Abstract
Prostaglandin E2 (PGE2) is a potent lipid mediator that effects changes in cell functions through ligation of four distinct G protein–coupled E prostanoid (EP) receptors (EP1–EP4). PGE2 inhibits bacterial killing and reactive oxygen intermediate (ROI) production by alveolar macrophages (AMs), although little is known about the operative molecular mechanisms. The aims of this study were to evaluate the molecular mechanisms and the specific EP receptors through which PGE2 inhibits killing of Klebsiella pneumoniae by AMs. The treatment of AMs with PGE2 suppressed the killing of K. pneumoniae, and this effect was blocked by an adenylyl cyclase inhibitor and mimicked by agonists for the stimulatory G protein (Gs)-coupled EP2 and EP4 receptors. Conversely, microbicidal activity was augmented by pretreatment with the cyclooxygenase inhibitor, indomethacin, and antagonists of EP2 and EP4. Similar results were found when ROI production was examined. PGE2 inhibition of killing and ROI generation was associated with its activation of the cAMP effectors, protein kinase A and exchange protein directly activated by cAMP-1, as well as attenuation of the phosphorylation and translocation of the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase component, p47phox, to the phagosomal membrane. We conclude that PGE2 suppresses the microbicidal activity of AMs through the Gs-coupled EP2/EP4 receptors, with increased cAMP inhibiting the assembly and activation of p47phox.
Keywords: bacterial killing, lipid mediators, macrophage, phagosome, prostaglandin E2, NADPH
CLINICAL RELEVANCE
This study identifies an important role of prostaglandin E2 acting through E prostanoid (EP) 2 and EP4 receptors to limit alveolar macrophage microbicidal activity, provides new insights into the regulation of innate immune cells, and suggests potential strategies for immunostimulant therapeutics.
Lower respiratory tract infections are the leading cause of death from infection in the United States, underscoring the necessity of increasing our understanding of lung innate immunity. The major resident cell responsible for the defense of the lung against infection is the alveolar macrophage (AM). AMs carry out this function by phagocytosing and killing microbes, and by releasing activating cytokines, chemokines, and lipid mediators (1).
Prostaglandin E2 (PGE2) is a lipid mediator derived from the cyclooxygenase (COX) metabolism of the cell membrane fatty acid, arachidonic acid. This prostanoid is produced in abundance at sites of inflammation, and suppresses many aspects of leukocyte activation (2, 3). The effects of PGE2 follow the ligation of four distinct cell membrane–associated G protein–coupled E prostanoid (EP) receptors (EP1–EP4). EP1 receptor activation provokes Gq (phospholipase C-coupled G protein)-coupled increases in intracellular Ca2+; EP3 receptor most often reduces cAMP via inhibitory G protein (Gi) coupling; and the EP2 and EP4 receptors signal predominantly through stimulatory G protein (Gs), increasing adenylyl cyclase (AC) activity and consequently cAMP formation (2, 4). cAMP is a second messenger that influences numerous cellular functions via the activation of two downstream effector molecules, protein kinase A (PKA) and the exchange proteins directly activated by cAMP (Epac-1 and -2) (5). The effects of PKA result from its ability to phosphorylate Ser and Thr residues on many cellular proteins, including the transcription factor, cAMP response element binding protein (CREB) (6). In phagocytes, the cAMP/PKA/CREB axis mediates the inhibition of TNF-α release and increase of IL-6 production (6). On the other hand, Epac-1, on binding cAMP, catalyzes the exchange of GTP for GDP, subsequently activating the GTPases, Rap1 and Rap2. Rap1 functions as an antagonist of Ras signaling by trapping the Ras effector, Raf1, in an inactive complex (7, 8). Rap1 also regulates several important cellular processes independent of Ras, such as integrin-mediated cell adhesion (8, 9).
The role of Epac-1 in phagocyte function has been investigated and presents conflicting data (10, 11). We recently reported that the suppression of Fcγ receptor (FcR)–mediated phagocytosis by PGE2 in AMs depended on cAMP signaling via EP2 rather than EP4 (12), and involved activation of Epac-1 (13). Interestingly, inhibition of AM bacterial killing by PGE2 involved both Epac-1 and PKA (13).
