pulmonary hypertension.
Efficacy of novel nitric oxide donors in acute and chronic sickle cell ischemic tissue injury.
Acknowledgement
Supported in part by NHLBI grants HL R01 68970, HL R01 70735 and 1U54 HL 0708819 (M.H.S.) and by intramural funding from the NHLBI and NIH Clinical Center (G.J.K. and M.T.G.). Vikki Nolan, Diego Wyszynski, John Farrell, Alice Bisbee, Lindsay Farrer and Paola Sebastiani participated in the collection and analysis of data reported from Dr. Steinberg’s laboratory.
References
- 1.Ohene-Frempong K, Steinberg MH. Clinical Aspects of Sickle Cell Anemia in Adults and Children. In: Steinberg BG, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge: Cambridge University Press; 2001. pp. 611–670. [Google Scholar]
- 2.De Ceulaer K, Higgs DR, Weatherall DJ, Hayes RJ, Serjeant GR, Serjeant GR. Alpha thalassemia reduces the hemolytic rate in homozygous sickle-cell disease. N Eng J Med. 1983;309:189–190. doi: 10.1056/NEJM198307213090320. [DOI] [PubMed] [Google Scholar]
- 3.Ballas SK, Marcolina MJ. Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia. Transfusion. 2006;46:105–110. doi: 10.1111/j.1537-2995.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 4.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Kato GJ. Cardiopulmonary complications of sickle cell disease: role of nitric oxide and hemolytic anemia. Hematology (Am Soc Hematol Educ Program) :51–57. doi: 10.1182/asheducation-2005.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebbel RP. Auto-oxidation and a membrane-associated ‘Fenton reagent’: a possible explanation for development of membrane lesions in sickle erythrocytes. Clinics in Haematology. 1985;14:129–140. [PubMed] [Google Scholar]
- 7.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, et al. Dysregulated Arginine Metabolism, Hemolysis-Associated Pulmonary Hypertension and Mortality in Sickle Cell Disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslan M, Freeman BA. Oxidant-mediated impairment of nitric oxide signaling in sickle cell disease–mechanisms and consequences. Cell Mol Biol (Noisy.-le-grand) 2004;50:95–105. [PubMed] [Google Scholar]
- 9.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19:989–991. doi: 10.1096/fj.04-3218fje. [DOI] [PubMed] [Google Scholar]
- 10.Atichartakarn V, Angchaisuksiri P, Aryurachai K, Onpun S, Chuncharunee S, Thakkinstian A, et al. Relationship between hypercoagulable state and erythrocyte phosphate-dylserine exposure in splenectomized haemoglobin E/beta-thalassaemic patients. Br J Haematol. 2002;118:893–898. doi: 10.1046/j.1365-2141.2002.03711.x. [DOI] [PubMed] [Google Scholar]
- 11.Atichartakarn V, Angchaisuksiri P, Aryurachai K, Chuncharunee S, Thakkinstian A. In vivo platelet activation and hyperaggregation in hemoglobin E/beta-thalassemia: a consequence of splenectomy. Int J Hematol. 2003;77:299–303. doi: 10.1007/BF02983790. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, DeLoughery TG. Recurrent thromboembolic disease following splenectomy for pyruvate kinase deficiency. Am J Hematol. 2001;67:197–199. doi: 10.1002/ajh.1107. [DOI] [PubMed] [Google Scholar]
- 13.Hayag-Barin JE, Smith RE, Tucker FC., Jr Hereditary spherocytosis, thrombocytosis, and chronic pulmonary emboli: a case report and review of the literature. Am J Hematol. 1998;57:82–84. doi: 10.1002/(sici)1096-8652(199801)57:1<82::aid-ajh15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Aessopos A, Farmakis D, Deftereos S, Tsironi M, Polonifi A, Moyssakis I, et al. Cardiovascular effects of splenomegaly and splenectomy in beta-thalassemia. Annals of Hematology. 2005;84:353–357. doi: 10.1007/s00277-004-1002-4. [DOI] [PubMed] [Google Scholar]
- 15.Stewart GW, Amess JA, Eber SW, Kingswood C, Lane PA, Smith BD, et al. Thrombo-embolic disease after splenectomy for hereditary stomatocytosis. Br J Haematol. 1996;93:303–310. doi: 10.1046/j.1365-2141.1996.4881033.x. [DOI] [PubMed] [Google Scholar]
