Abstract
Cyclooxygenase-2 (COX-2) is a key enzyme in the production of prostaglandins and thromboxanes from free arachidonic acid. Increasing evidence suggests that COX-2 plays a role in tumorigenesis. A variety of stimuli induce COX-2 and it is overexpressed in many tumors, including non–small cell lung cancer (NSCLC). We studied the regulation of COX-2 expression in immortalized human bronchial epithelial cells (HBECs) by transforming growth factor-β1 (TGF-β1) and epidermal growth factor (EGF) because these two growth factors are present in both the pulmonary milieu of those at risk for lung cancer as well as in the tumor microenvironment. EGF significantly enhanced TGF-β1–mediated induction of COX-2 and corresponding prostaglandin E2 (PGE2) production. TGF-β1 and EGF induced COX-2 at the transcriptional and post-transcriptional levels. EGF receptor (EGFR) inhibition, neutralizing antibody against amphiregulin, or mitogen-activated protein kinase kinase (MEK) inhibition blocked TGF-β1–mediated COX-2 induction. COX-2 induction by TGF-β1 depended upon Smad3 signaling and required the activity of EGFR or its downstream mediators. Autocrine amphiregulin signaling maintains EGFR in a constitutively active state in HBECs, allowing for COX-2 induction by TGF-β1. Thus, EGFR ligands, which are abundant in the pulmonary microenvironment of those at risk for lung cancer, potentiate and are required for COX-2 induction by TGF-β1 in HBEC. These findings emphasize the central role of EGFR signaling in COX-2 induction by TGF-β1 and suggest that inhibition of EGFR signaling should be investigated further for lung cancer prevention.
Keywords: cyclooxygenase-2, transforming growth factor-β1, epidermal growth factor receptor, lung cancer, Smad3
CLINICAL RELEVANCE
Our findings regarding cooperation between transforming growth factor-β1 and epidermal growth factor receptor (EGFR) signaling events in COX-2 regulation in human bronchial epithelial cells are novel and suggest that inhibition of EGFR signaling should be investigated further for lung cancer prevention.
Cyclooxygenase (COX) is the rate-limiting enzyme in production of prostaglandins and thromboxanes from free arachidonic acid. Two isoforms have been identified: COX-1 and COX-2. COX-1 is constitutively expressed in most tissues, whereas COX-2 is induced in response to several stimuli, such as IL-1β, transforming growth factor-β (TGF-β), or TNF-α (1). COX-2 is expressed at high levels in non–small cell lung cancer (NSCLC) and many other malignancies. Increasing evidence suggests that COX-2 plays a role in tumorigenesis involving numerous pathways, including enhanced angiogenesis and invasion, decreased immunity, and apoptosis resistance (2–10). In patients at risk of developing lung cancer, COX-2 expression has been detected in premalignant pulmonary lesions such as atypical adenomatous hyperplasia (11). Inbred mouse strains predisposed to lung cancer display elevated COX-2 in pre-malignant lesions and in alveolar type II cells, a potential precursor cell of NSCLC (12). These data suggest an important role for COX-2 in carcinogenesis, and inhibition of this pathway may be useful in lung cancer chemopreventive strategies (13). Indeed, the COX-2 inhibitor, celecoxib, is currently under evaluation in clinical trials in late-stage lung cancer in combination treatment with other chemotherapeutic agents (14) as well as in chemoprevention trials in individuals at risk of developing lung cancer (15, 16).
TGF-β (17, 18) and epidermal growth factor (EGF) (19–21) are two growth factors that influence COX-2 expression. TGF-β has diverse biological effects which, in part, are dependent upon cell type (22–24). For example, TGF-β inhibits proliferation in normal epithelial cells. However, tumor cells often lose this response to TGF-β and continue to proliferate despite abundant production of this cytokine. In the tumor environment, TGF-β can promote tumor growth by increasing angiogenesis and suppressing local and systemic immune responses (22–24).
To impede or delay the development of cancer, studies are now underway targeting molecules crucial for cancer cell proliferation and survival. One such target, epidermal growth factor receptor (EGFR), is a transmembrane glycoprotein with intrinsic tyrosine kinase activity that regulates cell growth in response to ligand binding. Overexpression of EGFR has been observed in several cancers, including NSCLC, prostate, breast, and colon cancer (25). EGFR has been shown to affect apoptosis, angiogenesis, and metastasis, encouraging tumor progression (25). Two drugs targeting EGFR, gefitinib and erlotinib, have recently been approved for lung cancer treatment (26–29), and these have shown promise in the clinic. It has been suggested that EGFR inhibition could also be used in lung cancer prevention (30).
Smoking increases lung concentrations of TGF-β and EGF (31–33), and the high levels of these growth factors may contribute to chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis, both of which are risk factors for subsequent lung cancer development (31, 34–36). EGFR mutations are also detected in the histologically normal respiratory epithelium of patients with lung cancer, suggesting a role for EGFR mutation in the early events of lung cancer development in some patients (37). Therefore, to study the involvement of TGF-β and EGF in COX-2 regulation in early stages of lung carcinogenesis, we evaluated COX-2 expression after treatment with TGF-β and EGF in immortalized human bronchial epithelial cells (HBECs). We report here that EGF potentiates TGF-β1–mediated COX-2 induction at the transcriptional and post-transcriptional levels in HBECs. Importantly, EGFR-dependent signaling is required for the induction of COX-2 by TGF-β1. These findings emphasize the central role of EGFR signaling in COX-2 induction by TGF-β1 and suggest that inhibition of EGFR signaling should be investigated further for lung cancer prevention.
MATERIALS AND METHODS
Cell Culture
Immortalized HBECs derived from different individuals (HBEC3 and HBEC4, normal HBECs transduced with telomerase and CDK4) were used for these studies (38). COX-2 induction by TGF-β1 and EGF was observed in both HBEC3 and HBEC4 cells, and subsequent studies were carried out with HBEC3 cells. HBECs were maintained in keratinocyte serum-free medium (K-SFM; Invitrogen, Carlsbad, CA) containing 0.2 ng/ml recombinant human EGF and 30 μg/ml bovine pituitary extract (KSFM complete medium). Twenty-four hours before and throughout the conduct of each experiment, the cells were cultured in growth factor–free K-SFM. Recombinant human TGF-β1 and EGF were purchased from Peprotech Inc (Rocky Hill, NJ) and Invitrogen, respectively.
Prostaglandin E2 Release
HBECs were cultured on 6-well plates. Immediately before collecting the sample, the cells were incubated in medium containing 15 μM arachidonic acid (Cayman Chemical Co., Ann Arbor, MI) for 1 hour, then supernatant from each well was collected and prostaglandin E2 (PGE2) was measured by specific enzyme immunoassay (EIA) (Cayman Chemical Co.). PGE2 concentration was normalized to total cellular protein and expressed as pg/μg protein.
COX-2 Expression
HBECs were cultured on 6-well plates. Cells were lysed in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, 1 mM phenylmethylsulfonylfluoride, and complete mini protease inhibitor cocktail) and total protein was quantified using the BCA protein assay kit (Pierce Biotechnology, Philadelphia, PA). COX-2 expression was determined by specific enzyme-linked immunosorbent assay (ELISA) (Assay Designs, Inc., Ann Arbor, MI). The cross reactivity of the COX-2 ELISA to human COX-1 is less than 0.1%. COX-2 concentration was normalized to total cellular protein and expressed as pg/μg protein.
