Abstract
Increased susceptibility to infections, including tuberculosis (TB), is a major cause of morbidity and mortality in patients with diabetes. Despite the clinical importance of this problem, little is known about how diabetes impairs protective immunity. We modeled this phenomenon by infecting acute (⩽ 1 mo) or chronic (⩾ 3 mo) diabetic mice with a low aerosol dose of Mycobacterium tuberculosis (Mtb) Erdman. Diabetes was induced by streptozotocin (STZ) treatment of C57BL/6 mice, while another mouse strain and diabetes model were used to confirm key observations. Lungs from acute diabetic and euglycemic mice had similar bacterial burdens, cytokine expression profiles, and histopathology. In contrast, chronic diabetic mice had > 1 log higher bacterial burden and more inflammation in the lung compared with euglycemic mice. The expression of adaptive immunity was delayed in chronic diabetic mice, shown by reduced early production of IFN-γ in the lung and by the presence of fewer Mtb antigen (ESAT-6)–responsive T cells compared with euglycemic mice within the first month of infection. However, after 2 months of TB disease proinflammatory cytokines levels were higher in chronic diabetic than euglycemic mice. Here we show that Mtb infection of STZ-treated mice provides a useful model to study the effects of hyperglycemia on immunity. Our data indicate that the initiation of adaptive immunity is impaired by chronic hyperglycemia, resulting in a higher steady-state burden of Mtb in the lung.
Keywords: diabetes mellitus, host defense, mouse, Mycobacterium tuberculosis
CLINICAL RELEVANCE
Diabetes increases susceptibility to infections, including tuberculosis (TB). We present a model of TB in diabetic mice to investigate the mechanisms underlying this complication of diabetes and to inform the rational development of therapies to overcome it.
People with diabetes mellitus are prone to infection with a broad range of pathogens, including Mycobacterium tuberculosis (Mtb) (1, 2). The World Health Organization estimates that 170 million people worldwide currently have diabetes, a figure that will double by 2030. Tuberculosis (TB) remains among the leading causes of death from infection (3, 4). Many of the countries with the highest incidence of diabetes also have a high incidence of TB. Even a modest improvement in the management of diabetes-related infections has the potential to yield large benefits in terms of alleviating morbidity and reducing medical costs. Despite these considerations, surprisingly little is known about the basis for the increased susceptibility of patients with diabetes to infection.
Limited clinical studies of immunity in patients with diabetes described impaired T cell proliferation and a reduced capacity of T cells to respond to appropriate stimulation (5). A relatively small number of animal studies exploring the effects of hyperglycemia on host defense have been published. Reading and coworkers (6) reported increased Influenza A virus susceptibility of diabetic mice, possibly due to compromised collectin-mediated defense. Hyperglycemic mice are also more susceptible to trypanosomiasis and trichinosis, but less susceptible than euglycemic mice to blood-stage malaria infection (7–10). Diabetic mice infected with Mtb by high-dose (⩾ 105 colony-forming units [cfu]) intravenous injection were reported to have increased mortality and moderately higher bacterial lung burden compared with euglycemic mice (11, 12). Insulin treatment of diabetic mice appeared to restore resistance to Mtb in one study (11).
Establishing a well-characterized diabetes infection model is a logical first step to investigate the mechanism of impaired host defense in diabetes. We used mice with acute (⩽ 1 mo) or chronic (⩾ 3 mo) streptozotocin (STZ)-induced diabetes to study TB immunity in a low dose (∼ 50 cfu) aerosol infection model with Mtb Erdman. The bulk of our work was done with C57BL/6 mice, but other mouse strains and a different diabetes model were used to provide corroborative data. Acute diabetes did not measurably impair TB defense. In contrast, chronic diabetic mice had a higher bacterial lung burden, increased lung inflammation, and a delayed adaptive immune response to Mtb compared with euglycemic mice.
MATERIALS AND METHODS
Animals
C57BL/6 and heterozygous Ins2Akita (Akita) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Akita mice spontaneously become hypoinsulinemic and hyperglycemic by 3 to 4 weeks of age (13). ICR mice, an outbred mouse strain, were purchased from Taconic Farms (Hudson, NY). Mice were housed within the Animal Medicine facility at UMass Medical School, and the University of Massachusetts Medical School Institutional Animal Care and Use Committee approved these experiments. All mice were at least 8 weeks old when treated with STZ.
Reagents
Culture-filtrate protein (CFP) was obtained by culturing Mtb Erdman in Sauton's Media for 1 month. The culture supernatant was concentrated and dialyzed against PBS with a cutoff of 6 kD.
Induction of Diabetes
Diabetes was established by intraperitoneal injection of STZ (Sigma-Aldrich, St Louis, MO) dissolved in phosphate citrate buffer (pH 4.5). Male mice received 150 mg/kg STZ and female mice received 200 mg/kg STZ. Random blood glucose measurements were performed with a BD Logic glucometer (BD, Franklin Lakes, NJ) 7 days after STZ treatment, on the day of infection, and at the conclusion of each experiment. Mice were considered diabetic if their blood glucose was greater than 200 mg/dl. Diabetic mice were tested for ketoacidosis by urine dip stick (LW Scientific, Inc., Lawrenceville, GA). Control mice had less than 200 mg/dl blood glucose.