PGE2 effects on innate immunity are important, as it is produced in the context of infection (14). Moreover, overproduction of this prostanoid has been observed in a number of clinical conditions associated with an increased susceptibility to bacterial infections (15, 16). For example, we recently established a causal role for PGE2 overproduction in the suppression of macrophage and neutrophil innate immune functions after hematopoietic stem cell transplantation in mice (14).
The killing of phagocytosed bacteria by AMs depends upon several distinct microbicidal mechanisms, including the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NADPHox)–mediated release of reactive oxygen intermediates (ROI). We and others have shown that PGE2 and cAMP can inhibit ROI generation (13, 17–19). However, the importance of endogenous PGE2 in regulating ROI generation, the receptors by which it acts, and its influence on NADPHox assembly and activation are all unknown. Here, we demonstrate that PGE2 suppresses bacterial killing in AMs through the limitation of ROI generation. This effect results from the activation by PGE2 of both Gs-coupled EP2 and EP4 receptors, followed by the cAMP-dependent impairment of phosphorylation and phagosomal membrane translocation of the NADPHox component, p47phox.
MATERIALS AND METHODS
Animals
Mice with a targeted disruption of the EP2 gene were obtained from Ono Pharmaceutical (Osaka, Japan) and bred in the University of Michigan Unit for Laboratory Animal Medicine. Strain-matched C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Female Wistar rats were obtained from Charles River Laboratories (Wilmington, MA). Animals were treated according to National Institutes of Health guidelines for the use of experimental animals, with the approval of the University of Michigan Committee for the Use and Care of Animals.
Reagents
Dulbecco's modified Eagle's medium without phenol red, RPMI 1640, and penicillin/streptomycin/amphotericin B solution were purchased from Life Technologies–Invitrogen (Carlsbad, CA). Tryptic soy broth was supplied by Difco (Detroit, MI). Indomethacin, o-phenylenediamine dihydrochloride, and SDS were from Sigma-Aldrich (St. Louis, MO). AH-6809, butaprost free acid, and PGE2 were from Cayman Chemicals (Ann Arbor, MI). Ono-AE1-329 and Ono-AE3-208 were generous gifts from Ono Pharmaceutical (Osaka, Japan), and SQ 22536 was purchased from Biomol (Palo Alto, CA). Compounds requiring reconstitution were dissolved in either ethanol or DMSO. Required dilutions of all compounds were prepared immediately before use, and equivalent quantities of vehicle were added to the appropriate controls. In this study, we used Klebsiella pneumoniae 43816, serotype 2 (American Type Culture Collection [ATCC], Rockville, MD).
Cell Isolation and Culture
Resident AMs from mice and rats were obtained by ex vivo lung lavage, as previously described (20, 21), and were resuspended in RPMI to a final concentration of 2 × 106 cells/ml. Cells were allowed to adhere to tissue culture–treated plates for 1 hour (37°C, 5% CO2), followed by one wash with warm RPMI. The resulting population of adherent cells was more than 99% AMs, as determined by a modified Wright-Giemsa stain. Cells were cultured overnight in RPMI containing 10% FBS and were washed twice the next day with warm medium before experimental incubations. Rat AM-derived NR8383 cells (ATCC) were cultured in RPMI containing 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were collected and resuspended in fresh medium at a concentration of approximately 1 × 106 cells/ml.
Tetrazolium Dye Reduction Assay of Bacterial Killing
The ability of bacteria to survive within the AM was quantified using a tetrazolium dye reduction assay, as described elsewhere (21, 22), and results are expressed as the percent survival of ingested bacteria. Because PGE2 modulates both phagocytosis and bacterial killing, we developed a method that separates the effects of PGE2 on phagocytosis from bacterial killing. During K. pneumoniae infection, PGE2 is produced during the first minutes after infection. Thus, in order to prevent PGE2 production and its effects on phagocytosis, we employed COX1/2 inhibitors and EP2/EP4 antagonists 20 minutes before the addition of the K. pneumoniae. In addition, to verify the effects of PGE2 and its receptors only on bacterial killing, we employed the EP2 and EP4 agonist as well as PGE2 after the phagocytosis time (30 minutes).