Phospho-EGFR Detection
Phospho-EGFR (p-EGFR) level in the cell lysate was determined by specific ELISA (R&D Systems, Minneapolis, MN) per manufacturer's instructions. Total protein was quantified using the BCA protein assay kit, and p-EGFR concentration was normalized to total cellular protein and expressed as pg/μg protein.
Kinase Inhibitor and Neutralizing Antibody Assays
HBECs were cultured to 60% confluence in 6-well plates and pretreated with small molecule inhibitors or neutralizing antibodies for 1 hour. TGF-β1 and /or EGF were then added to the culture and co-incubated with the inhibitors or the neutralizing antibodies for 24 hours. The cell lysates were collected and COX-2 protein levels were determined by specific ELISA or Western analysis. The mitogen-activated protein kinase kinase (MEK) inhibitor (PD98059), extracellular signal–regulated kinase (ERK) inhibitor II (FR180204), and EGFR tyrosine kinase inhibitors (PD153035, AG1478) were obtained from Calbiochem (La Jolla, CA). Human neutralizing antibodies against amphiregulin (catalog number: AF262), TGF-α (catalog number: AF-239-NA), EGF (catalog number: AF236), and HB-EGF (catalog number: AF-259-NA) were purchased from R&D Systems. Human neutralizing antibodies against EGFR (catalog number: 05-101) were purchased from Upstate (Lake Placid, NY).
Transient Transfection and Luciferase Assay
HBECs were cultured in 24-well plates. A DNA construct containing the human COX-2 promoter driving a luciferase reporter gene (hCOX-2 [−1437/+127]-Luc, kindly provided by Dr. Natarajan at Beckman Research Institute of the City of Hope, Duarte, CA [39]) was transiently introduced into HBECs using Effectene Transfection Reagent (Qiagen, Valencia, CA). Six hours later, transfection media was replenished with fresh KSFM and cells were cultured for 18 hours. Cells were then treated with TGF-β1 and/or EGF, and lysates collected over a 48-hour time course. COX-2 promoter activity was measured with the Dual Luciferase Assay Kit (Promega Corp., Madison, WI).
Transfection of siRNA
Cells in 6-well plates, at 40% confluence, were incubated with the siRNA transfection solution at 37°C for 6 hours, washed twice with PBS, and then incubated in KSFM complete medium for 48 hours. The medium was then changed to fresh KSFM, and TGF-β1 was added to the culture for 16 hours. Total RNA was collected using Rneasy Kit from Qiagen (Valencia, CA). siRNA for Smad2 (SMARTpool), Smad3 (SMARTpool and Upgrade), and nontargeting siRNA control were purchased from Dharmacon (Chicago, IL). The TransMessenger Transfection reagent was from Qiagen. Two of the siRNA sequences of Smad3 SMARTpool were used to verify the Smad3 SMARTpool results. These two siRNA sequences are: (1): sense, 5′-GAGUUCGCCUUCAAUAUGAUU-3′; antisense, 5′-UCAUAUUGAAGGCGAACUCUU-3′; (2) sense, 5′-UCAAGAGCCUGGUCAAGAAUU-3′; antisense, 5′-UUCUUGACCAGGCUCUUGAUU-3′.
Real-Time PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen). For quantitative real-time PCR (QPCR) analysis, RNA was extracted and cDNA prepared with a kit (Invitrogen) according to the manufacturer's instructions. Gene expression was quantified using the SYBR Green quantitative PCR kit in the iCycler (Bio-Rad, Hercules, CA) and normalized with human β-actin or human GAPDH housekeeping control amplifications. Amplification was carried out in a total volume of 25 μl for 40 cycles of 30 seconds at 95°C, 30 seconds at 60°C (57°C for Smad2), and 30 seconds at 72°C. Samples were run in duplicate and their relative expression was determined by normalizing expression of each target to human β-actin or human GAPDH and then comparing this normalized value to the normalized expression in a reference sample to calculate a fold change value. Primers were designed so that amplicons spanned intron/exon boundaries to minimize amplication of genomic DNA. A melting curve analysis was run at the end of the PCR to ensure a lack of primer-dimers. Primer sequences were as follows: human β-actin—forward, 5′-GATGAGATTGGCATGGCTTT -3′ and reverse, 5′-CACCTTCACCGTTCCAGTTT -3′; hCOX-2—forward, 5′-TCCTATTATACTAGAGCCCTTCCT-3′ and reverse, 5′-TTCCACAATCTCATTTGAATCAGG-3′; hSmad2—forward, 5′-GGACTGAGTACACCAAATACG-3′ and reverse, 5′-GCAATATATAACATGTGGCAATC-3′ (40); hSmad3—forward, 5′-CCCCAGAGCAATATTCCAGA-3′ and reverse, 5′-GACATCGGATTCGGGGATAG-3′ (41); human amphiregulin—forward, 5′-GGCTCAGGCCATTATGC-3′ and reverse, 5′-ACCTGTTCAACTCTGACTGA-3′ (42); and human GAPDH—forward, 5′-TGCACCACCAACTGCTTAGC-3′ and reverse, 5′-GGCATGGACTGTGGTCATGAG-3′ (43).
Western Analysis
The cell lysate was collected using lysis buffer containing 1 mM sodium orthovanadate and total protein was quantified using the BCA protein assay. 10% SDS-PAGE electrophoresis was performed. Protein was then transferred to PVDF membrane (Millipore, Bedford, MA). After incubation with the horseradish peroxidase–conjugated antibody, the signal was detected with an ECL Kit from Pierce. Phospho-Smad3 (Ser433/435) (catalog number: 9514) and phospho-p44/42 MAPK (Thr202/Tyr204) (catalog number: 9106) antibodies were purchased from Cell Signaling (Danvers, MA). COX-2 antibody (catalog number: 160112) was purchased from Cayman Chemical Co.
Statistical Analysis
For each study group, the outcome measurements were summarized using mean and SD. Statistical significance of data was determined by the two-tailed Student's t test. P values less than 0.05 were considered significant.