Infection with Mtb
Aliquots of frozen Mtb Erdman stock in PBS plus 0.05% Tween-80 (PBS-T) were thawed and then sonicated for 5 minutes in a cup-horn sonifier (Branson Ultrasonics Corporation, Danbury, CT). A volume of sonicated stock previously titrated to deliver approximately 50 cfu per mouse was added to the nebulizer of a Glas-Col Inhalation Exposure System (Glass-Col, LLC, Terre Haute, IN) and mice were exposed to the infectious aerosol for 30 minutes. In every experiment, two mice were killed 24 hours after infection to confirm the actual delivered dose.
Bacterial Load
At each time point lung homogenates from five mice were plated to measure bacterial burden. Lungs were homogenized in PBS-T, serially diluted 10-fold over 4 logs, and plated in duplicate on Middlebrook 7H11 agar (DIFCO; Becton Dickinson, Sparks, MD). Plates were cultured at 37°C for 3 weeks and then counted using a dissecting microscope to confirm colony morphology.
Lung Histology
Lungs were inflated and fixed with 10% buffered formalin for 24 hours and then processed for staining. Tissue sections were stained with hematoxylin and eosin (H&E) or for inducible nitric oxide synthase expression with anti-iNOS/NOS II rabbit polyclonal IgG diluted 1:100 (Millipore, Billerica, MA) and bound antibody detected with a peroxidase-based ABC staining kit according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). As a negative control sections were stained in the absence of the primary antibody. Sections were made at intervals spanning the whole lung and examined by light microscopy. Lung surface area and areas of inflammation were measured with a Nikon Eclipse E400 microscope (Nikon Instruments, Melville, NY) at ×20 magnification using Spot Insight v 3.5 software (Diagnostic Instruments, Inc., Sterling Heights, MN). Percent total lung area involved with inflammation was calculated by dividing the cumulative area of inflammation by the total lung surface area examined in all sections for each lung studied.
Flow Cytometry of Lung Leukocytes
Lung T cell, macrophage/monocyte, and granulocyte populations were measured by flow cytometry. To isolate parenchymal leukocytes, lungs were perfused through the heart with PBS immediately after mice were killed. The lungs were removed and then minced, digested (30 min, 37°C) in 1 mg/ml collagenase and 25μg/ml DNase (both from Sigma-Aldrich), passed through a 40-μm cell strainer, and remaining red blood cells lysed with Gey's solution. Viable cells were counted using a hemocytometer and trypan blue dye staining.
One to two million lung leukocytes were treated with Fc blocking mAb (clone 2.4G2; BD Bioscience Pharmingen, San Diego, CA) then stained with anti-CD3-APC-Cy7 (clone 145–2C11), CD4-PerCP (clone RM4–5), CD8-PE (clone 53.6–7), and Gr-1-PE-Cy7 (clone RB6–8C5; all from BD Bioscience Pharmingen, San Diego, CA) and F4/80-APC (clone BM8; eBioscience, San Diego, CA). Stained cells were analyzed on a LSRII flow cytometer (BD Bioscience Pharmingen). Fifty thousand leukocyte-gated events were collected, and data analysis was done with FlowJo PC (TreeStar, Inc., Ashland, OR). Isotype control antibodies were purchased from BD Bioscience Pharmingen and eBioscience.
Lung Cytokine Expression
Lungs were homogenized in PBS-T, an equal volume of cell lysis buffer (0.5% Triton X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl2 and 1 mM MgCl2, pH 7.4) was added, and the mixture was vortexed, incubated (20 min, 4°C), vortexed again, centrifuged (10 min 12,000–14,000 × g), and the supernatant was filterer sterilized. Lung lysates were pooled by experimental group before being assayed for IL-1α, IL-1β, IL-2, IL-4, IL-10, IL-12p70, IL-17, IL-18, IL-12p40, transforming growth factor (TGF)-β, macrophage inflammatory protein (MIP)-1α, and TNF by multiplex enzyme-linked immunosorbent assay (ELISA) (Searchlight; Pierce Biotechnology, Woburn, MA). IFN-γ in individual lung lysates was measured by ELISA according to the manufacturer's protocol (R&D Systems, Minneapolis, MN).
Ex Vivo Lung T Cell Restimulation
IFN-γ ELIspots (R&D Systems) were performed with lung leukocytes according to the manufacturer's protocol. Cells were plated in duplicate with 105 or 2 × 104 cells per well, incubated (37°C, 5% CO2) 24 hours with media, 4 μg/ml Con A, 2.5 μg/ml anti-CD3 (clone 145–2C11), 2 μg/ml Mtb Erdman CFP, or 10 μM of an ESAT-6 MHC class II-restricted epitope peptide (MTEQQWNFAGIEAAA; kindly provided by Dr. Samuel Behar, Harvard Medical School). Spot-forming cells (SFC) were counted manually with a dissecting microscope.
