Abstract
Rationale: Human immunodeficiency virus (HIV) infection has a major but unquantified impact on the risk of tuberculosis.
Objectives: To quantify the impact of HIV infection on the number of tuberculosis cases in San Francisco.
Methods: We studied all patients reported with tuberculosis in San Francisco from 1991 to 2002. The initial isolates of Mycobacterium tuberculosis were genotyped using IS6110 restriction fragment-length polymorphism genotyping as the primary method, and clustered cases (identical genotype patterns) were identified.
Measurements and Main Results: We determined the case number, case rate, and the fraction of tuberculosis attributable to HIV infection. Of 2,991 reported tuberculosis cases, 2,193 (73.3%) had a genotype pattern of M. tuberculosis available. Genotypic clusters with at least one HIV-positive person were larger, lasted longer, and had a shorter time between successive cases relative to clusters with only HIV-uninfected persons (P < 0.00005, P = 0.0009, P = 0.018, respectively). Overall, 13.7% of the tuberculosis cases were attributable to HIV infection and an estimated 405 excess tuberculosis cases occurred.
Conclusions: During a period encompassing the resurgence and decline of tuberculosis in San Francisco, a substantial number of the tuberculosis cases were attributable to HIV infection. Coinfection with HIV amplified the local tuberculosis epidemic.
Keywords: tuberculosis, HIV infection, transmission, genotyping
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Human immunodeficiency virus (HIV) infection has a major but unquantified impact on the risk of tuberculosis.
What This Study Adds to the Field
Genotypic clusters of tuberculosis with at least one HIV-positive person were larger, lasted longer, and had a shorter time between successive cases relative to clusters with only HIV-uninfected persons. Coinfection with HIV amplified the local tuberculosis epidemic in San Francisco from 1991 to 2002, as 13.7% of tuberculosis cases were attributed to HIV infection.
Infection with the human immunodeficiency virus (HIV) has an enormous impact on the epidemiologic and clinical features of tuberculosis worldwide, particularly in resource-poor countries (1–5). Advanced HIV infection alters the clinical manifestations of tuberculosis: it increases the risk of reactivation of latent tuberculosis infection and exogenous reinfection (6–9), the infection progresses more rapidly to active disease (10), pulmonary cavitation is less likely to occur (11), and death rates are higher (12, 13). Although an increased number of HIV-coinfected tuberculosis cases might increase tuberculosis case rates overall, a higher death rate among HIV-positive patients with tuberculosis could reduce the sources of infection with Mycobacterium tuberculosis within the community. The overall impact of HIV on tuberculosis may ultimately depend on the interplay of multiple, complex factors (5).
We sought to quantify the extent to which HIV infection affects a local tuberculosis epidemic over a relatively long period of time. We used data from a prospective, 12-year, population-based, molecular epidemiologic study of tuberculosis to determine the tuberculosis case rates in HIV-positive compared with HIV-uninfected patients, to estimate the magnitude of transmission from HIV-positive patients to HIV-uninfected individuals; and to estimate the fraction of tuberculosis cases attributable to HIV infection. Because highly active antiretroviral therapy (HAART) became widely available in San Francisco during late 1996, we also assessed its impact on tuberculosis case numbers and rates.
The study was conducted in San Francisco, a city that has been severely affected by the HIV and AIDS epidemics. In 2004, San Francisco had the third largest number of persons living with AIDS in the United States (14). In 2003, an estimated 18,000 to 19,000 people were living with HIV and AIDS out of 719,600 San Francisco residents, for an overall HIV prevalence of 2.5%. The AIDS epidemic in San Francisco has mainly affected homosexual men and a smaller proportion of heterosexuals, injection drug users (IDUs), and nonwhites (14). During the 1990s, the estimated HIV prevalence levels were 29% among homosexual men (15), 24% among homeless males (16), and 31% among IDUs (15) in San Francisco, similar to HIV prevalence levels in sub-Saharan Africa (17). The preliminary results of this study were previously reported as an abstract (18).
METHODS
Study Population
All patients with tuberculosis reported in San Francisco from January 1, 1991, through December 31, 2002, were enrolled as part of a prospective study of the molecular epidemiology of tuberculosis (19). The routine microbiologic evaluation included microscopy for acid-fast bacilli, mycobacterial culture, and drug susceptibility tests if the culture was positive. All patients, regardless of HIV serostatus, were treated with a regimen consisting of isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months followed by isoniazid and rifampin for 4 months unless modifications were made because of drug intolerance, underlying drug resistance, or to avoid drug interactions. The Committee on Human Research of the University of California, San Francisco, approved the study protocol.
Genotyping
The initial isolates of M. tuberculosis were analyzed by standardized IS6110 restriction fragment-length polymorphism (RFLP) genotyping (20) and were compared using computer software (BioImage Whole Band Analyzer, version 3.0; Millepore, Ann Arbor, MI) (21). We defined a genotypic cluster as a group of two or more patients whose isolates had at least six bands in identical IS6110 RFLP patterns and were collected within 12 months of each other (19, 20, 22). We further analyzed isolates with fewer than six bands in their IS6110 RFLP pattern using a probe for the polymorphic guanine-cytosine–rich sequence and compared the patterns visually (23). Patients whose isolate had fewer than six bands were clustered if their IS6110 RFLP patterns were identical; if the number, relative intensity, and molecular weights of polymorphic guanine-cytosine–rich sequence bands were identical; and if the isolates were collected within 12 months of each other (19, 20).
We chronologically ordered the tuberculosis cases in each genotypic cluster by the date that the first culture-positive specimen was obtained for each patient. We initially assumed that the first case of pulmonary tuberculosis in a cluster resulted from the reactivation of a latent tuberculosis infection and was the source case patient. Subsequent pulmonary and/or extrapulmonary cases in the same genotypic cluster likely resulted from recent transmission and rapid progression to disease, and were defined as secondary cases if they occurred within 12 months of the source case or each other. Because of temporal variation in the time to diagnosis and the possibility that case finding and diagnosis were more rapid among HIV-infected persons, it is likely that the first case of pulmonary tuberculosis was not the source case in every single cluster. Therefore, we performed a sensitivity analysis by assuming that the second, then the third, case of pulmonary tuberculosis to be diagnosed was the source case. Finally, we assumed that patients whose initial isolate had a unique genotype pattern resulted from reactivation of a latent tuberculosis infection (19, 20).