- 16.Setty BN, Kulkarni S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99:1564–1571. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- 17.Setty BN, Rao AK, Stuart MJ. Thrombophilia in sickle cell disease: the red cell connection. Blood. 2001;98:3228–3233. doi: 10.1182/blood.v98.12.3228. [DOI] [PubMed] [Google Scholar]
- 18.Stuart MJ, Setty BN. Hemostatic alterations in sickle cell disease: relationships to disease pathophysiology. Pediatr Pathol Mol Med. 2001;20:27–46. [PubMed] [Google Scholar]
- 19.Semenza GL. Involvement of hypoxia-inducible factor 1 in pulmonary pathophysiology. Chest. 2005;128:592S–594S. doi: 10.1378/chest.128.6_suppl.592S. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circulation Research. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 21.Castro O, Gladwin MT. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematology/Oncology Clinics of North America. 2005;19:881–896. doi: 10.1016/j.hoc.2005.07.007. vii. [DOI] [PubMed] [Google Scholar]
- 22.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New England Journal of Medicine. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 23.Ataga KI, Sood N, De Gent G, Kelly E, Henderson AG, Jones S, et al. Pulmonary hypertension in sickle cell disease. The American Journal of Medicine. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 24.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary Hypertension in SS, SC and S-beta Thalassemia: Prevalence, Associated Clinical Syndromes, and Mortality. ASH Annual Meeting Abstracts. 2004;104:1663. [Google Scholar]
- 25.Jardine DL, Laing AD. Delayed pulmonary hypertension following splenectomy for congenital spherocytosis. Intern Med J. 2004;34:214–216. doi: 10.1111/j.1444-0903.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 26.Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–1530. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 27.Atichartakarn V, Likittanasombat K, Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P, et al. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78:139–145. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 28.Aessopos A, Stamatelos G, Skoumas V, Vassilopoulos G, Mantzourani M, Loukopoulos D. Pulmonary hypertension and right heart failure in patients with beta-thalassemia intermedia. Chest. 1995;107:50–53. doi: 10.1378/chest.107.1.50. [DOI] [PubMed] [Google Scholar]
- 29.Chotivittayatarakorn P, Seksarn P, Pathmanand C, Thisyakorn C, Sueblinvong V. Cardiac dysfunction in beta-thalassemic children. J Med Assoc Thai. 1993;76:591–596. [PubMed] [Google Scholar]
- 30.Derchi G, Fonti A, Forni GL, Galliera EO, Cappellini MD, Turati F, et al. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1999;138:384. doi: 10.1016/s0002-8703(99)70129-8. [DOI] [PubMed] [Google Scholar]
- 31.Du ZD, Roguin N, Milgram E, Saab K, Koren A. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1997;134:532–537. doi: 10.1016/s0002-8703(97)70091-7. [DOI] [PubMed] [Google Scholar]
- 32.Grisaru D, Rachmilewitz EA, Mosseri M, Gotsman M, Lafair E, Okon E, et al. Cardiopulmonary assessment in beta-thalassemia major. Chest. 1990;98:1138–1142. doi: 10.1378/chest.98.5.1138. [DOI] [PubMed] [Google Scholar]
- 33.Hahalis G, Manolis AS, Apostolopoulos D, Alexopoulos D, Vagenakis AG, Zoumbos NC. Right ventricular cardiomyopathy in beta-thalassaemia major. Eur Heart J. 2002;23:147–156. doi: 10.1053/euhj.2001.2709. [DOI] [PubMed] [Google Scholar]
- 34.Koren A, Garty I, Antonelli D, Katzuni E. Right ventricular cardiac dysfunction in beta-thalassemia major. Am J Dis Child. 1987;141:93–96. [PubMed] [Google Scholar]
- 35.Hayag-Barin JE, Smith RE, Tucker FC., Jr Hereditary spherocytosis, thrombocytosis, and chronic pulmonary emboli: a case report and review of the literature. American Journal of Hematology. 1998;57:82–84. doi: 10.1002/(sici)1096-8652(199801)57:1<82::aid-ajh15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Verresen D, De Backer W, Van Meerbeeck J, Neetens I, Van Marck E, Vermeire P. Spherocytosis and pulmonary hypertension coincidental occurrence or causal relationship? Eur Respir J. 1991;4:629–631. [PubMed] [Google Scholar]