RESULTS
EGF Potentiates the TGF-β1–Mediated Increase in COX-2 Expression and PGE2 Production in HBECs
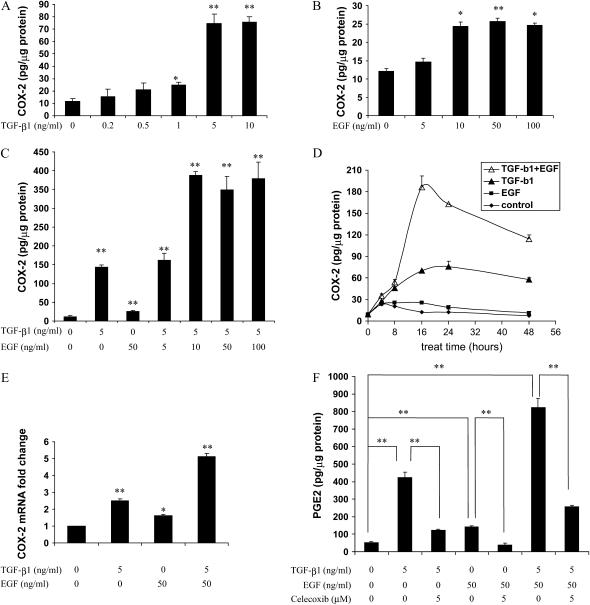
TGF-β has been reported to induce COX-2 in several cell types (17, 18). To investigate the effect of TGF-β on COX-2 expression in immortalized HBEC, cells were treated with several concentrations of TGF-β1 for 24 hours and COX-2 levels in the cell lysates were determined by specific ELISA. TGF-β1 caused a dose-dependent induction of COX-2 protein that was maximal (5-8 fold) at 5-10 ng/ml (Figure 1A). The COX-2 protein induction in response to TGF-β1 occurred as early as 8 hours and peaks between 16 and 24 hours (Figure 1D). Twenty-four hours was then chosen for most of the following studies, since it corresponded to the highest COX-2 induction by TGF-β1.
Figure 1.
Epidermal growth factor (EGF) potentiates transforming growth factor (TGF)-β1–mediated cyclooxygenase (COX)-2 induction in human bronchial epithelial cells (HBECs). HBEC3 cells were cultured in 6-well plates and treated with TGF-β1 and/or EGF. Cell lysates were collected and COX-2 protein was measured by specific enzyme-linked immunosorbent assay (ELISA). In A, B, and C, COX-2 concentrations were determined after 24-hour exposure to growth factors. (A) TGF-β1 induced COX-2 in a dose-dependent manner. (B) EGF induced COX-2 in a dose-dependent manner. (C) EGF potentiated TGF-β1–mediated COX-2 induction. (D) TGF-β1 (5 ng/ml) and EGF (50 ng/ml) induced COX-2 in a time-dependent manner. (E) TGF-β1 (5 ng/ml) and/or EGF (50 ng/ml) induced COX-2 mRNA expression. Cells were treated for 8 hours and total RNA was extracted. COX-2 mRNA level was determined by quantitative real-time PCR. (F) EGF potentiated TGF-β1–mediated PGE2 induction. HBEC3 cells were treated with TGF-β1 (5 ng/ml), EGF (50 ng/ml), and/or celecoxib (5 μM) for 24 hours (celecoxib was added 1 hour before the growth factors). Right before collecting the sample, cells were incubated with 15 μM arachidonic acid for 1 hour. PGE2 in the supernatant from each well was measured by specific enzyme immunoassay (EIA). One representative experiment out of three is shown. *P < 0.05, **P < 0.01.
Next we sought to determine if COX-2 expression could be augmented by EGF in HBEC. To evaluate the effects of EGF on COX-2 expression, HBEC3 cells were treated with doses spanning 0 to 100 ng/ml EGF, and COX-2 levels were determined by ELISA. EGF caused a 2-fold increase in COX-2 production, with maximal induction occurring at 10 to 100 ng/ml (Figure 1B). Interestingly, EGF strongly potentiated TGF-β1–induced COX-2 production (Figures 1C and 1D). Similar results were also obtained in HBEC4 cells (data not shown). COX-2 mRNA expression after each treatment was also assessed by quantitative real-time PCR. Exposure to either growth factor individually elevated COX-2 mRNA (Figure 1E), and combined treatment resulted in a greater increase in COX-2 mRNA than seen with either growth factor alone (Figure 1E). TGF-β1 also significantly induced PGE2 production as determined by ELISA, and this was also potentiated by EGF. Elevated COX-2 levels appeared to be responsible for the augmented PGE2 production from cells treated with TGF-β1 and EGF, as indicated by the capacity of celecoxib to prevent this increase (Figure 1F).
TGF-β1 and EGF Induce COX-2 at the Transcriptional and Post-Transcriptional Levels
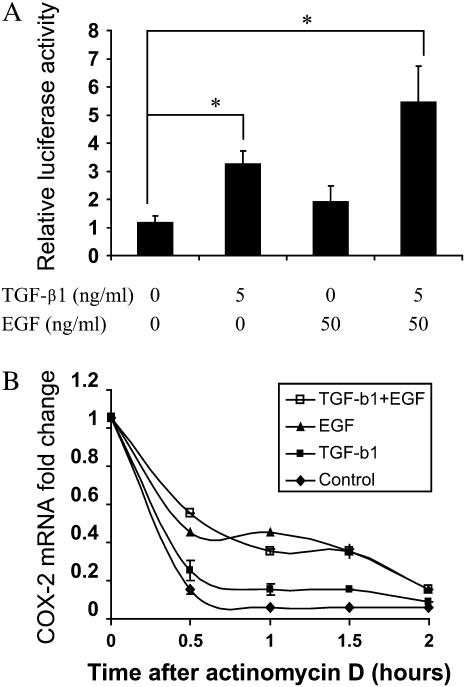
Possible mechanisms for COX-2 mRNA induction by TGF-β1 and EGF were evaluated. COX-2 transcription was assessed using a firefly luciferase reporter construct containing the COX-2 promoter region (−1437/+127)-Luc. HBEC3 cells were transiently transfected with pCOX-2 promoter-Luc and pRL-TK (renilla luiferase construct for the normalization of transfection efficiency), or the empty vector, pGL3-Luc. TGF-β1 caused a 2.8-fold increase of COX-2 promoter activity in HBEC3 cells at 24 hours, while EGF increased COX-2 transcription by 1.6-fold (Figure 2A). In combination, TGF-β1 and EGF induced COX-2 transcription to a greater extent than TGF-β or EGF alone. The empty vector had no effect (data not shown).
Figure 2.
TGF-β1 and EGF induce COX-2 at the transcriptional and post-transcriptional levels in HBECs. (A) HBEC3 cells were transfected with COX-2 promoter (−1437/+127)-Luc and pRL-TK. Twenty-four hours after the transfection, cells were treated with TGF-β1 and EGF for an additional 24 hours. Cell lysates were collected and luciferase activity was measured with dual-luciferase assay kit. (B) HBEC3 cells were treated with 5 ng/ml TGF-β1 and 50 ng/ml EGF for 22 hours and 5 μg/ml Actinomycin D was added to inhibit transcription. Total RNA was extracted over a 2-hour time course, and COX-2 mRNA was measured by quantitative real-time PCR. Each set of conditions was normalized to the “0” time point. One representative experiment out of three is shown. *P < 0.05.
To determine whether TGF-β1 and EGF influence COX-2 mRNA stability, HBEC3 cells were pre-treated with TGF-β1 (5 ng/ml) and/or EGF (50 ng/ml) for 22 hours and followed by inhibition of transcription by actinomycin D treatment. Two hours following actinomycin D treatment, COX-2 mRNA decay was monitored by quantitative real-time PCR. EGF treatment alone or in combination with TGF-β1 increased COX-2 mRNA half-life (Figure 2B); however, TGF-β1 treatment alone had little effect (Figure 2B).