Statistical Analysis
We used California state estimates of the population of San Francisco (24, 25) to calculate case rates and used the incidence rate data test or the χ2 test for trends. For the analysis, persons who were HIV negative and persons who did not have a test result were grouped as HIV uninfected. We calculated the tuberculosis case rate among persons coinfected with HIV using the estimated number of HIV-positive persons in San Francisco as the denominator (San Francisco Department of Public Health, unpublished data). Similarly, we calculated the tuberculosis case rate among HIV-uninfected patients using the estimated number of persons in San Francisco not known to be HIV positive as the denominator. The treatment success ratio was estimated as the percentage of patients with tuberculosis who were cured, defined by either bacteriologic confirmation, or completion of their drug treatment regimen with no clinical or radiographic evidence of active tuberculosis. The case fatality ratio (CFR) was estimated as the percentage of tuberculosis cases who died during treatment.
We used estimates of the number of HIV-positive persons in San Francisco during each year of the study to calculate the population attributable fraction (PAF), the attributable fraction of tuberculosis among the HIV-positive population (AFE), and the number of excess patients coinfected with M. tuberculosis and HIV that did occur but could have been prevented if HIV infection were absent. We calculated the PAF of tuberculosis cases due to the direct effect of HIV as follows:
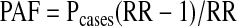 |
where Pcases is the prevalence of HIV among tuberculosis cases and RR is the rate ratio of tuberculosis among HIV-positive versus HIV-uninfected persons. We estimated the AFE using the following equation:
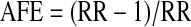 |
where RR is the rate ratio of tuberculosis among HIV-positive versus HIV-uninfected persons.
We performed univariate and multivariate logistic regression modeling to identify the patient characteristics associated with secondary tuberculosis cases and to estimate the odds ratios (ORs) or rate ratios (RRs) for associations with HIV infection, and their 95% confidence interval (CI).
RESULTS
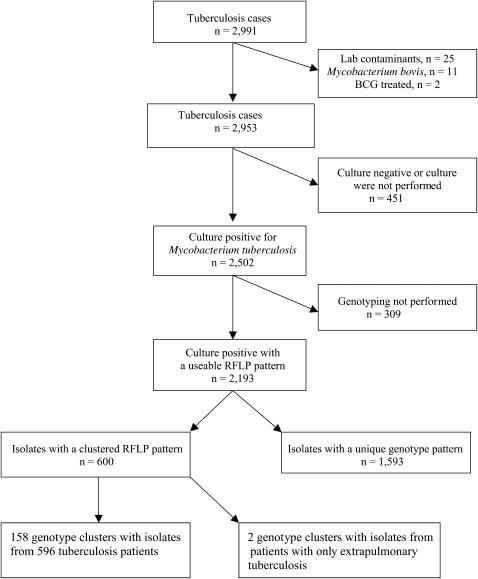
A total of 2,991 tuberculosis cases were reported to the Tuberculosis Section of the San Francisco Department of Public Health during the 12-year study period. We excluded 25 cases that met predefined criteria for laboratory cross-contamination (26), 11 cases of Mycobacterium bovis, and 2 patients who had been treated with M. bovis bacille Calmette-Guérin for bladder cancer that resulted in extrapulmonary disease, and analyzed the remaining 2,953 cases (Figure 1). The annual number of cases was greatest in 1992 (364 cases; case rate, 49.8 per 100,000 population) and progressively decreased thereafter (P < 0.000005) (Table E1 of the online supplement).
Figure 1.
Derivation of the study population for genotypic cluster analysis, San Francisco, 1991–2002. Boxes to the right indicate exclusions leading to a study population of 2,193 patients with genotyped isolates. We excluded 25 patients whose isolates were laboratory contaminants, 11 cases of Mycobacterium bovis, and 2 patients who were treated with bacille Calmette-Guèrin (BCG). RFLP = restriction fragment-length polymorphism.
An HIV test result was available for 44.1% of all patients with tuberculosis and 61.6% of patients aged between 18 and 55 years. Patients who did not have HIV counseling and testing were more likely to have characteristics of low–HIV risk groups in San Francisco: for example, being female, foreign born, or of Asian race; younger than 25 years or older than 44 years; and with no report of substance abuse or homelessness (Table E2). In a multivariate model, being a young adult (25–44 yr) was the patient characteristic most strongly associated with having an HIV test result (OR, 3.8; 95% CI, 3.2–4.4; P < 0.0001). Patients were more likely to have an HIV test result during 1997–2002, when antiretroviral therapy for HIV disease was widely available, than during 1991–1996 (P < 0.0005). By age, 70.9% of patients with tuberculosis 18–55 years old who were reported during 1997–2002 had an HIV test result versus 56.9% of those reported during 1991–1996 (P < 0.0001).
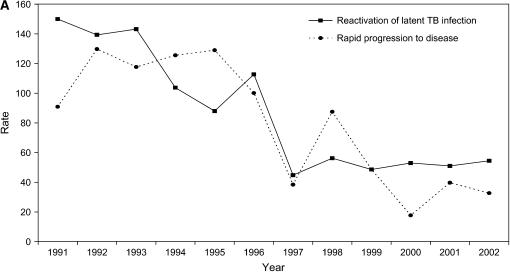
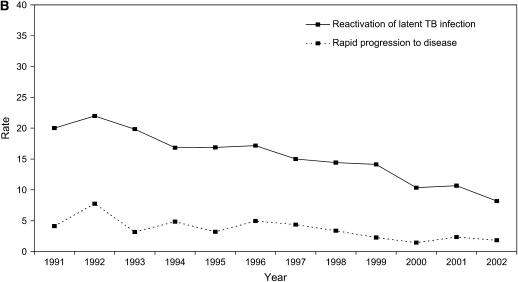
Tuberculosis case rates in San Francisco were nearly eight times higher among persons infected with HIV than among persons who were not infected with HIV (RR, 7.7; 95% CI, 6.9–8.5). During the 12-year period, the tuberculosis case rate decreased significantly among HIV-positive patients by 56.6% (P < 0.00006), and it decreased, but not significantly, among HIV-uninfected patients by 54.4% (P = 0.086) (Figures 2A and 2B, and Table E1).