Statistical Analysis
F-test was performed to confirm that variances between treatment groups were not statistically significant. Student's t test for samples with equal variance or unequal variance was performed as appropriate for comparisons between treatment groups. P values < 0.05 were considered significant. Error bars represent one standard deviation.
RESULTS
Induction of Diabetes in Mice
Nonfasting blood glucose was measured after STZ treatment (except for heterozygous Akita mice, which become spontaneously diabetic), before Mtb Erdman infection, and at the conclusion of an experiment. Nonfasting blood glucose was measured because we felt this was a better indicator than fasting values of typical blood glucose levels during infection. Nonfasting blood glucose of euglycemic C57BL/6 was less than 200 mg/dl. Some of the STZ-treated C57BL/6 mice had nonfasting blood glucose above the upper limit of glucometer detection (> 600 mg/dl), while the remaining mice had an average blood glucose of 463 mg/dl. Interestingly, half of the ICR mice not treated with STZ had nonfasting blood glucose over 200 mg/dl (range, 175–329 mg/dl), but their blood glucose was less than 200 mg/dl after a 16-hour fast, consistent with previously reported fasting blood glucose values for this strain (11). With the exception of one STZ-treated ICR mouse that became euglycemic, all of the STZ-treated ICR mice had a blood glucose greater than 200 mg/dl after a 16-hour fast. None of the diabetic mice developed ketoacidosis as determined by urine ketone dipstick tests. Blood glucose of acute and chronic diabetic mice was not significantly influenced by Mtb Erdman infection (data not shown).
TB Susceptibility of Diabetic Mice
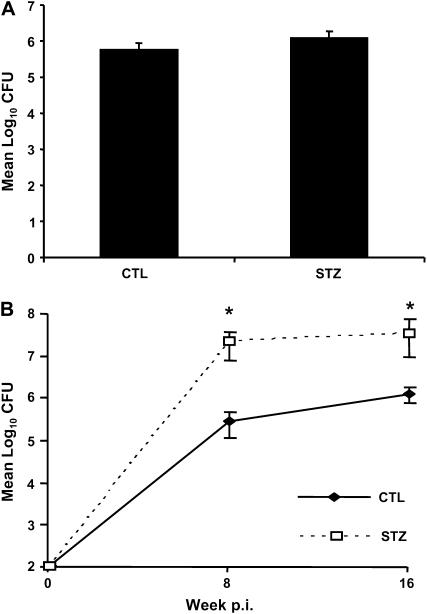
Acute diabetic C57BL/6 and ICR mice had bacterial lung burdens comparable to euglycemic mice 4 weeks after infection (Figure 1A and data not shown). These findings contrast with results published by Yamashiro and colleagues (11), who reported increased bacterial lung burden for acutely diabetic ICR mice compared with euglycemic controls as early as 14 days after high-dose (105 cfu) intravenous challenge. In our low-dose (∼ 50 cfu) aerosol study, chronic diabetic C57BL/6 mice had more than 1 log higher bacterial lung burden than euglycemic mice at 8 weeks after infection (Figure 1B), but the bacterial burden was stabilized at a plateau of approximately 107 cfu out to 16 weeks after infection. These data suggest a delay in the expression of adaptive immunity during the early logarithmic expansion of Mtb, while the stable cfu plateau indicates that the adaptive effector response was functional once it was expressed in the lungs of diabetic mice. Bacterial burden was also higher in chronic diabetic Akita mice compared with nondiabetic controls 16 weeks after aerosol Mtb infection (mean cfu ± SD of 1.01 × 105 ± 0.27 × 105 versus 3.71 × 105 ± 0.27 × 105; n = 5, P < 0.05).
Figure 1.
Chronic but not acute diabetes increases tuberculosis (TB) susceptibility in mice. We grouped mice as acute or chronic based on the duration of streptozotocin (STZ)-induced diabetes, ⩽ 1 mo or ⩾ 3 mo, respectively, before aerosol infection with Mycobacterium tuberculosis (Mtb) Erdman (∼ 50 colony-forming units [cfu]). Lung Mtb load was determined by plating serial dilutions of lung homogenates at the indicated times and counting cfu after 3 wk incubation. (A) Acute diabetic (STZ) versus euglycemic control (CTL) mice 4 weeks after infection. (B) Chronic STZ diabetic versus euglycemic mice 8 and 16 weeks after infection. Data are presented as mean log10 cfu ± SD. Open squares: STZ-treated mice with chronic diabetes; closed diamonds: euglycemic control mice. Data are presented as mean cfu ± SD. *P < 0.05, n = 5.