- 37.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125:1436–1441. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- 38.Taher A, Abou-Mourad Y, Abchee A, Zalouaa P, Shamseddine A. Pulmonary thromboembolism in beta-thalassemia intermedia: are we aware of this complication? Hemoglobin. 2002;26:107–112. doi: 10.1081/hem-120005447. [DOI] [PubMed] [Google Scholar]
- 39.Hoeper MM, Niedermeyer J, Hoffmeyer F, Flemming P, Fabel H. Pulmonary hypertension after splenectomy? Annals of Internal Medicine. 1999;130:506–509. doi: 10.7326/0003-4819-130-6-199903160-00014. [DOI] [PubMed] [Google Scholar]
- 40.Robinette CD, Fraumeni JF. Splenectomy and subsequent mortality in veterans of the 1939–45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 41.Westerman MP, Pizzey A, Hirschmann JV, Cerino M, Eze A, Ramoton P, et al. Plasma ‘free’ Hb is related to red cell derived vesicle numbers in sickle cell anemia and thalassemia intermedia: Implications for nitric oxide (NO) scavenging and pulmonary hypertension. Blood. 2004;104(11):465a. [Google Scholar]
- 42.Kuypers FA, de JK. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy.-le-grand) 2004;50:147–158. [PubMed] [Google Scholar]
- 43.Kato GJ, McGowan VR, Machado RF, Little JA, Taylor VJ, Morris CR, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter WH, Schenke WH, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 46.Morris CR, Kuypers FA, Kato GJ, Lavrisha L, Larkin S, Singer T, et al. Hemolysis-associated pulmonary hypertension in thalassemia. Annals of the New York Academy of Sciences. 2005;1054:481–485. doi: 10.1196/annals.1345.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dore F, Bonfigli S, Pardini S, Pirozzi F, Longinotti M. Priapism in thalassemia intermedia. Haematologica. 1991;76:523. [PubMed] [Google Scholar]
- 49.Edney MT, Schned AR, Cendron M, Chaffee S, Ellsworth PI. Priapism in a 15-year-old boy with congenital dyserythropoietic anemia type II (hereditary erythroblastic multinuclearity with positive acidified serum lysis test) J Urol. 2002;167:309–310. [PubMed] [Google Scholar]
- 50.Goulding FJ. Priapism caused by glucose phosphate isomerase deficiency. Journal of Urology. 1976;116:819–820. doi: 10.1016/s0022-5347(17)59030-8. [DOI] [PubMed] [Google Scholar]
- 51.Jackson N, Franklin IM, Hughes MA. Recurrent priapism following splenectomy for thalassaemia intermedia. Br J Surg. 1986;73:678. doi: 10.1002/bjs.1800730832. [DOI] [PubMed] [Google Scholar]
- 52.Macchia P, Massei F, Nardi M, Favre C, Brunori E, Barba V. Thalassemia intermedia and recurrent priapism following splenectomy. Haematologica. 1990;75:486–487. [PubMed] [Google Scholar]
- 53.Montalban J, Lozano P, Lu L, Gonzalez A. Paroxysmal nocturnal hemoglobinuria and priapism. Med Clin (Barc.) 1986;87:394. [PubMed] [Google Scholar]
- 54.Rao KR, Patel AR. Priapism and thalassaemia intermedia. British Journal of Surgery. 1986;73:1048. doi: 10.1002/bjs.1800731236. [DOI] [PubMed] [Google Scholar]
- 55.Thuret I, Bardakdjian J, Badens C, Wajcman H, Galacteros D, Vanuxem D, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu->Arg. American Journal of Hematology. 1996;51:133–136. doi: 10.1002/(SICI)1096-8652(199602)51:2<133::AID-AJH6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 56.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci USA. 2005;102:1661–1666. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bialecki ES, Bridges KR. Sildenafil relieves priapism in patients with sickle cell disease. Am J Med. 2002;113:252. doi: 10.1016/s0002-9343(02)01165-8. [DOI] [PubMed] [Google Scholar]
- 58.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Longterm oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67:1043–1048. doi: 10.1016/j.urology.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 59.Nolan VG, Adewoye A, Baldwin C, Wang L, Ma Q, Wyszynski DF, et al. Sickel cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol. 2006;133:570–578. doi: 10.1111/j.1365-2141.2006.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bordi B, Rosaria DM, Guariglia R, Capobianco G, Bordi E, Tirelli A. A case of congenital dyserythropoietic anemia type II, Gilbert’s syndrome and malleolar trophic ulcers. Hematology. 2002;7:197–199. doi: 10.1080/1024533021000008146. [DOI] [PubMed] [Google Scholar]