COX-2 Induction by TGF-β1 in HBECs Requires Constitutive p-EGFR Signaling, Maintained by Amphiregulin in HBEC
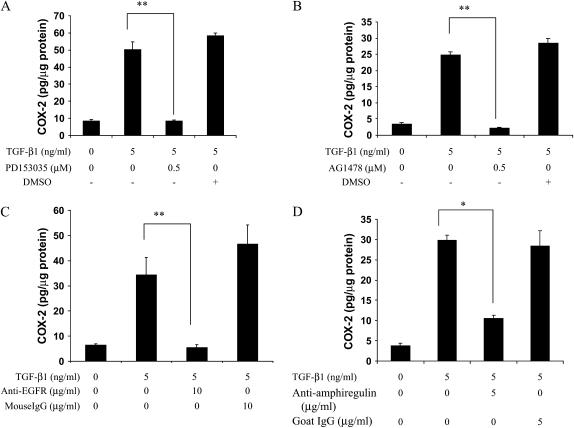
The induction of COX-2 by IFN-γ or tobacco smoke has been reported to be mediated via EGFR activation (44–46). Here we sought to determine whether TGF-β1 induced COX-2 in an EGFR-dependent manner. Two specific EGFR inhibitors (PD153035, AG1478) completely abolished COX-2 induction by TGF-β1 (Figures 3A and 3B), and an anti-EGFR antibody had the same effect (Figure 3C). This suggests that EGFR signaling is critical for COX-2 induction by TGF-β1. Because EGFR activation is typically accomplished via ligand binding, neutralizing antibodies against four common EGFR ligands (TGF-α, EGF, amphiregulin, and heparin binding-EGF [HB-EGF]) were used to assess their roles in this process, while goat IgG served as a negative control. The amphiregulin-neutralizing antibody significantly inhibited COX-2 induction by TGF-β1 (Figure 3D). In contrast, neutralizing antibodies against TGF-α, EGF, or HB-EGF, when evaluated over a wide range of antibody concentration, had no effect (data not shown). This finding indicates that EGFR activation by amphiregulin is required for COX-2 induction by TGF-β1. To test the hypothesis that TGF-β1 itself induces production of amphiregulin, which in turn could activate EGFR, we measured amphiregulin protein levels in HBEC-conditioned media. Amphiregulin was present in the culture supernatant (30–40 pg/μg cellular protein) as determined by specific ELISA (data not shown). However, consistent with a previous report (47), neither secretory amphiregulin nor amphiregulin mRNA expression increased above this high constitutive level after TGF-β1 exposure (data not shown). Thus, although there is no induction of amphiregulin by TGF-β1, the basal level of amphiregulin produced by HBECs appears to play a critical role for TGF-β1–mediated COX-2 induction.
Figure 3.
Inhibition of EGF receptor (EGFR) signaling blocks TGF-β1–mediated COX-2 induction. HBEC3 cells were pretreated with (A) PD153035 (EGFR inhibitor, 0.5μM), (B) AG1478 (EGFR inhibitor, 0.5μM), or one of the following neutralizing antibodies: (C) anti-EGFR (10 μg/ml) or (D) anti-amphiregulin (5 μg/ml) for 1 hour. A quantity of 5 ng/ml TGF-β1 was then added and co-incubated with the inhibitor or neutralizing antibody for 24 hours. Cell lysates were collected and COX-2 protein expression was determined using specific ELISA. One representative experiment out of three is shown. *P < 0.05, **P < 0.01.
The constitutive levels of COX-2 in these epithelial cells were low. Under the experimental conditions used in these studies, EGFR inhibition also modestly decreased constitutive COX-2 level (data not shown). This indicates that EGFR-dependent signaling affects COX-2 in both constitutive and stimulated states, which is in agreement with a previous report (44).
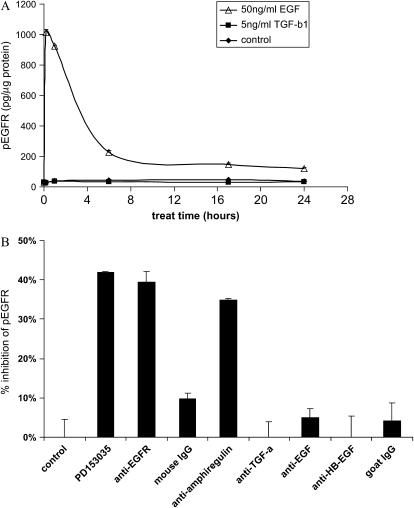
To study the effect of TGF-β1 on EGFR phosphorylation, p-EGFR levels were determined by specific ELISA. EGF treatment was used as a positive control and demonstrated that EGFR phosphorylation peaked at 10 minutes and remained elevated up to 24 hours (Figure 4A). In contrast, TGF-β1 treatment (10 minutes to 24 hours) had no effect on constitutive p-EGFR (30 to 40 pg/μg protein) (Figure 4A). The constitutive EGFR phosphorylation was significantly reduced by the EGFR tyrosine kinase inhibitor (PD153035), anti-EGFR, or anti-amphiregulin neutralizing antibody, while the neutralizing antibodies against EGF, HB-EGF, or TGF-α had no effect (Figure 4B). Thus, amphiregulin, but not EGF, HB-EGF, or TGF-α, plays an important role in maintaining the basal level of EGFR signaling, which, in turn, is required for COX-2 induction by TGF-β1 in HBECs. Although exogenous EGF induces COX-2 production in HBECs, the endogenous EGF, HB-EGF, or TGF-α does not contribute significantly to the basal level of EGFR activation and thus neutralizing antibodies against EGF, HB-EGF, or TGF-α have no effect on TGF-β1–mediated COX-2 induction.
Figure 4.
HBEC3 cells maintain a constitutive level of EGFR phosphorylation, which is inhibited by the EGFR inhibitor, PD153035, or neutralizing antibodies against EGFR or amphiregulin. (A) HBEC3 cells were treated with 5 ng/ml TGF-β1 or 50 ng/ml EGF, and cell lysates were collected at various time points (10 minutes to 24 hours). (B) HBEC3 cells were treated with PD153035 (0.5 μM), neutralizing antibodies against EGFR (10μg/ml), or against EGFR ligands (anti-amphiregulin [5 μg/ml], anti–TGF-α [5 μg/ml], anti-EGF [1μg/ml], anti–HB-EGF [20μg/ml]) and cell lysates were collected 24 hours later. p-EGFR in the cell lysates was determined by specific ELISA. One representative experiment out of three is shown.
Smad3 Mediates COX-2 Induction by TGF-β1
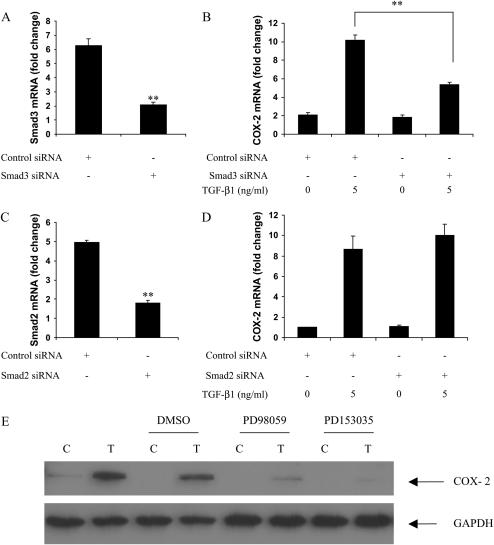
Because TGF-β1 effects are predominantly mediated through activation of the Smad family of signaling molecules, we measured COX-2 induction by TGF-β1 after knocking down Smad2 or Smad3 with siRNA. Smad3 siRNA blocked Smad3 mRNA expression, as well as COX-2 induction by TGF-β1 (Figures 5A and 5B). Similar results were obtained using either Smad3 SMARTpool siRNA or two individual siRNA duplexes from the SMARTpool. In contrast, although Smad2 siRNA significantly inhibited Smad2 mRNA expression, it had no effect on TGF-β1–mediated COX-2 induction (Figures 5C and 5D). These findings indicate that TGF-β1 induces COX-2 through Smad3 in HBECs.