Figure 2.
Tuberculosis case rates as the number of tuberculosis cases per 100,000 population, by HIV test result. (A) Rates of HIV-positive tuberculosis cases due to reactivation of latent tuberculosis infection (solid line) versus secondary cases caused by recent transmission and rapid progression to disease (dashed line). We calculated the tuberculosis case rate among persons coinfected with HIV and Mycobacterium tuberculosis using the estimated number of HIV-positive persons in San Francisco as the denominator (San Francisco Department of Public Health, unpublished data). (B) Rates of HIV-uninfected tuberculosis cases due to reactivation of latent tuberculosis infection (solid line) versus secondary cases caused by recent transmission and rapid progression to disease (dashed line). We calculated the tuberculosis case rate among HIV-uninfected patients using the estimated number of persons living in San Francisco minus the estimated number of HIV-positive persons in San Francisco as the denominator. TB = tuberculosis.
Genotyping
Approximately 85% of the 2,953 tuberculosis cases had a positive culture for M. tuberculosis. A genotyping result was available among 87.7% of the culture-positive cases (Figure 1). Culture-positive patients who lacked a genotype result were more likely to be Asian/Pacific Islanders, younger than 25 years old or at least 45 years old, or foreign born; to have extrapulmonary tuberculosis only; and to have no reported substance abuse (injecting drugs, non-injecting drugs, and/or excessive alcohol) or homelessness during the 12 months before their diagnosis (P < 0.005 for each comparison).
There were 1,593 tuberculosis cases whose isolate had a unique fingerprint pattern (72.6%) and 600 cases (27.4%) whose isolate had a fingerprint pattern that was clustered. A putative source case could not be identified for two clusters of two persons each with only extrapulmonary tuberculosis, and all four patients were excluded from further analyses of genotyping. We analyzed the remaining 2,189 patients, including 596 patients in 158 clusters. Among HIV-infected persons, 59.0% (230/390) of the culture-positive genotyped tuberculosis cases were due to reactivation of latent tuberculosis infection and 41.3% (161/390) were due to recent transmission that generated HIV-positive secondary cases. Among HIV-uninfected persons, 84.5% (1,521/1,799) of the culture-positive genotyped cases were likely due to reactivation of a latent tuberculosis infection and 15.5% (278/1,799) were secondary cases due to recent tuberculosis transmission (P < 0.0005). Approximately 11% (230/2,189) of all culture-positive genotyped tuberculosis cases were due to reactivation of a latent tuberculosis infection in an HIV-positive person and 7.4% (161/2,189) were due to recent transmission that generated an HIV-positive secondary case.
Of the 596 clustered cases, 438 were secondary cases, and 36.6% of the secondary cases were HIV-positive persons. The strongest independent risk factors associated with being a secondary case of tuberculosis in a genotypic cluster were as follows: if the source case was sputum smear positive (adjusted OR [AOR], 1.8; 95% CI, 1.2–2.5; P < 0.0005), reported noninjecting drug use (AOR, 3.7; 95% CI, 1.4–9.4; P = 0.007), and reported substance abuse (AOR, 13.5; 95% CI, 5.3–34.1; P < 0.0005); and if the secondary case individual was of Asian race (AOR, 14.5; 95% CI, 4.4–47.4; P < 0.0005) or African-American race (AOR, 4.0; 95% CI, 1.6–9.4; P = 0.003), born in the United States (AOR, 31.8; 95% CI, 10.7–93.9; P < 0.0005), or HIV positive (AOR, 6.1; 95% CI, 3.0–12.7; P < 0.0005) (Table 1). There is confounding in the multivariate model by place of birth, substance abuse, increasing age, and race.
TABLE 1.
FACTORS ASSOCIATED WITH BEING A SECONDARY CASE OF TUBERCULOSIS, SAN FRANCISCO, 1991–2002
| Unadjusted OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics of the Source Cases | ||||||||||||
| Male | 2.7 | 2.0–3.6 | <0.00005 | |||||||||
| Age < 45 yr | 1.5 | 1.0–2.2 | 0.0418 | |||||||||
| Race | ||||||||||||
| Asian | Ref | 0.1 | 0.0–0.2 | <0.0005 | ||||||||
| White | 1.0 | 0.7–1.6 | 0.9574 | |||||||||
| African American | 2.6 | 1.5–4.5 | 0.0002 | 0.2 | 0.1–0.6 | 0.002 | ||||||
| Latino | 0.1 | 0.1–0.2 | <0.00005 | |||||||||
| Native American | 0.5 | 0.0–36.3 | 0.5699 | |||||||||
| U.S. born | 2.8 | 2.2–3.5 | <0.00005 | 0.1 | 0.0–0.2 | <0.0005 | ||||||
| HIV positive | 4.1 | 3.2–5.2 | <0.00005 | 0.2 | 0.1–0.4 | <0.0005 | ||||||
| Sputum smear positive | 2.1 | 1.6–2.6 | <0.00005 | 1.8 | 1.2–2.5 | <0.0005 | ||||||
| Initial resistance to isoniazid | 0.3 | 0.2–0.5 | <0.00005 | |||||||||
| Homeless* | 4.0 | 2.9–5.6 | <0.00005 | |||||||||
| Injecting drug use* | 0.9 | 0.4–1.9 | 0.7061 | |||||||||
| Noninjecting drug use* | 6.6 | 4.7–9.1 | <0.00005 | 3.7 | 1.4–9.4 | 0.007 | ||||||
| Excessive alcohol use* | 3.0 | 2.1–4.2 | <0.00005 | |||||||||
| Substance abuse* | 5.3 | 3.9–7.1 | <0.00005 | 13.5 | 5.3–34.1 | <0.0005 | ||||||
| Characteristics of the Secondary Cases | ||||||||||||
| Male | 1.5 | 1.2–1.9 | <0.0006 | |||||||||
| Age < 45 yr | 2.1 | 1.7–2.6 | <0.00005 | |||||||||
| Race | ||||||||||||
| Asian | Ref | 14.5 | 4.4–47.4 | <0.0005 | ||||||||
| African American | 10.3 | 7.6–14.0 | <0.00005 | 4.0 | 1.6–9.4 | 0.003 | ||||||
| White | 3.9 | 2.9–5.1 | <0.00005 | |||||||||
| Latino | 2.9 | 2.0–4.2 | 0.00005 | |||||||||
| Native American | 8.1 | 2.3–27.2 | <0.00005 | |||||||||
| U.S. born | 6.6 | 5.2–8.3 | <0.00005 | 31.8 | 10.7–93.9 | <0.0005 | ||||||
| HIV positive | 3.7 | 2.9–4.7 | <0.00005 | 6.1 | 3.0–12.7 | <0.0005 | ||||||
| Sputum smear positive | 1.6 | 1.2–2.0 | 0.0002 | |||||||||
| Initial resistance to isoniazid | 0.3 | 0.2–0.6 | <0.00005 | 0.2 | 0.1–0.7 | 0.007 | ||||||
| Homeless* | 4.5 | 3.4–6.0 | <0.00005 | |||||||||
| Injecting drug use* | 6.1 | 4.0–9.1 | <0.00005 | |||||||||
| Noninjecting drug use* | 4.5 | 3.3–6.1 | <0.00005 | 0.3 | 0.1–0.9 | 0.024 | ||||||
| Excessive alcohol use* | 3.0 | 2.2–4.4 | <0.00005 | |||||||||
| Substance abuse*† | 4.5 | 3.4–5.8 | <0.00005 | 0.1 | 0.1–0.3 | <0.0005 | ||||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio; Ref = reference value.