- 61.Gimmon Z, Wexler MR, Rachmilewitz EA. Juvenile leg ulceration in beta-thalassemia major and intermedia. Plastic and Reconstructive Surgery. 1982;69:320–325. doi: 10.1097/00006534-198202000-00023. [DOI] [PubMed] [Google Scholar]
- 62.Giraldi S, Abbage KT, Marinoni LP, Oliveira V, Pianowski AE, Lehmkuhl AE, et al. Leg ulcer in hereditary spherocytosis. Pediatric Dermatology. 2003;20:427–428. doi: 10.1046/j.1525-1470.2003.20512.x. [DOI] [PubMed] [Google Scholar]
- 63.Muller-Soyano A, Tovar dR, Duke PR, De Acquatella GC, Arends T, Guinto E, et al. Pyruvate kinase deficiency and leg ulcers. Blood. 1976;47:807–813. [PubMed] [Google Scholar]
- 64.Peachey RD. Leg ulceration and haemolytic anaemia: an hypothesis. British Journal of Dermatology. 1978;98:245–249. doi: 10.1111/j.1365-2133.1978.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 65.Pope FM, Hodgson GA. Leg ulceration and thalassaemia. British Journal of Dermatology. 1968;80:840. [PubMed] [Google Scholar]
- 66.Stevens DM, Shupack JL, Javid J, Silber R. Ulcers of the leg in thalassemia. Archives of Dermatology. 1977;113:1558–1560. [PubMed] [Google Scholar]
- 67.Tanaka KR, Paglia DE. Pyruvate kinase deficiency. Seminars in Hematology. 1971;8:367–396. [PubMed] [Google Scholar]
- 68.Vanscheidt W, Leder O, Vanscheidt E, Wokalek H, Wilmer E, Hofmann E, et al. Leg ulcers in a patient with spherocytosis: a clinicopathological report. Dermatologica. 1990;181:56–59. doi: 10.1159/000247863. [DOI] [PubMed] [Google Scholar]
- 69.Lawrence P, Aronson I, Saxe N, Jacobs P. Leg ulcers in hereditary spherocytosis. Clinical and Experimental Dermatology. 1991;16:28–30. doi: 10.1111/j.1365-2230.1991.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 70.Ballas SK. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. American Journal of Hematology. 1991;36:122–130. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- 71.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 72.Adams RJ, Kutlar A, McKie V, Carl E, Nichols FT, Liu JC, et al. Alpha thalassemia and stroke risk in sickle cell anemia. American Journal of Hematology. 1994;45:279–282. doi: 10.1002/ajh.2830450402. [DOI] [PubMed] [Google Scholar]
- 73.Hsu LL, Miller ST, Wright E, Kutlar A, McKie V, Wang W, et al. Alpha Thalassemia is associated with decreased risk of abnormal transcranial Doppler ultrasonography in children with sickle cell anemia. J Pediatr Hematol Oncol. 2003;25:622–628. doi: 10.1097/00043426-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 74.Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37:1424–1426. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 75.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 76.Kinney TR, Sleeper LA, Wang WC, Zimmerman RA, Pegelow K, Ohene-Frempong K, Wethers DL, Bello JA, Vichinsky EP, Moser FG, Gallagher DM, DeBaun MR, Platt OS, Miller ST. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103:640–645. doi: 10.1542/peds.103.3.640. [DOI] [PubMed] [Google Scholar]
- 77.Kato GJ, Hsieh M, Machado RF, Taylor JG, Butman JA, Lehky T, et al. Cerebrovascular disease associated with sickle cell pulmonary hypertension. American Journal of Hematology. 2006;81:503–510. doi: 10.1002/ajh.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hawker H, Neilson H, Hayes RJ, Serjeant GR. Haematological factors associated with avascular necrosis of the femoral head in homozygous sickle cell disease. British Journal of Haematology. 1982;50:29–34. doi: 10.1111/j.1365-2141.1982.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 79.Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott P, Gillette P, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 80.Bailey K, Morris JS, Thomas P, Serjeant GR. Fetal haemoglobin and early manifestations of homozygous sickle cell disease. Arch Dis Child. 1992;67:517–520. doi: 10.1136/adc.67.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinberg MH. Predicting clinical severity in sickle cell anaemia. Br J Haematol. 2005;129:465–481. doi: 10.1111/j.1365-2141.2005.05411.x. [DOI] [PubMed] [Google Scholar]