Figure 5.
Smad3 and ERK phosphorylation mediate COX-2 induction after exposure of HBEC to TGF-β1. (A–D) HBECs were transfected with siRNA SMARTpool for Smad3 (100 nM, A, B) or Smad2 (50 nM, C, D). Forty-eight hours after the transfection, TGF-β1 (5 ng/ml) was added for 16 hours, and then total RNA was collected for quantitative real-time PCR analysis. (E) HBEC3 cells were pretreated with 10 μM PD98059 (MEK inhibitor) or 0.5 μM PD153035 (EGFR inhibitor) for 1 hour, then 5 ng/ml TGF-β1 was added and co-incubated with either inhibitor for 18 hours. COX-2 expression was determined by Western blot analysis. C: control; T: 5 ng/ml TGF-β1. **P < 0.01. One representative experiment out of three is shown.
Active ERK Is Required for COX-2 Induction by TGF-β1
In response to a variety of stimuli, ERK signaling impacts COX-2 induction (48). The cooperative effects of the Smad3 and ERK pathways have also been described in the regulation of some other genes (49). We therefore determined the involvement of ERK signaling in TGF-β1–mediated COX-2 induction. ERK phosphorylation was determined in HBEC by Western analysis. Untreated HBECs maintained a detectable level of ERK phosphorylation, which was increased by TGF-β1 at approximately 18 to 24 hours (data not shown). To study the requirement for ERK in COX-2 induction by TGF-β1, HBEC3 cells were pretreated with the MEK inhibitor PD98059 for 1 hour, and then incubated for 18 hours with TGF-β1 in the presence of PD98059. PD98059 significantly inhibited the induction of COX-2 by TGF-β1 (Figure 5E), indicating a requirement for active ERK. Further support for the importance of ERK in this process was indicated by the fact that the selective ERK inhibitor FR180204 (50) significantly inhibited TGF-β1–mediated COX-2 induction, while the same concentration of a structurally related negative control compound, FR180289, did not (data not shown). Thus, active ERK must be present for COX-2 induction by TGF-β1; however, because the elevation in COX-2 expression due to TGF-β1 precedes the increase in p-ERK by several hours, ERK signaling itself may not provide the direct stimulus to amplify COX-2. Importantly, constitutive ERK phosphorylation is required for COX-2 induction by TGF-β1. We also found that PD98059 modestly inhibited basal level of COX-2 (data not shown), suggesting that ERK phosphorylation is important for both constitutive and stimulated COX-2 in HBEC.
The Constitutive Level of ERK Phosphorylation Is Dependent on EGFR Signaling in HBEC
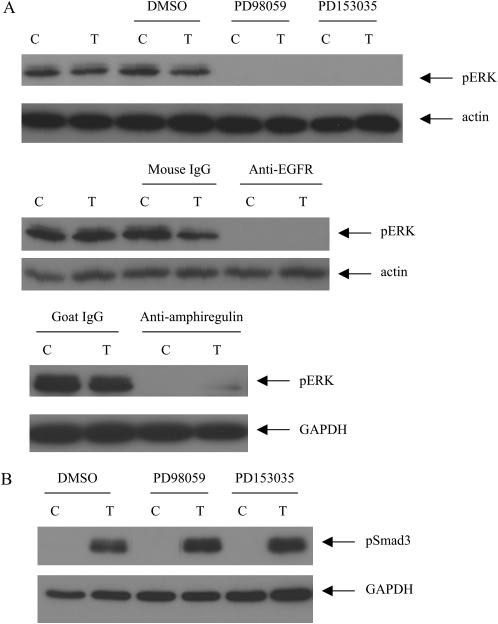
Because we found that both EGFR and ERK inhibition significantly inhibited TGF-β1 mediated COX-2 induction, the impact of EGFR signaling on ERK phosphorylation was investigated. The cells were pretreated with the EGFR inhibitor PD153035 for 1 hour and TGF-β1 was added to the culture and co-incubated with the inhibitor for 1 hour. We found that the constitutive level of ERK phosphorylation was abolished by PD153035 (Figure 6A), which would in turn inhibit TGF-β1–mediated COX-2 induction. The neutralizing antibody against EGFR and amphiregulin also showed similar effects as seen with PD153035 (Figure 6A).
Figure 6.
Constitutive ERK phosphorylation depends upon EGFR activity in HBECs. (A) HBEC3 cells were pretreated with inhibitor (10 μM PD98059 or 0.5 μM PD153035) or neutralizing antibody (10 μg/ml anti-EGFR or 5 μg/ml anti-amphiregulin) for 1 hour, then 5 ng/ml TGF-β1 was added and co-incubated with the inhibitor or neutralizing antibody for 1 hour. (B) HBEC3 cells were pretreated with inhibitor (10 μM PD98059 or 0.5 μM PD153035) for 1 hour, then 5 ng/ml TGF-β1 was added and co-incubated with the inhibitor for 1 hour. p-ERK and phospho-Smad3 expression was determined by Western analysis. C: control; T: 5 ng/ml TGF-β1. One representative experiment out of three is shown.
Because we have also found that Smad3 was required for TGF-β1–mediated COX-2 induction, the impact of EGFR or ERK inhibition on Smad3 phosphorylation was investigated. HBEC3 cells were pretreated with PD153035 or PD98059 for 1 hour and TGF-β was then added to the culture for 1 hour. TGF-β1 induced Smad3 phosphorylation, which was not inhibited by either PD153035 or PD98059 (Figure 6B).
DISCUSSION
A multifaceted role for COX-2 in tumorigenesis has been suggested, as its enzymatic products encourage many aspects of the malignant phenotype, including increased angiogenesis, resistance to apoptosis, and suppression of anti-tumor immune responses (2–10). COX-2 is overexpressed in many tumors and likely contributes to neoplastic transformation at early stages, as it is also highly expressed in pre-neoplastic lesions (11). Based on its role in promoting survival and expansion of transformed cells, the cyclooxygenase pathway has been targeted in lung cancer chemoprevention strategies (15, 16); however, the risk/benefit ratio of specific COX-2 antagonists has been called into question (51, 52). Understanding the mechanisms by which upstream regulators increase COX-2 expression at the early stages of lung tumor development may provide alternative avenues for pharmacologic intervention in preventive strategies.