Values are based on univariate and multivariate logistic regression analysis.
Within the 12 months before the diagnosis of tuberculosis.
Substance abuse was defined as reporting at least one of the following behaviors: injecting drug use, noninjecting drug use, or excessive alcohol.
Of 158 genotypic clusters, 8 (5.1%) had only HIV-positive patients, 66 (41.8%) had a mixture of HIV-positive and HIV-uninfected patients, and 85 (53.8%) had only HIV-uninfected patients (Table 2). However, 63.6% of the 596 clustered patients were in genotypic clusters with a mixture of both HIV-positive and HIV-uninfected patients. We combined all of the genotypic clusters that had at least one HIV-positive patient and compared them with the genotypic clusters of only HIV-uninfected patients. Genotypic clusters that had at least one HIV-positive patient were significantly larger than genotypic clusters with only HIV-uninfected patients (P < 0.00005) (Table 2).
TABLE 2.
COMPARISON OF GENOTYPE CLUSTERS ACCORDING TO THE HIV TEST RESULT OF THE PATIENTS WITH TUBERCULOSIS IN THE CLUSTERS, SAN FRANCISCO, 1991–2002
| Characteristics of Clusters | Total | Clusters with at Least One HIV-positive Patient with TB | Clusters of Only HIV-uninfected Patients with TB | P Value |
|---|---|---|---|---|
| No. of clusters (%) | 158 (100.0) | 73 (46.2) | 85 (53.8) | <0.0005 |
| No. of cases in cluster (%) | 596 (100.0) | 377 (63.3) | 219 (36.7) | <0.0005 |
| Median cluster size (range) | 2 (2–31) | 3 (2–31) | 2 (2–12) | <0.00005 |
| Median cluster duration, d* (range) | 178 (1–2,346) | 243 (1–2,346) | 96 (1–1,422) | 0.009 |
| Median time between successive cases, d (range) | 62.0 (1–361) | 57.0 (1–354) | 78.5 (1–361) | 0.018 |
Definition of abbreviations: TB = tuberculosis.
First case to last case.
The median time from the first to the last tuberculosis cases of the genotypic clusters was 178.0 days, and the longest time that a strain was prevalent in the 12-year period was 2,346 days or 6.6 years (Table 2). The median duration of genotypic clusters with at least one HIV-positive person was significantly greater (243 d) than that of genotypic clusters with only HIV-uninfected patients (96 d) (P = 0.009). In addition, the median time between successive cases in the 158 genotypic clusters was 62.0 days, but was significantly shorter in clusters with at least one HIV-positive patient (57 d) compared with genotypic clusters with only HIV-uninfected patients (78.5 d) (P = 0.018).
We further examined the 438 secondary cases in genotypic clusters. Assuming that the first pulmonary case diagnosed in the cluster was the source case, 80 (18.3%) HIV-positive individuals and 90 (20.5%) HIV-uninfected individuals were in clusters with an HIV-positive source case. Seventy-eight (17.8%) HIV-positive individuals and 190 (43.4%) HIV-uninfected individuals were in clusters with an HIV-uninfected source case. Overall, 61.2% of the secondary tuberculosis cases had the same HIV serostatus as their respective source case. By sensitivity analysis, the percentages of HIV-positive and HIV-uninfected source cases and secondary cases changed little (<3% in each category) (Table E3).
The tuberculosis case rate decreased between 1991 and 2002 by 58.7% (P = 0.00081) among tuberculosis cases due to reactivation of a latent tuberculosis infection, and by 62.4% (P = 0.00002) among the secondary cases due to recent transmission. During 1991–1996, the tuberculosis case rate due to reactivation of a latent tuberculosis infection and secondary cases decreased (13.7 and 47.8%, respectively), but not significantly (P = 0.277 and P = 0.052, respectively). However, during 1997–2002, after the introduction of HAART, the tuberculosis case rate due to reactivation of a latent tuberculosis infection decreased significantly by 44.9% (P = 0.017) and tuberculosis case rates due to recent transmission declined by 34.4%, but this decline was not significant (P = 0.154).