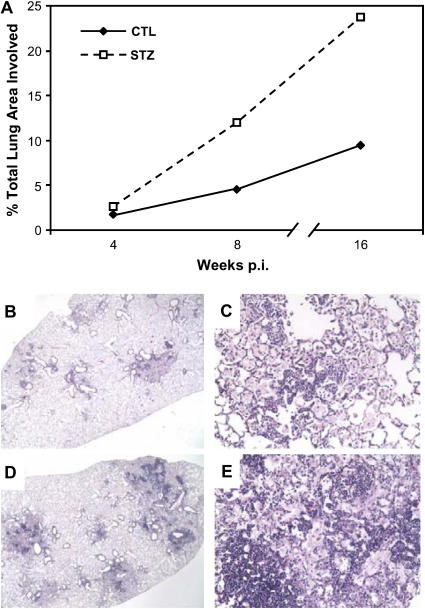
We saw no noticeable difference in the pattern or extent of TB pathology in the lungs of acute diabetic and euglycemic mice (data not shown). Similarly, there was no consistent difference in the pattern of lung TB pathology between euglycemic and chronic diabetic C57BL/6 mice, but the extent of inflammation was increased in the diabetic group. We compared the extent of inflammation by analyzing serial sections through the entire lung of two mice per group, measuring the total cross-sectional area of lung tissue sections for each mouse and the total area within those sections involved with inflammation. A considerably greater area of the lung was occupied with inflammation in chronic diabetic mice with than in euglycemic controls (Figures 2A–2E). The histopathology results demonstrate that mice with diabetes are capable of responding to Mtb infection and recruiting a normal appearing but exaggerated leukocyte response to the lung, consistent with the 10-fold greater bacillary burden.
Figure 2.
Chronic diabetic mice with TB have a similar pattern but increased extent of lung inflammation compared with euglycemic controls. (A) Lung area involved with inflammation of euglycemic and chronic diabetic C57BL/6 mice at different time points after aerosol challenge with Mtb Erdman. Total cross-sectional lung area from all tissues sections was examined and the combined areas of inflammation within these sections were delineated using a Nikon Eclipse E400 microscope (×20 magnification) with Spot Insight v 3.5 software. Percent total area involved with inflammation for two mice per group was calculated as (total area of inflammation/total lung area surveyed) ×100. Open squares: STZ-treated mice with chronic diabetes; closed diamonds: euglycemic control mice. (B, C) Representative hematoxylin and eosin (H&E)-stained lung sections from euglycemic C57BL/6 mouse 12 weeks after infection taken at ×20 and ×200 magnification, respectively. (D, E) Representative H&E-stained lung section from chronic diabetic C57BL/6 mouse 12 weeks after infection at ×20 and ×200 magnification, respectively.
Lung Leukocyte Populations
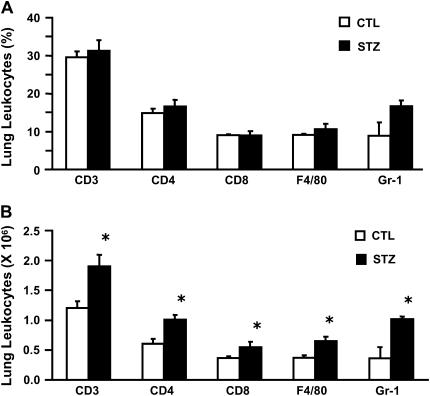
To further characterize the inflammatory response of chronic diabetic and eugylcemic mice with pulmonary TB, we isolated lung leukocytes from the left lung and right caudal lung lobe by enzymatic digestion and measured T cell (CD3+, CD4+, CD8+), macrophage/monocyte (F4/80+), and granulocyte (GR-1+, CD3−, F4/80−) populations by flow cytometry 16 weeks after infection. Chronic diabetic and euglycemic mice had the same relative proportion of T cell subsets, macrophages/monocytes, and granulocytes. However, chronic diabetic had more total lung leukocytes and therefore more total cells of each population than euglycemic controls (Figures 3A and 3B). This finding is consistent with the histopathology results. We saw no evidence of increased early inflammation in chronic diabetic mice, as they had a number of lung leukocytes and splenocytes comparable with those of euglycemic mice 7, 14, and 21 days after infection (data not shown). A comparison of acute diabetic and euglycemic C57BL/6 mice with TB revealed no difference in total lung leukocytes or proportion of different leukocyte subsets at any time point (data not shown).
Figure 3.
Chronic diabetic mice have greater leukocyte recruitment to the lungs than euglycemic mice after 16 weeks of TB disease. Isolated lung leukocytes were stained with anti-CD3, -CD4, -CD8, −Gr-1, and -F4/80 mAb for analysis by flow cytometry. (A) The proportion of different leukocyte populations is presented as the mean % surface marker positive cells ± SD (n = 3). Open columns: euglycemic C57BL/6 mice; closed columns: STZ-treated mice with chronic diabetes. (B) The total number of leukocytes of each subset in the lung was determined by multiplying the % surface marker positive cells by the total number of leukocytes isolated from each mouse. Open columns: euglycemic mice; closed columns: STZ-treated mice with chronic diabetes. Data are presented as the mean cell number (×106) ± SD. *P < 0.05, n = 3.