The growth factors TGF-β1 and EGF have each individually been reported to influence COX-2 expression, and these molecules are highly expressed in individuals at risk of developing lung cancer (31–33). The seminal studies of Sieweke and coworkers indicated a critical role for TGF-β in tumorigenesis (53). In subsequent studies, TGF-β has been found to promote tumor invasion and metastasis, induce angiogenesis, and promote immune suppression (54). TGF-β and Ras synergistically induce COX-2 in rat intestinal epithelial cells and immortalized mouse colonocytes (17, 18). TGF-β was shown to stabilize COX-2 mRNA in rat intestinal epithelial cells (RIE-1) and human lung fibroblasts (1, 17). Also relevant to our findings, the capacity for EGF to induce COX-2 has been demonstrated in several cell lines (19–21). EGFR signaling has been shown to promote apoptosis resistance, angiogenesis, and metastasis, encouraging tumor progression (25). Furthermore, TGF-β1 and EGF synergistically induce COX-2 in mink lung epithelial cells (55); however, this has not been examined in human cells, and to date, no mechanism to explain the synergistic effects of TGF-β and EGF on COX-2 expression has been presented. We have investigated COX-2 regulation by TGF-β1 and EGF signaling pathways in HBECs immortalized by stable introduction of Cdk4 and hTERT (38). As previously reported, these cells did not form colonies in soft agar or tumors in nude mice. The gene expression profiles of these cell lines clustered together with nonimmortalized bronchial epithelial cells (38), suggesting their utility as a model of the normal bronchial epithelium. Our findings, discussed below, are consistent with the hypothesis that signals from the TGF-β1 and EGF pathways cooperate to increase COX-2 mRNA and protein expression and that components of the EGFR pathway must be active in order for TGF-β1 to have an effect. Based on the importance of TGF-β1, EGF, and COX-2 in lung cancer development, pharmacologic manipulation of these pathways warrants further investigation in lung cancer chemopreventive settings.
In HBECs, inhibitors of the MEK/ERK pathway blocked COX-2 induction by TGF-β1. While a direct role for ERK in COX-2 elevation after TGF-β1 treatment might be postulated, we also found that HBECs maintained a constitutive level of p-ERK levels that was not further induced by TGF-β1 until several hours after COX-2 mRNA and protein had already increased. Accordingly, it is unlikely that ERK itself transmits the signal from TGF-β1 to increase COX-2 expression. In contrast, Smad3 is phosphorylated shortly after exposure to TGF-β1, and Smad3 inhibition prevents subsequent COX-2 induction. Thus, while active ERK must be present for induction of COX-2, Smad3 signaling appears to be directly mediating the effects of TGF-β1 in this setting. These findings are consistent with previous reports documenting cooperation between Smad and ERK pathways in the regulation of other genes (49, 56, 57). The point(s) at which TGF-β and EGFR signaling converge are not completely defined. Activation of Smad3 by TGF-β is well established to occur by phosphorylation within the C-terminus (58); however, here we found that inhibition of the EGFR/ERK activity did not alter phosphorylation at this site in HBECs. It has also been reported that TGF-β1 may induce Smad3 phosphorylation in the linker region in an ERK-dependent manner and ERK inhibition may reduce total serine phosphorylation of Smad3 (57). We found that inhibition of the EGFR or ERK pathway did not alter total serine phosphorylation of Smad3 induced by TGF-β1 in HBECs (data not shown), suggesting that downstream pathways might be involved in TGF-β1–mediated COX-2 induction. Several possible mechanisms will require further investigation. For example, TGF-β–mediated Smad2/3 nuclear translocation have been suggested to be modulated by the MAP kinase pathway (59, 60) which may play a role in regulating TGF-β–mediated COX-2 induction. It has also been shown that Smad3 and MAPK/ERK can cooperatively increase AP1 activity (61), and this transcription factor may play a role in COX-2 induction through EGFR and TGF-β pathways. Further investigations will be required to assess these possibilities.
We found that ERK phosphorylation was increased by TGF-β1 at 18 to 24 hours in HBEC (data not shown). It has been previously reported that TGF-β1 could induce ERK phosphorylation in a delayed manner (62, 63). Secondary mediators may play a role in this delayed pERK induction by TGF-β1 in HBEC. Additional studies will be required to assess these possibilities.
We observed that secretion of the EGFR ligand, amphiregulin, results in autocrine signaling through EGFR to maintain ERK phosphorylation, providing a permissive state for TGF-β1 to influence COX-2 expression. In immortalized HBECs, TGF-β1–induced COX-2 expression is completely abolished by EGFR tyrosine kinase inhibition or EGFR-neutralizing antibody. Neutralizing antibody against amphiregulin also significantly inhibited COX-2 induction by TGF-β1. However, TGF-β1 did not increase amphiregulin production or EGFR phosphorylation in HBECs. HBECs maintain a basal level of p-EGFR, which is significantly inhibited by an EGFR tyrosine kinase inhibitor, anti-EGFR– or anti-amphiregulin–neutralizing antibodies. TGF-β1 activates the Smad pathway and increases COX-2 transcription in HBECs. Although TGF-β1 does not increase amphiregulin production, the constitutive presence of HBEC-derived amphiregulin leads to a basal level of constitutive EGFR phosphorylation that is required for TGF-β1–mediated COX-2 induction. Further investigation demonstrated that constitutive ERK phosphorylation was abolished by EGFR inhibition, which in turn prevented COX-2 induction by TGF-β1. Thus, the requirement for amphiregulin/EGFR signaling in TGF-β1–mediated COX-2 elevation may be due to its capacity to maintain constitutive ERK phosphorylation. Our data suggests that amphiregulin/EGFR signaling may be important at early stages of lung tumor development. Consistent with our findings, enhanced levels of amphiregulin have also been correlated with advanced tumor stage in NSCLC (64). An amphiregulin-mediated autocrine loop also contributes to the transformed phenotype of human hepatocellular carcinoma cells (65), thus suggesting that these pathways are involved in the pathogenesis of lung cancer as well as other malignancies.
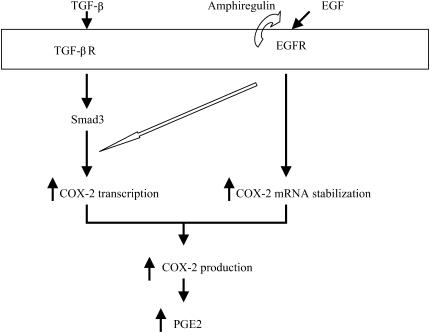
EGF alone has been reported to influence COX-2 expression in several cell lines (19–21), and here modestly elevated COX-2 expression in HBECs. Combined treatment with TGF-β1 and EGF resulted in a marked increase in COX-2 expression. This may occur through effects on both the transcription rate of the COX-2 gene as well as stability of the mRNA product. In luciferase reporter gene assays using the COX-2 promoter, TGF-β1 and EGF increased transcription. In addition, EGF increased the stability of COX-2 mRNA, while TGF-β1 had minimal effects. Combined effects of these two cytokines on transcription and degradation could account for the dramatic increase in COX-2 expression when HBECs are exposed to TGF-β1 and EGF together. In addition, as noted above, although EGFR inhibition abolished TGF-β1–mediated COX-2 induction, TGF-β1 did not induce EGFR phosphorylation. Constitutive EGFR phosphorylation thus plays an important permissive role for COX-2 induction by TGF-β1. Our current model showing the possible mechanism of COX-2 induction by TGF-β1 and EGF in HBECs is depicted in Figure 7.