Attributable Fraction of Tuberculosis Cases Caused by HIV Coinfection
The average prevalence of HIV coinfection among patients with tuberculosis in San Francisco during 1991–2002 was 15.7%, ranging from 21.6% in 1991 to a low of 7.4% in 1997 (Table 3). The PAF for HIV infection over the entire study period was 13.7%, but it declined by 30.6% from 19.2% in 1991 to 14.7% in 2002 and was lowest in 1997 (5.4%). Among tuberculosis cases caused by reactivation of latent infection, the PAF for HIV infection was 11.1%, ranging from 16.4% in 1991 to 5.3% in 1999. Among secondary cases in genotypic clusters, who had a higher prevalence of HIV than persons with reactivated disease, the average PAF for HIV infection was 35.1%, ranging from 50.1% in 1995 to 10.6% in 2000. An excess of 405 tuberculosis cases attributable to HIV infection occurred during the study period. Approximately 56% of the excess cases were from reactivation of latent tuberculosis infection, whereas 44% were secondary tuberculosis cases from recent transmission of M. tuberculosis and rapid progression to disease. Although the PAF for HIV infection was greater for secondary tuberculosis cases, the absolute number of excess cases of tuberculosis due to HIV infection in San Francisco was greater for reactivation of latent tuberculosis infection.
TABLE 3.
ESTIMATES OF THE RATE RATIO OF HIV-POSITIVE TUBERCULOSIS CASES VERSUS HIV-UNINFECTED TUBERCULOSIS CASES, POPULATION ATTRIBUTABLE FRACTION, AND EXCESS TUBERCULOSIS CASES, SAN FRANCISCO, 1991–2002
| Year* | RR | 95% CI | Prevalence of HIV+ (%) | AFE (%) | PAF (%) | Total No. of TB Cases | No. of TB Cases if HIV Were Absent | Excess Cases (Total Cases × PAF) |
|---|---|---|---|---|---|---|---|---|
| 1991 | 8.8 | 6.7–11.6 | 21.6 | 88.7 | 19.2 | 319 | 257 | 62 |
| 1992 | 7.0 | 5.2–9.2 | 17.0 | 85.7 | 14.6 | 364 | 311 | 53 |
| 1993 | 9.1 | 6.8–12.0 | 19.7 | 89.0 | 17.5 | 320 | 264 | 56 |
| 1994 | 9.9 | 7.2–13.4 | 20.0 | 89.9 | 18.0 | 270 | 221 | 49 |
| 1995 | 8.6 | 6.1–12.0 | 16.9 | 88.4 | 15.0 | 260 | 221 | 39 |
| 1996 | 8.4 | 5.9–11.7 | 15.5 | 88.0 | 13.6 | 271 | 234 | 37 |
| 1997 | 3.7 | 2.1–6.2 | 7.4 | 73.2 | 5.4 | 217 | 205 | 12 |
| 1998 | 7.1 | 4.6–10.5 | 13.3 | 85.9 | 11.4 | 218 | 193 | 25 |
| 1999 | 5.7 | 3.6–8.7 | 11.2 | 82.5 | 9.2 | 224 | 203 | 21 |
| 2000 | 5.3 | 2.7–8.9 | 10.6 | 81.1 | 8.6 | 161 | 147 | 14 |
| 2001 | 4.9 | 2.8–8.0 | 10.1 | 79.5 | 8.0 | 179 | 164 | 15 |
| 2002 | 8.4 | 5.2–13.0 | 16.7 | 88.1 | 14.7 | 150 | 128 | 22 |
| Total | 7.7 | 7.0–8.47 | 15.7 | 87.0 | 13.7 | 2,953 | 2,548 | 405 |
Definition of abbreviations: AFE = attributable fraction among the exposed (HIV+ persons); CI = confidence interval; PAF = population attributable fraction, 100 × [Pcases(RR − 1)/RR]; Pcases = prevalence of HIV-positive persons in the population; RR = rate ratio of HIV+:HIV-uninfected patients with TB; TB = tuberculosis.
P < 0.00005 for all years.
The percentage of tuberculosis cases among HIV-positive patients in San Francisco that was attributable to their HIV infection (AFE) during the 12-year period was 87.0%, and was highest in 1994 (89.9%) and lowest in 1997 (73.2%) (Table 3).
Treatment Success and Case Fatality Ratios
Treatment success for all patients with tuberculosis was 85.9%, ranging from a low of 70.5% in 1993 to a high of 89.2% in 2001, and has been greater than 85% since 1996. Treatment success was significantly lower among HIV-positive compared with HIV-uninfected patients (76.5 vs. 85.9%, P = 0.001).
The case fatality ratio for all patients with tuberculosis was 12.0%, but was higher among patients coinfected with HIV than among HIV-uninfected patients (22.0 vs. 10.3%, respectively; P = 0.035). HIV-positive patients with tuberculosis were just as likely to die with tuberculosis during 1997–2002, after the introduction of HAART, as during 1991–1996 (P = 0.204). Similarly, HIV-positive patients were just as likely as HIV-uninfected patients to be diagnosed at death and before initiating antituberculosis treatment (P = 0.62), but HIV-positive patients with tuberculosis were twice as likely to die during treatment (RR, 2.1; 95% CI, 1.7–2.6; P < 0.00005) and had a shorter survival time (P < 0.00005, data not shown).
DISCUSSION
Our study demonstrates the impact that HIV infection has had on tuberculosis transmission dynamics, treatment, and mortality in San Francisco during a 12-year period. Genotypic clusters with at least one HIV-positive patients with tuberculosis in the cluster were larger, lasted longer, and had a shorter time interval between successive cases, relative to clusters with only HIV-uninfected persons. Our study suggests that the high HIV prevalence in San Francisco amplified the local tuberculosis epidemic: 13.7% of San Francisco's tuberculosis cases were attributable to HIV in the population and there occurred an excess of 405 tuberculosis cases, most of them among patients with reactivation of latent tuberculosis infection. The PAF in San Francisco is similar to the PAF (14%) in Harare, Zimbabwe (27).