- 82.Franco RS, Yasin Z, Palascak MB, Ciraolo P, Joiner CH, Rucknagel DL. The effect of fetal hemoglobin on the survival characteristics of sickle cells. Blood. 2006;108:1073–1076. doi: 10.1182/blood-2005-09-008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sebastiani P, Ramoni MF, Nolan V, Baldwin CT, Steinberg MH. Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia. Nature Genetics. 2005;37:435–440. doi: 10.1038/ng1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Embury SH, Dozy AM, Miller J, Davis JR, Jr, Kleman KM, Preisler H, et al. Concurrent sickle-cell anemia and alpha-thalassemia: effect on severity of anemia. N Engl J Med. 1982;306:270–274. doi: 10.1056/NEJM198202043060504. [DOI] [PubMed] [Google Scholar]
- 85.Steinberg MH, Rosenstock W, Coleman MB, Adams JG, Platica O, Cedeno M, et al. Effects of thalassemia and microcytosis on the hematologic and vasoocclusive severity of sickle cell anemia. Blood. 1984;63:1353–1360. [PubMed] [Google Scholar]
- 86.Koshy M, Entsuah R, Koranda A, Kraus AP, Johnson R, Bellvue R, et al. Leg ulcers in patients with sickle cell disease. Blood. 1989;74:1403–1408. [PubMed] [Google Scholar]
- 87.Higgs DR, Aldridge BE, Lamb J, Clegg JB, Weatherall DJ, Hayes RJ, et al. The interaction of alpha-thalassemia and homozygous sickle-cell disease. N Engl J Med. 1982;306:1441–1446. doi: 10.1056/NEJM198206173062402. [DOI] [PubMed] [Google Scholar]
- 88.Neonato MG, Guilloud-Bataille M, Beauvais P, Begue P, Belloy M, Benkerrou M, et al. Acute clinical events in 299 homozygous sickle cell patients living in France. French Study Group on Sickle Cell Disease. Eur J Haematol. 2000;65:155–164. doi: 10.1034/j.1600-0609.2000.90210.x. [DOI] [PubMed] [Google Scholar]
- 89.Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- 90.Billett HH, Nagel RL, Fabry ME. Paradoxical increase of painful crises in sickle cell patients with alpha-thalassemia. Blood. 1995;86:4382. [PubMed] [Google Scholar]
- 91.Billett HH, Kim K, Fabry ME, Nagel RL. The percentage of dense red cells does not predict incidence of sickle cell painful crisis. Blood. 1986;68:301–303. [PubMed] [Google Scholar]
- 92.Bailey S, Higgs DR, Morris J, Serjeant GR. Is the painful crisis of sickle-cell disease due to sickling? Lancet. 1991;337:735. doi: 10.1016/0140-6736(91)90322-g. [DOI] [PubMed] [Google Scholar]
- 93.Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- 94.Ballas SK, Talacki CA, Rao VM, Steiner RM. The prevalence of avascular necrosis in sickle cell anemia: correlation with alpha-thalassemia. Hemoglobin. 1989;13:649–655. doi: 10.3109/03630268908998842. [DOI] [PubMed] [Google Scholar]
- 95.Hebbel RP, Mohandas N. Cell Adhesion and Microrheaology in Sickle Cell Disease. In: Steinberg MH, Forget BG, Higgs RL, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge: Cambridge University Press; 2001. pp. 527–549. [Google Scholar]
- 96.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302:992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 97.Hoover R, Rubin R, Wise G, Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979;54:872–876. [PubMed] [Google Scholar]
- 98.Hebbel RP. Perspectives series: cell adhesion in vascular biology. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Invest. 1997;99:2561–2564. doi: 10.1172/JCI119442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH. P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood. 2001;98:1955–1962. doi: 10.1182/blood.v98.6.1955. [DOI] [PubMed] [Google Scholar]
- 100.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. Journal of Clinical Investigation. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells; Proceedings of the National Academy of Sciences of the United States of America; 1996. pp. 9114–9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spiecker M, Darius H, Kaboth K, Hubner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. Journal of Leukocyte Biology. 1998;63:732–739. [PubMed] [Google Scholar]