Figure 7.
Possible mechanisms of COX-2 induction by TGF-β and EGF in HBECs. TGF-β1 activates Smad3 and increases COX-2 transcription. EGF predominantly increases the stability of COX-2 mRNA and causes a minor increase in COX-2 transcription. Combined effects of these two cytokines on transcription and mRNA stability account for the significant increase in COX-2 expression after exposure to both TGF-β1 and EGF. Importantly, the constitutive presence of HBEC-derived amphiregulin leads to a basal level of constitutive EGFR phosphorylation that is required for TGF-β1–mediated COX-2 induction.
Based on our current findings and previously reported high levels of TGF-β1 and EGFR ligands found in the pulmonary microenvironment of individuals at risk of developing lung cancer (31–36), the induction of COX-2 by TGF-β1 and components of the EGFR pathway may contribute to early stages of lung carcinogenesis. COX-2 induction in HBEC may have a broad array of biological effects, impacting tumor growth and development (66). For example, vascular endothelial growth factor (VEGF), an important mediator of angiogenesis, has been previously related to COX-2 activity (67). We found that TGF-β1 and EGF significantly induced VEGF in HBEC, and this was partially inhibited by celecoxib (data not shown), implying COX-2, induced by TGF-β1 and EGF contributed to this process. Above all, our data stress the central role of EGFR signaling in COX-2 induction by TGF-β1. EGFR inhibition has previously been suggested for lung cancer prevention (30). Our findings suggest that further investigation of EGFR pathway inhibition is warranted in lung cancer prevention.
Acknowledgments
The authors thank Hejing Wang for her assistance with statistical analysis.
This work was supported by the UCLA SPORE in Lung Cancer, National Institutes of Health Grants P50 CA90388, the American Lung Association of California-Research Program, Tobacco-Related Disease Research Program, VA Merits, UT Southwestern SPORE in Lung Cancer P50 CA70907, Gillson Longenbaugh and Anderson Charitable Foundations, and Department of Defense VITAL grant W81XWH041014202PP.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0100OC on June 28, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA. Differential regulation of cyclooxygenases 1 and 2 by interleukin-1 beta, tumor necrosis factor-alpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res 1998;241:222–229. [DOI] [PubMed] [Google Scholar]
- 2.Dubinett SM, Sharma S, Huang M, Dohadwala M, Pold M, Mao JT. Cyclooxygenase-2 in lung cancer. Prog Exp Tumor Res 2003;37:138–162. [DOI] [PubMed] [Google Scholar]
- 3.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res 1998;58:1208–1216. [PubMed] [Google Scholar]
- 4.Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, Huang M, Pold M, Batra RK, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem 2001;276:20809–20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pold M, Dohadwala M, Luo J, Lin Y, Dubinett S. Microarray identifies cyclo-oxygenase-2-dependent modulation of insulin-like growth factor binding protein-3 in non-small cell lung cancer cells. Chest 2002;121:29S–30S. [DOI] [PubMed] [Google Scholar]
- 6.Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem 2002;277:50828–50833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuze-Vourc'h N, Zhu L, Krysan K, Batra RK, Sharma S, Dubinett SM. Abnormal interleukin 10Ralpha expression contributes to the maintenance of elevated cyclooxygenase-2 in non-small cell lung cancer cells. Cancer Res 2003;63:766–770. [PubMed] [Google Scholar]
- 8.Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res 2003;9:961–968. [PubMed] [Google Scholar]
- 9.Krysan K, Merchant FH, Zhu L, Dohadwala M, Luo J, Lin Y, Heuze-Vourc'h N, Pold M, Seligson D, Chia D, et al. COX-2-dependent stabilization of survivin in non-small cell lung cancer. Faseb J 2004;18:206–208. [DOI] [PubMed] [Google Scholar]
- 10.Pold M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, Pold A, Luo J, Krysan K, Dohadwala M, et al. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res 2004;64:1853–1860. [DOI] [PubMed] [Google Scholar]
- 11.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 1998;58:3761–3764. [PubMed] [Google Scholar]
- 12.Wardlaw SA, March TH, Belinsky SA. Cyclooxygenase-2 expression is abundant in alveolar type II cells in lung cancer-sensitive mouse strains and in premalignant lesions. Carcinogenesis 2000;21:1371–1377. [PubMed] [Google Scholar]
- 13.Mao JT, Cui X, Reckamp K, Liu M, Krysan K, Dalwadi H, Sharma S, Hazra S, Strieter R, Gardner B, et al. Chemoprevention strategies with cyclooxygenase-2 inhibitors for lung cancer. Clin Lung Cancer 2005;7:30–39. [DOI] [PubMed] [Google Scholar]
- 14.Reckamp KL, Krysan K, Morrow JD, Milne GL, Newman RA, Tucker C, Elashoff RM, Dubinett SM, Figlin RA. A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced non-small cell lung cancer. Clin Cancer Res 2006;12:3381–3388. [DOI] [PubMed] [Google Scholar]
- 15.Riedl K, Krysan K, Pold M, Dalwadi H, Heuze-Vourc'h N, Dohadwala M, Liu M, Cui X, Figlin R, Mao JT, et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat 2004;7:169–184. [DOI] [PubMed] [Google Scholar]
- 16.Mao JT, Roth MD, Serio KJ, Baratelli F, Zhu L, Holmes EC, Strieter RM, Dubinett SM. Celecoxib modulates the capacity for prostaglandin E2 and interleukin-10 production in alveolar macrophages from active smokers. Clin Cancer Res 2003;9:5835–5841. [PubMed] [Google Scholar]
- 17.Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, Beauchamp RD. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem 2000;275:6628–6635. [DOI] [PubMed] [Google Scholar]
- 18.Roman CD, Morrow J, Whitehead R, Beauchamp RD. Induction of cyclooxygenase-2 and invasiveness by transforming growth factor-beta(1) in immortalized mouse colonocytes expressing oncogenic Ras. J Gastrointest Surg 2002;6:304–309. [DOI] [PubMed] [Google Scholar]
- 19.Slice LW, Hodikian R, Zhukova E. Gastrin and EGF synergistically induce cyclooxygenase-2 expression in Swiss 3T3 fibroblasts that express the CCK2 receptor. J Cell Physiol 2003;196:454–463. [DOI] [PubMed] [Google Scholar]
- 20.Slice LW, Chiu T, Rozengurt E. Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J Biol Chem 2005;280:1582–1593. [DOI] [PubMed] [Google Scholar]
- 21.Ackerman WE 4th, Rovin BH, Kniss DA. Epidermal growth factor and interleukin-1beta utilize divergent signaling pathways to synergistically upregulate cyclooxygenase-2 gene expression in human amnion-derived WISH cells. Biol Reprod 2004;71:2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akhurst RJ. TGF-beta antagonists: why suppress a tumor suppressor? J Clin Invest 2002;109:1533–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117–129. [DOI] [PubMed] [Google Scholar]
- 24.Roman C, Saha D, Beauchamp R. TGF-beta and colorectal carcinogenesis. Microsc Res Tech 2001;52:450–457. [DOI] [PubMed] [Google Scholar]