Lung Cytokine Expression
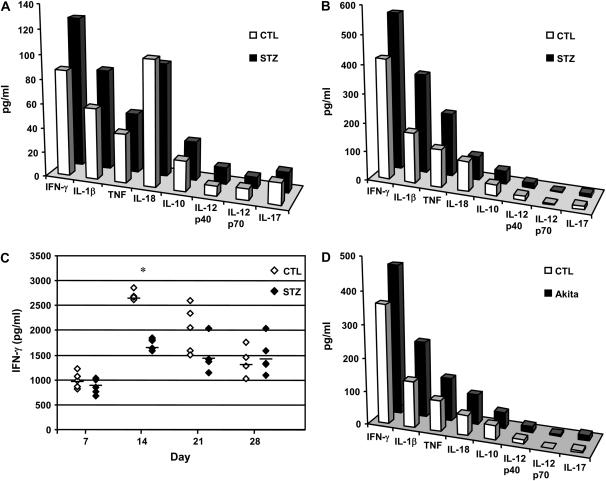
Protective immunity to TB depends on a T helper (Th)1 cell–mediated immune response. It is well established that IFN-γ is critically important for TB defense in mice and in the human host (14–16). We assayed pooled lung lysates from acute diabetic, chronic diabetic, and euglycemic mice with TB for Th1, Th2, and pro-inflammatory cytokines. Expression of IFN-γ and IL-1β was higher in lungs from acute diabetic C57BL/6 mice compared with euglycemic mice 8 weeks after infection (Figure 4A). Acute diabetic ICR mice had lower IL-12p40 expression in their lungs than euglycemic mice but a comparable amount of IFN-γ 8 weeks after infection, which agrees with previously reported observations (11, and data not shown).
Figure 4.
Lung IFN-γ and inflammatory cytokine expression are increased in acute and chronic diabetic mice late in Mtb infection, while chronic diabetic mice have reduced IFN-γ expression shortly after infection. Lung homogenates were prepared as described in Materials and Methods. (A) Cytokines present in pooled lung lysates prepared from acute diabetic or euglycemic C57BL/6 mice 8 weeks after infection. Closed bars: STZ-treated mice with acute diabetes; open bars: euglycemic control mice; n = 5. (B) Cytokines present in pooled lung lysates prepared from chronic diabetic or euglycemic mice 16 weeks after infection. Closed bars: STZ-treated mice with chronic diabetes; open bars: euglycemic control mice; n = 5. (C) IFN-γ content in lung lysates from individual chronic diabetic C57BL/6 mice 7, 14, 21, and 28 days after infection. Open diamonds: euglycemic control mice; closed diamonds: STZ-treated mice with chronic diabetes; *P < 0.05, n = 5. (D) Cytokines present in pooled lung lysates prepared from chronic diabetic Akita mice or euglycemic C57BL/6 mice 16 weeks after infection. Closed bars: Akita mice with chronic diabetes; open bars: euglycemic control mice; n = 5.
We measured IFN-γ in lung lysates from individual C57BL/6 mice with chronic STZ-induced diabetes on Weeks 1, 2, 3, and 4 after Mtb infection. IFN-γ levels were comparable between groups at 1 week after infection and increased in both groups by 2 weeks after infection, but the increase in the eugylcemic mice was significantly greater than in the diabetic mice (Figure 4C). While IFN-γ increased more slowly in the diabetic than in euglycemic mice during the first 3 weeks after Mtb infection, the levels measured in pooled lung lysates from chronic diabetic C57BL/6 and Akita mice were higher than eugylcemic controls 16 weeks after infection. The levels of IL-1β and TNF were also higher in chronic diabetic mice relative to euglycemic mice 16 weeks after infection (Figures 4B and 4D). These data indicate that mice with chronic diabetes are fully capable of mounting a strong IFN-γ response to chronic Mtb infection in the lung, albeit with delayed kinetics.
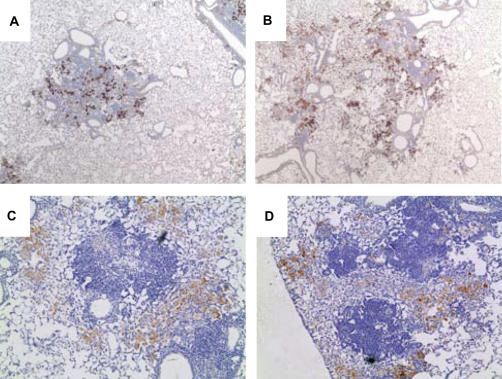
One possible explanation for impaired control of Mtb growth in the lungs despite the high level of IFN-γ expression could be a reduced capacity of lung macrophages in chronic diabetic mice to respond to IFN-γ stimulation. Among the many antimicrobial effector functions stimulated by IFN-γ, inducible nitric oxide synthase (iNOS) may be most important for TB defense (17). We therefore examined iNOS expression by immunohistochemistry in lung tissue sections taken 8 weeks after infection. There was abundant iNOS expression in the TB lesions of mice with chronic diabetes (both C57BL/6 and Akita) that was comparable to the expression in euglycemic controls (Figure 5). Chronic hyperglycemia does not impair the capacity of lung macrophages to be activated for iNOS expression in the context of a cell-mediated immune response.
Figure 5.