- 103.Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of I-kappa Balpha. Journal of Biological Chemistry. 1997;272:30969–30974. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- 104.Shin WS, Hong YH, Peng HB, De Caterina R, Libby P, Liao JK. Nitric oxide attenuates vascular smooth muscle cell activation by interferon-gamma. The role of constitutive NF-kappa B activity. Journal of Biological Chemistry. 1996;271:11317–11324. doi: 10.1074/jbc.271.19.11317. [DOI] [PubMed] [Google Scholar]
- 105.Lee SK, Kim JH, Yang WS, Kim SB, Park SK, Park JS. Exogenous nitric oxide inhibits VCAM-1 expression in human peritoneal mesothelial cells. Role of cyclic GMP and NF-kappaB. Nephron. 2002;90:447–454. doi: 10.1159/000054733. [DOI] [PubMed] [Google Scholar]
- 106.Christakis J, Vavatsi N, Hassapopoulou H, Papadopoulou M, Mandraveli K, Loukopoulos D, et al. Comparison of homozygous sickle cell disease in northern Greece and Jamaica. Lancet. 1990;335:637–640. doi: 10.1016/0140-6736(90)90419-6. [DOI] [PubMed] [Google Scholar]
- 107.Kar BC, Satapathy RK, Kulozik AE, Kulozik M, Sirr S, Serjeant BE, et al. Sickle cell disease in Orissa State, India. Lancet. 1986;2:1198–1201. doi: 10.1016/s0140-6736(86)92205-1. [DOI] [PubMed] [Google Scholar]
- 108.Dash BP, Kar BC. Priapism is rare in sickle cell disease in India. J Assoc Physicians India. 2000;48:255. [PubMed] [Google Scholar]
- 109.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131:129–134. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Homi J, Levee L, Higgs D, Thomas P, Serjeant G. Pulse oximetry in a cohort study of sickle cell disease. Clin Lab Haematol. 1997;19:17–22. doi: 10.1046/j.1365-2257.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 111.Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood. 1993;81:3422–3427. [PubMed] [Google Scholar]
- 112.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- 113.Milner PF. Oxygen transport in sickle cell anemia. Archives of Internal Medicine. 1974;133:565–572. [PubMed] [Google Scholar]
- 114.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiner DL, Hibberd PL, Betit P, Cooper AB, Botelho CA, Brugnara C. Preliminary assessment of inhaled nitric oxide for acute vaso-occlusive crisis in pediatric patients with sickle cell disease. JAMA. 2003;289:1136–1142. doi: 10.1001/jama.289.9.1136. [DOI] [PubMed] [Google Scholar]
- 116.Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? American Journal of Critical Care and Respiratory Medicine. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 117.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Derchi G, Forni GL, Formisano F, Cappellini MD, Galanello G, D’Ascola G, et al. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:452–458. [PubMed] [Google Scholar]
- 119.Gordeuk VR. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:433b–4434. [PubMed] [Google Scholar]
- 120.Mack AK, Kato GJ. Sickle cell disease and nitric oxide: A paradigm shift? International Journal of Biochemistry and Cell Biology. 2005;38:1237–1243. doi: 10.1016/j.biocel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alexander N, Higgs D, Dover G, Serjeant GR. Are there clinical phenotypes of homozygous sickle cell disease? British Journal of Haematology. 2004;126:606–611. doi: 10.1111/j.1365-2141.2004.05025.x. [DOI] [PubMed] [Google Scholar]
- 122.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol. 2005;129:449–464. doi: 10.1111/j.1365-2141.2005.05432.x. [DOI] [PubMed] [Google Scholar]
- 123.De Franceschi L, Bachir D, Galacteros F, Tchernia G, Cynober T, Alper S, et al. Oral magnesium supplements reduce erythrocyte dehydration in patients with sickle cell disease. J Clin Invest. 1997;100:1847–1852. doi: 10.1172/JCI119713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- 125.Quinn CT, Miller ST. Risk factors and prediction of outcomes in children and adolescents who have sickle cell anemia. Hematology/Oncology Clinics of North America. 2004;18:1339–1354. doi: 10.1016/j.hoc.2004.07.004. ix. [DOI] [PubMed] [Google Scholar]