- 25.Lonardo F, Dragnev KH, Freemantle SJ, Ma Y, Memoli N, Sekula D, Knauth EA, Beebe JS, Dmitrovsky E. Evidence for the epidermal growth factor receptor as a target for lung cancer prevention. Clin Cancer Res 2002;8:54–60. [PubMed] [Google Scholar]
- 26.Sridhar SS, Seymour L, Shepherd FA. Inhibitors of epidermal-growth-factor receptors: a review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol 2003;4:397–406. [DOI] [PubMed] [Google Scholar]
- 27.Levitzki A. EGF receptor as a therapeutic target. Lung Cancer 2003;41:S9–14. [DOI] [PubMed] [Google Scholar]
- 28.Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med 2004;55:433–457. [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol 2006;33:369–385. [DOI] [PubMed] [Google Scholar]
- 30.Saba N, Jain S, Khuri F. Chemoprevention in lung cancer. Curr Probl Cancer 2004;28:287–306. [DOI] [PubMed] [Google Scholar]
- 31.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2001;163:1476–1483. [DOI] [PubMed] [Google Scholar]
- 32.Li JQ, Wen Y, Zhao H, Liu ZL, Song MJ, Xu YJ, Zhang ZX. [The effects of bilirubin concentration on laminin and epidermal growth factor expression in lung tissue and type II pneumocytes in smoking rats model.] Zhonghua Nei Ke Za Zhi 2005;44:129–132. (In Chinese.) [PubMed] [Google Scholar]
- 33.de Boer WI, Hau CM, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol 2006;125:184–192. [DOI] [PubMed] [Google Scholar]
- 34.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 2000;69:145–182. [DOI] [PubMed] [Google Scholar]
- 35.Artinian V, Kvale PA. Cancer and interstitial lung disease. Curr Opin Pulm Med 2004;10:425–434. [DOI] [PubMed] [Google Scholar]
- 36.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl 2001;34:50s–59s. [PubMed] [Google Scholar]
- 37.Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, Gazdar AF, Wistuba II. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res 2005;65:7568–7572. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 2004;64:9027–9034. [DOI] [PubMed] [Google Scholar]
- 39.Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem 2003;278:34834–34844. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi Y, Creek KE, Pirisi L, Kalus R, Young SR. RNA degradation in human breast tissue after surgical removal: a time-course study. Exp Mol Pathol 2004;77:98–103. [DOI] [PubMed] [Google Scholar]
- 41.Kjellman C, Honeth G, Jarnum S, Lindvall M, Darabi A, Nilsson I, Edvardsen K, Salford LG, Widegren B. Identification and characterization of a human smad3 splicing variant lacking part of the linker region. Gene 2004;327:141–152. [DOI] [PubMed] [Google Scholar]
- 42.Ejskjaer K, Sorensen BS, Poulsen SS, Mogensen O, Forman A, Nexo E. Expression of the epidermal growth factor system in human endometrium during the menstrual cycle. Mol Hum Reprod 2005;11:543–551. [DOI] [PubMed] [Google Scholar]
- 43.Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res 2006;66:5338–5345. [DOI] [PubMed] [Google Scholar]
- 44.Asano K, Nakamura H, Lilly CM, Klagsbrun M, Drazen JM. Interferon gamma induces prostaglandin G/H synthase-2 through an autocrine loop via the epidermal growth factor receptor in human bronchial epithelial cells. J Clin Invest 1997;99:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J, Jetten AM. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells: role of mitogen-activated protein kinases. J Biol Chem 1999;274:29138–29148. [DOI] [PubMed] [Google Scholar]
- 46.Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, Carew JF, Altorki NK, Kopelovich L, Subbaramaiah K, et al. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res 2005;65:664–670. [PubMed] [Google Scholar]
- 47.Bennett KL, Plowman GD, Buckley SD, Skonier J, Purchio AF. Regulation of amphiregulin mRNA by TGF-beta in the human lung adenocarcinoma cell line A549. Growth Factors 1992;7:207–213. [DOI] [PubMed] [Google Scholar]
- 48.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol 2004;68:1089–1100. [DOI] [PubMed] [Google Scholar]
- 49.Leivonen SK, Hakkinen L, Liu D, Kahari VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol 2005;124:1162–1169. [DOI] [PubMed] [Google Scholar]
- 50.Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, Nishimura S, Inamura N, Nakajima H, Neya M, et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun 2005;336:357–363. [DOI] [PubMed] [Google Scholar]
- 51.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 2006;355:873–884. [DOI] [PubMed] [Google Scholar]
- 52.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006;355:885–895. [DOI] [PubMed] [Google Scholar]
- 53.Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science 1990;248:1656–1660. [DOI] [PubMed] [Google Scholar]
- 54.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol 2005;23:2078–2093. [DOI] [PubMed] [Google Scholar]
- 55.Saha D, Datta PK, Sheng H, Morrow JD, Wada M, Moses HL, Beauchamp RD. Synergistic induction of cyclooxygenase-2 by transforming growth factor-beta1 and epidermal growth factor inhibits apoptosis in epithelial cells. Neoplasia 1999;1:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem 2005;95:918–931. [DOI] [PubMed] [Google Scholar]
- 57.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 2003;17:1576–1578. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA 1997;94:10669–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois CM. Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor beta 1-induced furin gene transactivation. J Biol Chem 2001;276:33986–33994. [DOI] [PubMed] [Google Scholar]
- 60.Hayes SA, Huang X, Kambhampati S, Platanias LC, Bergan RC. p38 MAP kinase modulates Smad-dependent changes in human prostate cell adhesion. Oncogene 2003;22:4841–4850. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature 1998;394:909–913. [DOI] [PubMed] [Google Scholar]
- 62.Finlay GA, Thannickal VJ, Fanburg BL, Paulson KE. Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem 2000;275:27650–27656. [DOI] [PubMed] [Google Scholar]
- 63.Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol 2004;36:1482–1491. [DOI] [PubMed] [Google Scholar]
- 64.Fontanini G, De Laurentiis M, Vignati S, Chine S, Lucchi M, Silvestri V, Mussi A, De Placido S, Tortora G, Bianco AR, et al. Evaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage I-IIIA non-small-cell lung cancer: amphiregulin and microvessel count are independent prognostic indicators of survival. Clin Cancer Res 1998;4:241–249. [PubMed] [Google Scholar]
- 65.Castillo J, Erroba E, Perugorria MJ, Santamaria M, Lee DC, Prieto J, Avila MA, Berasain C. Amphiregulin contributes to the transformed phenotype of human hepatocellular carcinoma cells. Cancer Res 2006;66:6129–6138. [DOI] [PubMed] [Google Scholar]
- 66.Dalwadi H, Krysan K, Heuze-Vourc'h N, Dohadwala M, Elashoff D, Sharma S, Cacalano N, Lichtenstein A, Dubinett S. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res 2005;11:7674–7682. [DOI] [PubMed] [Google Scholar]
- 67.Eibl G, Bruemmer D, Okada Y, Duffy JP, Law RE, Reber HA, Hines OJ. PGE(2) is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem Biophys Res Commun 2003;306:887–897. [DOI] [PubMed] [Google Scholar]