Lung iNOS expression in established TB lesions is similar between euglycemic and chronic diabetic mice. A euglycemic control C57BL/6 mouse (A), an STZ-treated chronic diabetic mouse (B), a euglycemic wild-type C57BL/6J control for the Akita mice (C), and a chronic diabetic Akita mouse (D) were infected with Mtb Erdman by aerosol. Lung sections prepared 16 weeks after infection were stained with anti-iNOS/NOS II. Cells expressing iNOS are identified by brown staining. Images are ×40 magnification.
Ex Vivo Antigen Restimulation
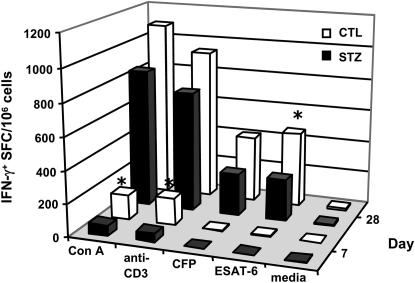
Compared with euglycemic mice, IFN-γ expression was reduced in the lungs of chronic diabetic mice 14 days after infection but increased 16 weeks after infection, suggesting that the increased TB susceptibility of these mice stems from delayed expression of adaptive immunity rather than an intrinsically faulty effector response. Consistent with that concept, Mtb growth is held at a plateau level in the lungs of chronic diabetic mice but at a higher bacillary burden than in euglycemic controls. To evaluate the kinetics of adaptive immunity, we isolated lung leukocytes from chronic diabetic and euglycemic C57BL/6 mice 7 and 28 days after infection. Lung leukocytes were cultured 24 hours on IFN-γ ELIspot plates and stimulated with Con A, anti-CD3 mAb, Mtb Erdman CFP, an MHC class II restricted Mtb ESAT-6 epitope peptide, or control media. As shown in Figure 6, by 7 days after infection there was a higher frequency of IFN-γ–producing lung T cells responsive to Con A or anti-CD3 in euglycemic C57BL/6 mice compared with chronic diabetic mice. By 28 days after infection lung leukocytes from euglycemic mice had a significantly higher frequency of IFN-γ–producing lung T cells responsive to the Mtb-specific antigen ESAT-6 than mice with chronic diabetes. These results demonstrate a temporal delay in the expression of protective immunity to Mtb in chronic diabetic mice.
Figure 6.
Antigen-specific and nonspecific IFN-γ responses are reduced in chronic diabetic mice at early time points in TB disease. Chronic diabetic C57BL/6 mice (STZ) and euglycemic controls (CTL) were infected with Mtb Erdman by aerosol. Lung leukocytes harvested 7 and 28 days after infection were cultured on IFN-γ ELIspot plates in the presence of control media, Con A, anti-CD3 mAb, Mtb Erdman CFP, or an ESAT-6 MHC class II peptide. Results are expressed as the mean number of spot-forming cells (SFC) per million cells. *P < 0.05, n = 4.
DISCUSSION
Diabetes mellitus increases human TB susceptibility, but the immunological basis for this diabetic complication remains poorly understood. We combined STZ treatment and low-dose aerosol Mtb challenge of mice to investigate the impact of hyperglycemia on protective immunity. We found that control of Mtb infection was significantly impaired by chronic diabetes, while acute STZ-induced diabetes had no discernible effect on TB susceptibility. Mtb challenge of chronic diabetic mice resulted in a > 1 log higher plateau lung bacillary burden compared with euglycemic mice (Figure 1). This increased TB susceptibility could hypothetically result from defects in leukocyte recruitment to the lung, reduced expression of cytokines essential for TB defense, or a reduced capacity of macrophages to respond to activating cytokines. We found no noticeable differences in the proportion of lung T cells, macrophages/monocytes or granulocytes between diabetic and euglycemic mice with TB (Figure 3A), no evidence for deficient IFN-γ levels in established TB disease (16 wk after infection), nor any evidence of decreased macrophage responsiveness to IFN-γ in diabetic mice as reflected by iNOS expression in vivo (Figures 4A, 4B, 4D, and 5). The increased lung leukocytes and histopathology seen in chronic diabetic mice at 16 weeks after infection presumably reflects their higher bacterial load rather than any direct effect of hyperglycemia on inflammation, since there was no difference in leukocyte recruitment to the lungs of acute diabetic versus euglycemic mice with TB that had comparable lung bacillary burden. While chronic diabetic mice ultimately expressed a robust immune response to Mtb in the chronic phase of TB disease, a key finding in our study was a relative delay in IFN-γ responses detected between 1 and 4 weeks after infection (Figure 6).