Although intensified tuberculosis control efforts were underway in San Francisco and the tuberculosis case rates declined among HIV-positive and HIV-uninfected individuals (19), tuberculosis case rates remained significantly higher among HIV-positive individuals. Tuberculosis case rates decreased more rapidly among HIV-positive persons, particularly those with reactivation of latent tuberculosis infection (Figures 2A and 2B). During the study period, targeted interventions, such as directly observed therapy and isoniazid to treat latent tuberculosis, were used to interrupt transmission of M. tuberculosis, particularly among homeless and HIV-positive persons (19). By 1997, the Department of Public Health began location-based screening and active case finding among IDUs and homeless and HIV-positive persons, and the incidence rate ratios decreased when fully adjusting for confounding by injection drug use, substance abuse, and homelessness in the multivariate model. HAART was widely available in San Francisco by 1997 and likely reduced the risk of rapid progression to disease, thereby decreasing the tuberculosis case rate among HIV-positive persons. Several other studies have documented decreases in the tuberculosis incidence rate soon after the introduction of antiretroviral therapy (28–31). However, such an improvement may be short-lived if it coincides with increased risk-taking behaviors that enhance the opportunities for HIV transmission (9, 28).
There are currently three different approaches to evaluate the infectiousness of M. tuberculosis in HIV-positive versus HIV-uninfected persons. First, the annual risk of tuberculosis infection (ARTI) estimates the number of new infections occurring among young schoolchildren, and one would expect the ARTI to increase in populations with an increasing prevalence of HIV. However, the ARTI can be influenced by many factors and provides an ecological analysis at best (32–34). For example, as HIV prevalence increased, the prevalence of tuberculosis infection increased in Kenya (35) but not in Tanzania (36). Second, one can look at the period of infectiousness of a patient with tuberculosis and make inferences about the transmission potential. Corbett and colleagues showed that HIV-positive gold miners in South Africa had a significantly shorter mean duration of smear positivity or infectiousness (0.17 yr) than HIV-negative persons (1.15 yr), a sixfold difference likely due to increased presentation to health care providers and increased case-finding rates among HIV-positive persons (37). In contrast, the estimated duration of infectiousness was similar for HIV-positive and HIV-negative individuals in a community-based study in South Africa (38). A third approach is to assess tuberculosis infections among the contacts of the source cases. A meta-analysis concluded that patients with tuberculosis and HIV-1 infection are not intrinsically more infectious to their contacts than are HIV-1–negative patients with tuberculosis (39), and a study in Brazil showed that HIV-positive index cases may even be less infectious to their contacts (40). Taken together, multiple studies using the first three approaches demonstrate that patients with HIV-related tuberculosis are not more infectious, and may even be less infectious, than HIV-uninfected patients.
Our study was unable to determine the infectiousness of HIV-positive patients with tuberculosis compared with HIV-uninfected patients. However, by using molecular genotyping methods, we were able to estimate the number of secondary cases that arose from HIV-positive and HIV-uninfected source cases. Glynn and coworkers used a molecular epidemiologic approach to evaluate recent infection with M. tuberculosis in northern Malawi, an area in Africa with a high HIV prevalence, and reported the PAF was 57%; more than half of the sputum smear–positive pulmonary tuberculosis cases were attributed to HIV infection (41). Furthermore, nearly half of the tuberculosis cases arising from recent infection in their study population had acquired the infection from an HIV-positive source case (42). Similarly, by assuming that the first case in a cluster is the source case, we estimated that 38.8% of the tuberculosis cases due to recent transmission in San Francisco acquired the infection from an HIV-positive source case. In addition, one in every five secondary cases was caused by transmission from an HIV-positive source case to an HIV-uninfected person, an estimate upheld by sensitivity analysis. HIV-positive persons account for a significant amount of tuberculosis transmission to both HIV-positive and HIV-uninfected populations (5).
Our data suggest that transmission to HIV-positive contacts is important for the amplification of a local tuberculosis epidemic. The prevalence of HIV infection is often greater among the contacts of HIV-positive patients with tuberculosis than among the contacts of HIV-uninfected patients (43, 44). Transmission of M. tuberculosis to HIV-positive persons often leads to rapid progression to disease in the newly infected individuals (10), partially explaining why HIV-positive persons were likely to become secondary cases of tuberculosis and within a shorter time interval than HIV-uninfected contacts and HIV-uninfected patients. In a retrospective cohort study among gold miners in South Africa, the tuberculosis case rate was 2.9 cases per 100 person-years in HIV-positive miners and 0.8 cases per 100 person-years in HIV-negative miners (adjusted RR of tuberculosis, 2.9), and unexpectedly, the tuberculosis incidence was doubled (adjusted RR, 2.1) within 1 year of HIV seroconversion (45). In our 12-year study, genotypic clusters with at least one HIV-positive person were larger, lasted longer, and had a shorter time between successive cases relative to clusters with only HIV-uninfected persons in the genotypic cluster. Therefore, unless they compensate for the rapid progression to disease among HIV-positive contacts, current models of tuberculosis incidence could underestimate the effect of HIV infection in areas where tuberculosis is endemic (46, 47).
Our findings document and quantify the relative transmission of M. tuberculosis by patients with tuberculosis who are coinfected with HIV before they die or are rendered noninfectious by antituberculosis therapy. The overall CFR was high (12%) and was higher still among HIV-positive patients with tuberculosis (P < 0.035). The high CFRs could be due to the difficulties and delays in diagnosing tuberculosis. However, there was no significant difference in the proportion of HIV-positive and HIV-uninfected persons who were diagnosed at death, and theoretically, such untreated persons could still be infectious and transmit M. tuberculosis before their death. In addition, confounding factors such as homelessness, higher rates of exposure to HIV-positive contacts, and social networks linked to substance abuse may also facilitate tuberculosis transmission into populations where the HIV prevalence is high.