H2O2 Detection
AMs were plated in 96-well dishes at 2 × 105 cells/well. H2O2 secretion from AMs was determined colorimetrically using the Amplex Red reagent (Molecular Probes, Eugene, OR) according to the manufacturer's instructions (21). The detection limit of this method was 0.625 nM. To assess the effects of PGE2 signaling on H2O2 production, AMs were pretreated with compounds of interest for 10 minutes before infection with opsonized K. pneumoniae (multiple of infection, 50:1).
Semiquantitative Real-Time RT-PCR
Semi-quantitative RT-PCR was performed on an ABI Prism 7000 thermocycler (Applied Biosystems, Foster City, CA). Gene-specific primers were designed using Primer Express software (Applied Biosystems). The sequences for all primers used can be found in Table 1. Briefly, the reaction mixture contained 300 ng of cDNA, 12.5 μl of SYBR Green PCR Master Mix (Applied Biosystems), and forward and reverse primers at 300 nM in a final volume of 25 μl. For each experiment, samples (n = 2) were run in triplicate. The average cycle threshold (CT) was determined for each rat from a given experiment. Relative gene expression (using the formula 2−ΔΔCT) was calculated using the comparative CT method, which assesses the difference in gene expression between the gene of interest and an internal standard gene (β-actin) for each sample to generate the ΔΔCT. The average of the control sample was set to 1 for each experiment, and the relative gene expression for each experimental sample was compared with that.
TABLE 1.
PRIMERS AND PROBES FOR SEMI-QUANTITATIVE REAL-TIME PCR
| Sequences | ||
|---|---|---|
| β-actin | Forward | CCTAAGGCCAACCGTGAAAA |
| Reverse | AGGGACAACACAGCCTGGAT | |
| EP2 | Forward | CCTGGCCATTATGACCATCAC |
| Reverse | TCGGGAAGAGGTTTCATCCA | |
| EP4 | Forward | ACGCGGGCTTCAGTTCCT |
| Reverse | CGCACACCAGCACATTGC | |
Rap1 Activation Assay
BL21 Escherichia coli (Amersham Biosciences, Piscataway, NJ) transformed with the pGEX 2T plasmid containing the gene, GST-RalGDS (constructed by Dr. Johannes Bos and kindly provided by Dr. Daniel Altschuler, University of Pittsburgh, Pittsburgh, PA) were inoculated in Luria Broth (LB)-ampillicin and cultured for 6 hours. The culture was diluted into fresh LB-ampicillin and grown until the optical density at 600 nm was between 0.6 and 1.0, and 0.1 mM isopropyl-β-D-thiogalactoside was added for 5.5 hours to induce protein expression. Bacteria were pelleted at 4,000 × g for 10 minutes, resuspended in lysis buffer (PBS, 10 μg/ml aprotinin/leupeptin, protease inhibitor cocktail), and sonicated. Proteins were solubilized with 1% Triton X-100 for 30 minutes at 4°C and centrifuged at 10,000 × g for 10 minutes. Resulting supernatants were incubated with glutathione agarose beads (Invitrogen) for 1 hour at 4°C. Beads were washed three times and diluted 1:1 in PBS, and a 50% slurry was added 1:1 to glycerol and stored at −80°C.
Active levels of Rap1 in NR8383 rat AMs were measured by a modified version of a protocol previously described (23). Briefly, cells were lysed on ice in Rap1 lysis buffer (25 mM Tris-HCl, pH 7.5, 1% NP-40, 5 mM MgCl2, 150 mM NaCl, 0.1 mM DTT, 5% glycerol, protease inhibitor cocktail, 10 ug/ml aprotinin and leupeptin, 1 mM phenylmethylsulfonyl fluoride) for 15 minutes. Lysates were clarified by centrifugation at 16,000 × g for 10 minutes and incubated with glutathione agarose beads coupled to GST-RalGDS for 1 hour at 4°C. Positive control lysates were treated with GTPγS while negative control lysates were treated with GDP for 30 minutes before incubation with glutathione agarose beads coupled to GST-RalGDS. Beads were washed three times in lysis buffer and resuspended in 1× sample buffer. Samples were submitted to SDS-PAGE and the membranes were probed with polyclonal rabbit anti-Rap1 antibody (1:500; Upstate Biotechnology, Lake Placid, NY) and bands detected as described below. Total Rap1 levels were determined by removing aliquots from cell lysates before incubation with beads and blotting for Rap1.