Host control of TB requires an effective Th1 adaptive immune response in the lungs. After aerosol challenge, Mtb grows logarithmically in the lung for about 3 weeks until cell-mediated immunity causes bacillary load to plateau, largely due to IFN-γ activation of macrophages to restrict bacillary replication. Any delay in the expression of Th1 immunity translates into a higher plateau lung bacillary burden. In our study, chronic diabetic mice reached a higher plateau cfu level than euglycemic mice, but they retained the capacity to ultimately restrict logarithmic Mtb growth (Figure 1B). As compared with euglycemic controls, chronic diabetic mice had a reduced level of IFN-γ in lung homogenates 2 weeks after infection, and reduced IFN-γ production by purified lung T cells re-stimulated ex vivo with anti-CD3 mAb, Con A, or ESAT-6 peptide at 1 and 4 weeks after infection (Figures 4C and 6). These results point to an adverse effect of chronic diabetes on the acquisition of immunity to Mtb, and that could be due to impaired dendritic cell function. The TB susceptibility phenotype of chronic diabetic mice in our study was quite similar to that reported by Tian and colleagues (18) in experiments in which dendritic cells were transiently depleted in nondiabetic mice before Mtb challenge.
We used the STZ diabetes model as it uniformly produces hyperglycemia and because it allowed us to control the duration of diabetes. Further, the STZ diabetes model let us focus on the immunosuppressive effects of hyperglycemia without any of the confounding immunologic factors associated with the NOD mice, including defects in antigen-presenting cell function, T cell repertoire regulation, and natural killer cell function (19–21). It was recently reported that STZ treatment produces a transient (∼ 9 d) period of lymphopenia and reversible immune suppression in mice (22). Our finding of impaired TB defense in mice with chronic but not acute diabetes is the opposite of what would be expected if STZ was somehow directly responsible for the TB susceptibility phenotype, since the interval between STZ treatment and Mtb challenge was the shortest in the acute diabetes group. Moreover, Akita mice with chronic diabetes exhibited increased TB susceptibility in the absence of STZ treatment.
Within the limits of our experimental system, acute diabetes had no adverse impact on TB defense. Lung bacterial burden, histopathology, and iNOS expression were similar between acute diabetic and euglycemic C57BL/6 or ICR mice (Figure 1 and data not shown). These data indicate that hyperglycemia per se does not directly promote Mtb growth or degrade protective immunity. Irreversible formation of advanced glycation end products (AGE) has been linked to many of the complications associated with diabetes, including atherosclerosis, glomerulopathy, impaired wound healing, and depressed neutrophil function (23–27). Metabolites from the polyol and hexosamine pathways as well as dysfunctional protein kinase C activation may also contribute to these diabetic complications (28). AGE accumulate over time in humans with hyperglycemia and in mice. Our finding that TB susceptibility increases with the duration of diabetes suggests that AGE might contribute to the observed impairment of protective immunity. Prior studies indicate that 3 months of hyperglycemia, the period we used to model chronic diabetes in our studies, is sufficient for significant AGE accumulation and AGE-related pathology to develop in mice (29, 30).
Recently, Yamashiro and coworkers (11) reported increased TB susceptibility of ICR mice with acute STZ-induced hyperglycemia, as evidenced by increased lung Mtb burden as early as 14 days after infection. The conditions of that study differed significantly from ours. They used a 1,000-fold higher dose of Mtb and delivered the bacteria by intravenous injection. The conditions of diabetes were also different. Most STZ-treated ICR mice reported by Yamashiro and colleagues had a fasting blood glucose over 600 mg/dl, while STZ-treated ICR mice in our study had average fasting blood glucose of 403 mg/dl. We excluded the possibility of ketoacidosis influencing TB susceptibility the STZ-treated mice for our study, while this parameter was not mentioned in the report by Yamashiro and coworkers. Diabetic ketoacidosis might cause immune suppression by mechanisms different than hyperglycemia, as exemplified by its distinct association with rhinocerebral mucormycosis (2).
Diabetes and TB are globally important diseases with far-reaching health and economic consequences. We have established a reliable mouse model to study protective immunity against Mtb in the context of diabetes. This study extends previously reported findings of TB susceptibility in diabetic mice by contrasting acute and chronic diabetes, by evaluating lung histopathology and lung leukocyte recruitment, by excluding diabetic ketoacidosis as a contributing factor to immunosuppression, by surveying a broad array of cytokines relevant to TB defense, and by using a pathophysiologically relevant dose and route of Mtb infection. To our knowledge, this is the first report of increased TB susceptibility caused by chronic hyperglycemia in mice. Our data argue against a direct adverse effect of acute hyperglycemia and suggest instead that impaired host defense is a consequence of persistent hyperglycemia, as is the case for the vascular and renal complications of diabetes. Specifically, the data point to an adverse impact of chronic hyperglycemia on the initiation of adaptive immunity rather than on the magnitude or efficacy of its eventual expression. Based on these findings, our future studies will focus on dendritic cell trafficking and antigen presentation after aerosol Mtb challenge of diabetic mice. Although STZ treatment produces insulin-deficient diabetes, the resulting hyperglycemia is a common feature of type 1 and 2 diabetes and one that has been linked to a shared spectrum of diabetic complications. It is therefore reasonable to hypothesize that the adverse effect of chronic hyperglycemia on host defense could occur in the context of type1 or type 2 diabetes.
Acknowledgments
The authors thank Birgit Stein, Madhumathi Thiruvengadam, Jonathan Eskander, and Linda Paquin for technical assistance with data collection and animal care.