There are some limitations in the present study. First, not all patients with tuberculosis received HIV counseling and testing; thus, some misclassification bias by HIV serostatus may have occurred. However, patients who were not tested for HIV came from populations and risk groups that are known to have a low prevalence of HIV infection. Nevertheless, the exclusion of a group at low risk for tuberculosis transmission may have led us to overestimate transmission-related tuberculosis in the entire population. If some individuals who did not have HIV counseling and testing and were classified as HIV uninfected were actually HIV positive, their reclassification would strengthen our conclusions. Second, a genotyping pattern was available for most, but not all, of the culture-confirmed cases. Although undersampling is likely to underestimate the true rate of clustering (22), it is unlikely to change our conclusions, because the availability of genotyping did not differ by HIV serostatus. Third, we initially assumed that the first case in each cluster was the source case for the cluster. Because it is likely that the first patient diagnosed with tuberculosis was not the source case in every single cluster, we performed a sensitivity analysis in which we altered the source case between the first, second, and third case. The sensitivity analysis did not change our results significantly (Table E3). Fourth, children younger than 5 years were a very small portion of our study population (1.9%), so our results have limited inferences for pediatric populations. Finally, we do not have information about the HIV status of all of the contacts in our study, but if HIV-positive persons were more likely to have other HIV-positive persons among their close contacts, we could have overestimated the number of secondary cases attributed to HIV-positive patients with tuberculosis (44).
Our findings have important public health implications. Intensified tuberculosis case finding and treatment can reduce tuberculosis transmission and lower the case number and case rate over time, even in settings with high HIV prevalence. Routine HIV counseling, testing, early diagnosis of infection, and assessment for HAART are appropriate interventions for patients with tuberculosis, their contacts, and populations with a high risk of exposure to tuberculosis and HIV, such as the homeless (48–50). Targeted interventions that prevent infection with HIV, and prevent tuberculosis in persons coinfected with HIV and M. tuberculosis, will reduce the burden of disease in communities (5).
Supplementary Material
Acknowledgments
The staff and collaboration of the Tuberculosis Clinic in the Tuberculosis Section of the San Francisco Department of Public Health made this work possible. The authors thank the members of Stanford University's molecular genotyping laboratory who performed the RFLP; Ling Hsu, M.P.H., and Sandra Schwarcz, M.D., HIV/AIDS Statistics and Epidemiology Section, San Francisco Department of Public Health, for estimating the numbers of HIV-positive persons in San Francisco during 1991–2002; and AJRCCM Associate Editor Dr. W. W. Yew and several anonymous reviewers, whose comments significantly improved the manuscript.
Supported by National Institutes of Health grants K01 TW000001 (K.D.) and NIAID AI 34238 (P.C.H., C.L.D.).
A summary of this study was presented at the Centers for Disease Control and Prevention's Public Health Poster Forum at the 100th International Conference of the American Thoracic Society held May 20–25, 2005, in San Diego, California.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200603-440OC on August 9, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003;163:1009–1021. [DOI] [PubMed] [Google Scholar]
- 2.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis 1999;3:457–465. [PubMed] [Google Scholar]
- 3.Currie CS, Williams BG, Cheng RC, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS 2003;17:2501–2508. [DOI] [PubMed] [Google Scholar]
- 4.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA 2005;293:2767–2775. [DOI] [PubMed] [Google Scholar]
- 5.Nunn P, Williams BG, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev Immunol 2005;5:819–826. [DOI] [PubMed] [Google Scholar]
- 6.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, Battegay M. Isoniazid prophylaxis for tuberculosis in HIV infection: meta-analysis of randomized controlled trials. AIDS 1999;13:501–507. [DOI] [PubMed] [Google Scholar]
- 7.Selwyn PA, Schoenbaum EE, Davenny K, Robertson VJ, Feingold AR, Shulman JF, Mayers MM, Klein RS, Friedland GH, Rogers MF. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 1989;32:545–550. [DOI] [PubMed] [Google Scholar]
- 8.Antonucci G, Girardi E, Raviglione MC, Ippolito C. Risk factors for tuberculosis in HIV-infected persons: a prospective cohort study. The Gruppo Italiano di Studio Tuberculosi e AIDS (GISTA). JAMA 1995;274:143–148. [DOI] [PubMed] [Google Scholar]
- 9.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science 2003;301:1535–1537. [DOI] [PubMed] [Google Scholar]
- 10.Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR Jr, Hopewell PC. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction fragment length polymorphisms. N Engl J Med 1992;326:321–325. [DOI] [PubMed] [Google Scholar]
- 11.Geng E, Kreiswirth B, Burznyski J, Schluger N. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA 2005;293:2740–2745. [DOI] [PubMed] [Google Scholar]
- 12.Whalen CC, Nsubuga P, Okwera A, Johnson JL. HoM DL, Michael NL, Mugerwa RD, Ellner JJ. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 2000;14:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafer RW, Bloch AB, Larkin C, Vasudavan V, Seligman S, Dehovitz JD, DiFerdinando G, Stoneburner R, Cauthen G. Predictors of survival in HIV-infected tuberculosis patients. AIDS 1996;10:269–272. [DOI] [PubMed] [Google Scholar]
- 14.San Francisco HIV Prevention Planning Council. Apr 2004. 2004 San Francisco HIV Prevention Plan. Centers of Disease Control and Prevention: Cooperative Agreement No. U62CCU923478-01. Available from: San Francisco Department of Public Health (SFDPH) AIDS Office, San Francisco, CA.
- 15.San Francisco Department of Public Health (SFDPH) AIDS Office. 2004. HIV Consensus Meeting Report. San Francisco, CA: SFDPH AIDS Office.
- 16.Zolopa AR, Hahn JA, Gorter R, Miranda J, Wlodarczyk D, Petereson J, Pilote L, Moss AR. HIV and tuberculosis infection in San Francisco's homeless adults: prevalence and risk factors in a retrospective sample. JAMA 1994;272:455–461. [PubMed] [Google Scholar]
- 17.Joint United Nations Programme on HIV/AIDS (UNAIDS). 2004. Report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS; 2004.
- 18.DeRiemer K, Kawamura LM, Hopewell PC, Daley CL. Impact of HV on tuberculosis dynamics in San Francisco [abstract 26]. Late Breaker Abstracts Session. 100th International Conference, American Thoracic Society, May 20–25, San Diego, CA; 2005.