PKA Activity
The PKA activity experiments were done in NR8383 rat AMs. Cells (2 × 106) were pretreated for different times with PGE2, EP2 agonist, and EP4 agonist, and the cells were lysed in lysis buffer as described previously here. Protein extracts were submitted to SDS-PAGE and the membranes were probed with polyclonal rabbit anti–phosho-CREB (1:500; Upstate Biotechnology) and bands detected as described subsequently here. To assure equal amounts of protein, the membranes were probed for α-tubulin (1:1,000; Sigma).
Cell Fractionation, Immunoprecipitation, and Western Blotting
AMs (4 × 106) were plated in 6-well tissue culture dishes and pretreated for 20 minutes with EP2 or EP4 antagonists, or for 5 minutes with PGE2. After this, AMs were lysed by sonication in ice-cold lysis buffer containing 150 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, and 1 μg/ml leupeptin, followed by ultracentrifugation at 100,000 × g for 20 minutes at 4°C. The cytosolic (soluble) fraction was harvested, and the membrane (insoluble) fraction was washed and subjected to another ultracentrifugation step as described previously here. The resultant pellet was resuspended in lysis buffer and sonicated. Protein concentrations were determined by a modified Coomassie dye–binding assay (Pierce Chemical, Rockford, IL). The cytosolic fraction was used for immunoprecipitation, as described previously (21), with some modifications. It was incubated overnight at 4°C with anti-p47phox antibody (1:80; Upstate Biotechnology). Protein A-Sepharose was added to each sample and was incubated for 3 hours with rotation at 4°C. Beads were washed briefly three times with lysis buffer without Triton X-100, and samples containing protein were separated on 10% SDS-PAGE gels, then transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, membranes were probed with anti-p47phox (1:500 dilution; Upstate Biotechnology); anti-gp91phox (1:500; BD Bioscience, San Jose, CA) or anti-phosphoserine (1:1,000; BD Bioscience) antibodies for 90 minutes, followed by peroxidase-conjugated anti-rabbit (Amersham Biosciences) or anti-mouse secondary antibody (1:5,000; Zymed, South San Francisco, CA). Bands were detected by enhanced chemiluminescence detection (Amersham Biosciences). Relative band densities were determined by densitometric analysis using National Institutes of Health Image Software, and densities in experimental conditions expressed as the percentage of untreated control densities. The results were expressed as normalized phospho-p47phox/total-p47phox, which represent the value of band density obtained from the antiphosphoserine blot divided by the band density from the anti-p47phox blot. In all instances, density values of bands were corrected by subtraction of the background values.
Phagosome Isolation
Macrophages (3 × 106/well) plated in a 6-well dish were washed twice with PBS and incubated for 10 minutes with 1 μM PGE2 before the addition of 10:1 IgG-conjugated, 3 μm, paramagnetic beads (Dynal-Invitrogen). Cells were held on ice for 10 minutes to synchronize phagocytosis. Cells were then incubated at 37°C and IgG-bead phagosomes were isolated according to a published method at 5-, 15-, and 30-minute stages for isolation of early, intermediate, and late phagosomal proteins (24). To isolate IgG bead–containing phagosomes, macrophages were rinsed twice in PBS and scraped into 0.5 ml/dish ice-cold homogenization buffer (250 mM sucrose, 10 mM HEPES, 1 mM EDTA, pH 7.2; protease inhibitors and 1% Triton X-100). AMs were lysed during a 30-minute incubation on ice, and the bead phagosomes were isolated from cellular debris using a magnet (Qiagen, Valencia, CA) and washed twice in 0.5 ml of homogenization buffer without Triton X-100. Phagosomal proteins were removed from the beads by sonication (2 minutes with cooling on ice), followed by boiling for 3 minutes. The beads were collected with the magnet, and the solubilized material from phagosomes was used as a source of phagosomal proteins for subsequent electrophoresis and immunoblot analysis. We confirmed the presence of phagosomal proteins by quantifying the late phagosome protein, flotillin-1 (25, 26). To exclude possible plasma membrane contamination, we evaluated the presence of the integral plasma membrane protein, CD45 (data not shown).
Statistical Analysis
Data are represented as mean (± SE) and were analyzed with the Prism 3.0 statistical program (GraphPad Software, San Diego, CA). Comparisons between two experimental groups were performed with Student's t test. Comparisons among three experimental groups were performed with analysis of variance followed by Bonferroni analysis. Differences were considered significant if P values were 0.05 or less. All experiments were performed on three separate occasions, unless otherwise specified.