This work was supported by NIH grants DK 32520 and DK 53006 (to Aldo Rossini and D.G.), and HL 081149 (to H.K.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0478OC on June 21, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ponce-De-Leon A, Garcia-Garcia Md ML, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, Rojas R, Ferreyra-Reyes L, Cano-Arellano B, Bobadilla M, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care 2004;27:1584–1590. [DOI] [PubMed] [Google Scholar]
- 2.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med 1999;341:1906–1912. [DOI] [PubMed] [Google Scholar]
- 3.Enarson DA, Chretien J. Epidemiology of respiratory infectious diseases. Curr Opin Pulm Med 1999;5:128–135. [DOI] [PubMed] [Google Scholar]
- 4.White F, Nanan D. Status of national diabetes programmes in the Americas. Bull World Health Organ 1999;77:981–987. [PMC free article] [PubMed] [Google Scholar]
- 5.Spatz M, Eibl N, Hink S, Wolf HM, Fischer GF, Mayr WR, Schernthaner G, Eibl MM. Impaired primary immune response in type-1 diabetes: functional impairment at the level of APCs and T-cells. Cell Immunol 2003;221:15–26. [DOI] [PubMed] [Google Scholar]
- 6.Reading PC, Allison J, Crouch EC, Anders EM. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol 1998;72:6884–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanowitz HB, Amole B, Hewlett D, Wittner M. Trypanosoma cruzi infection in diabetic mice. Trans R Soc Trop Med Hyg 1988;82:90–93. [PubMed] [Google Scholar]
- 8.Amole BO, Wittner M, Hewlett D, Tanowitz HB. Trypanosoma brucei: infection in murine diabetes. Exp Parasitol 1985;60:342–347. [DOI] [PubMed] [Google Scholar]
- 9.Antonios SN, Galal AA, Salem SA, Elmarhomy SM. Experimental trichinosis in alloxan induced diabetes in mice. J Egypt Soc Parasitol 1989;19:149–156. [PubMed] [Google Scholar]
- 10.Elased K, De Souza JB, Playfair JH. Blood-stage malaria infection in diabetic mice. Clin Exp Immunol 1995;99:440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashiro S, Kawakami K, Uezu K, Kinjo T, Miyagi K, Nakamura K, Saito A. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol 2005;139:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saiki O, Negoro S, Tsuyuguchi I, Yamamura Y. Depressed immunological defence mechanisms in mice with experimentally induced diabetes. Infect Immun 1980;28:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathews CE, Langley SH, Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation 2002;73:1333–1336. [DOI] [PubMed] [Google Scholar]
- 14.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol 2001;19:93–129. [DOI] [PubMed] [Google Scholar]
- 15.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today 1998;19:491–494. [DOI] [PubMed] [Google Scholar]
- 16.Lammas DA, De HE, Edgar JD, Novelli V, Ben-Smith A, Baretto R, Drysdale P, Binch J, MacLennan C, Kumararatne DS, et al. Heterogeneity in the granulomatous response to mycobacterial infection in patients with defined genetic mutations in the interleukin 12-dependent interferon-gamma production pathway. Int J Exp Pathol 2002;83:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North RJ, Jung Y-J. Immunity to tuberculosis. Annu Rev Immunol 2004;22:599–623. [DOI] [PubMed] [Google Scholar]
- 18.Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol 2005;175:3268–3272. [DOI] [PubMed] [Google Scholar]
- 19.Serreze DV, Gaedeke JW, Leiter EH. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci USA 1993;90:9625–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000;12:431–440. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 2003;18:41–51. [DOI] [PubMed] [Google Scholar]
- 22.Luo B, Chan WF, Lord SJ, Nani SA, Rajotte RV, Shapiro AM, Anderson CC. Diabetes induces a rapid suppression of adaptive immunity followed by homeostatic T-cell proliferation. Scand J Immunol 2007;65:22–31. [DOI] [PubMed] [Google Scholar]
- 23.Lin RY, Choudhury RP, Cai W, Lu M, Fallon JT, Fisher EA, Vlassara H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2003;168:213–220. [DOI] [PubMed] [Google Scholar]
- 24.Pugliese G, Pricci F, Iacobini C, Leto G, Amadio L, Barsotti P, Frigeri L, Hsu DK, Vlassara H, Liu FT, et al. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J 2001;15:2471–2479. [DOI] [PubMed] [Google Scholar]
- 25.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol 2001;159:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu BF, Miyata S, Kojima H, Uriuhara A, Kusunoki H, Suzuki K, Kasuga M. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes 1999;48:2074–2082. [DOI] [PubMed] [Google Scholar]
- 27.Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, Stern DM, Al-Mohanna FA. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J Leukoc Biol 2002;71:433–444. [PubMed] [Google Scholar]
- 28.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest 2001;108:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He CJ, Zheng F, Stitt A, Striker L, Hattori M, Vlassara H. Differential expression of renal AGE-receptor genes in NOD mice: possible role in nonobese diabetic renal disease. Kidney Int 2000;58:1931–1940. [DOI] [PubMed] [Google Scholar]