- 19.Jasmer RM, Hahn JA, Small PM, Daley CL, Behr MA, Moss AR, Creasman JM, Schecter GF, Paz EA, Hopewell PC. A molecular epidemiologic analysis of tuberculosis in San Francisco, 1991–1997. Ann Intern Med 1999;130:971–978. [DOI] [PubMed] [Google Scholar]
- 20.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993;31:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woelffer GB, Bradford WZ, Paz A, Small PM. A computer-assisted molecular epidemiologic approach to confronting the reemergence of tuberculosis. Am J Med Sci 1996;311:17–22. [DOI] [PubMed] [Google Scholar]
- 22.Murray M, Alland D. Methodological problems in the molecular epidemiology of tuberculosis. Am J Epidemiol 2002;155:565–571. [DOI] [PubMed] [Google Scholar]
- 23.Chaves F, Yang Z, el Hajj H, Alonso M, Burman WJ, Eisenach KD, Dronda F, Bates JH, Cave MD. Usefulness of the secondary probe pTBN12 in DNA fingerprinting of Mycobacterium tuberculosis. J Clin Microbiol 1996;34:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.State of California, Department of Finance. Race/ethnic population with age and sex detail, 1990–1999. Sacramento, CA; 2004.
- 25.State of California, Department of Finance. Race/ethnic population with age and sex detail, 2000–2050. Sacramento, CA; 2004.
- 26.Small PM, McClenny NB, Singh SP, Schoolnik GK, Tompkins LS, Mickelsen PA. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J Clin Microbiol 1993;31:1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett, Hayes R, Churchyard G, Butterworth A, Mason P. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 2007;4(e22):e0164–0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porco TC, Martin JN, Page-Shafer KA, Cheng A, Charlebois E, Grant RM, Osmond DH. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS 2004;18:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santoro-Lopes G, Felix de Pinho AM, Harrison LH, Schecter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis 202;34:543–546. [DOI] [PubMed]
- 30.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long-term incidence and risk factors in a South African cohort. AIDS 2005;19:2109–2116. [DOI] [PubMed] [Google Scholar]
- 31.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002;359:2059–2064. [DOI] [PubMed] [Google Scholar]
- 32.Rieder HL. Methodological issues in the estimation of the tuberculosis problem from tuberculin surveys. Tuber Lung Dis 1995;76:114–121. [DOI] [PubMed] [Google Scholar]
- 33.Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis 1999;3:962–975. [PubMed] [Google Scholar]
- 34.Enarson DA. Measuring tuberculosis: lessons from Afghanistan. Int J Tuberc Lung Dis 2004;8:1041–1042. [PubMed] [Google Scholar]
- 35.Odhiambo JA, Borgdorff MW, Kiambih FM, Kibuga DK, Kwamanga DO, Ng'ang'a L, Agwanda R, Kalisvaart NA, Misljenovic O, Nagelkerke NJ, et al. Tuberculosis and the HIV epidemic: increasing annual risk of tuberculous infection in Kenya, 1986–1996. Am J Public Health 1999;89:1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egwaga SM, Cobelens FG, Muwinge H, Verhage C, Kalisvaart, Borgdorff MW. The impact of the HIV epidemic on tuberculosis transmission in Tanzania. AIDS 2006;20:915–921. [DOI] [PubMed] [Google Scholar]
- 37.Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant AD, Dye C, De Cock K, Hayes RJ, Williams BG, Churchyard GJ. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004;170:673–679. [DOI] [PubMed] [Google Scholar]
- 38.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker L-G. Undiagnosed tuberculosis in a community with high HIV prevalence. Am J Respir Crit Care Med 2007;175:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruciani M, Malena B, Bosco O, Gatti G, Serpelloni G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin Infect Dis 2001;33:1922–1930. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho AC, DeRiemer K, Nunes ZB, Martins M, Comelli M, Marinoni A, Kritski AL. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med 2001;164:2166–2171. [DOI] [PubMed] [Google Scholar]
- 41.Glynn JR, Crampin AC, Ngwira BMM, Mwaungulu FD, Mwafulirwa DT, Floyd S, Pönnighaus JM, Warndorff DK, Fine PEM. Trends in tuberculosis and the influence of HIV infection in northern Malawi, 1988–2001. AIDS 2004;18:1459–1463. [DOI] [PubMed] [Google Scholar]
- 42.Crampin AC, Glynn JR, Traore H, Yates MD, Mwaungulu L, Mwenebabu M, Steven D, Chaguluka SD, Floyd S, Drobniewski F, et al. Tuberculosis transmission attributable to close contacts and HIV status, Malawi. Emerg Infect Dis 2006;12:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suggaravetsiri P, Yani H, Chongsuvivatwong V, Naimpasan O, Akaresewi P. Integrated counseling and screening for tuberculosis and HIV among household contacts of tuberculosis patients in an endemic area of HIV infection: Chiang Rai, Thailand. Int J Tuberc Lung Dis 2003;7(12, Suppl 3):S424–S431. [PubMed] [Google Scholar]
- 44.Reichler MR, Bur S, Reeves R, Mangura B, Thompson V, Ford J, Castro KG. Results of testing for human immunodeficiency virus infection among recent contacts of infectious cases in the United States. Int J Tuberc Lung Dis 2003;7(12, Suppl 3):S471–S478. [PubMed] [Google Scholar]
- 45.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 2005;191:150–158. [DOI] [PubMed] [Google Scholar]
- 46.Porco TC, Small PM, Blower SM. Amplification dynamics: predicting the effect of HIV on tuberculosis outbreaks. J Acquir Immune Defic Syndr 2001;28:437–444. [DOI] [PubMed] [Google Scholar]
- 47.Godfrey-Faussett P, Mahler D, Mukadi YD, Nunn P, Perriëns J, Raviglione M. How human immunodeficiency virus voluntary testing can contribute to tuberculosis control. Bull World Health Organ 2002;80:939–945. [PMC free article] [PubMed] [Google Scholar]
- 48.American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. Am J Respir Crit Care Med 2005;172:1169–1227. [DOI] [PubMed] [Google Scholar]
- 49.Paltiel DA, Weinstein MC, Kimmel AD, Seage GR III, Losine E, Zhang H, Freedberg KA, Walensky RP. Expanding HIV screening in the United States: an analysis of cost-effectiveness. N Engl J Med 2005;352:586–595. [DOI] [PubMed] [Google Scholar]
- 50.Sanders GD, Gayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, Douglass LR, Lazzeroni LC, Holodniy M, Owens DK. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005;352:570–585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.