RESULTS
Exogenous and Endogenous PGE2 Suppresses AM Bacterial Killing
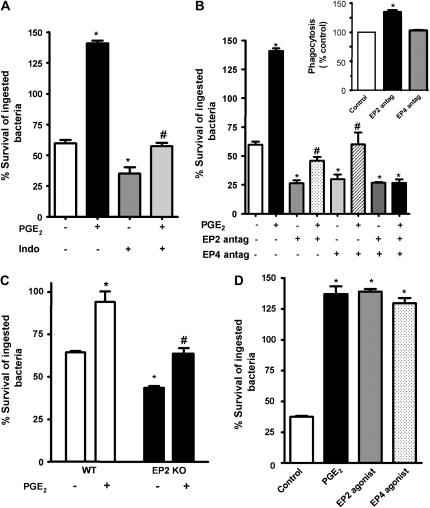
PGE2 is a major COX-derived eicosanoid produced at sites of infection. As reported previously, exogenous PGE2 inhibited bacterial killing by AMs (13, 14). To assess the influence of endogenous prostanoids on killing of K. pneumonia, we employed the dual COX-1/COX-2 inhibitor, indomethacin. Inhibition of COX activity by indomethacin increased AM microbicidal activity by approximately 50% (Figure 1A). This same effect was also observed in rat peritoneal macrophages and the rat AM cell line, NR3838 (data not shown). That PGE2 is the major COX arachidonic acid metabolite responsible for the effect of indomethacin treatment was suggested by the fact that exogenous PGE2 restored AM microbicidal activity in AMs treated with indomethacin treatment (Figure 1A). Preliminary dose–response experiments were conducted for PGE2 (10–1,000 nM). In all cases, data presented were used at a concentration of 1 μM, which showed the greatest inhibitory effect on AM microbicidal activity (data not shown)
Figure 1.
Role of endogenous prostanoids and specific E prostanoid (EP) receptors in alveolar macrophage (AM) killing of opsonized Klebsiella pneumoniae. (A) Rat AMs were pretreated for 30 minutes with the cyclooxygenase (COX) inhibitor, indomethacin (10 μM) or vehicle. At 30 minutes after infection with opsonized K. pneumoniae (50:1), the inhibitor was added back, with or without prostaglandin E2 (PGE2; 1 μM). (B) Rat AMs were treated with the EP2 receptor antagonist, AH-6809 (100 μM), the EP4 antagonist, Ono-AE3-208 (1 μM), or vehicle for 20 minutes before the addition of opsonized K. pneumoniae. At 30 minutes after infection, the antagonists were added back, with or without 1 μM PGE2. (C) Rat AMs were infected with opsonized K. pneumoniae. At 30 minutes after infection, the cells were incubated with the EP2 agonist, butaprost free acid (1 μM), the EP4 agonist, Ono-AE1-329 (1 μM), PGE2 (1 μM), or vehicle. (D) AMs from wild-type or EP2 knockout (KO) mice were infected, and 1 μM PGE2 was added 30 minutes after infection. Microbicidal activity was assessed as described in Materials and Methods. Data are expressed as the mean (± SE) percentage survival of ingested bacteria from three independent experiments (A–C) or a representative experiment of two performed in triplicate (D). *P < 0.05 compared with control; #P < 0.05 versus indomethacin, EP2 antagonist, EP4 antagonist, or EP2 KO group by analysis of variance (ANOVA).
Both EP2 and EP4 Mediate PGE2-Induced Suppression of Bacterial Killing
We have previously found that the EP2 receptor mediates PGE2 inhibitory effects on FcR-mediated phagocytosis in AMs (12). To verify that the endogenous prostanoid responsible for suppression of bacterial killing was indeed PGE2, and, to determine the receptors through which endogenous PGE2 acts to suppress bacterial killing, we employed the EP2 antagonist, AH-6809, and the EP4 antagonist, Ono-AE3-208, in concentrations previously determined to be specific to such receptors (12, 27–32). The pretreatment of AMs with either the EP2 antagonist or the EP4 antagonist alone increased intracellular killing, as evidenced by a decrease in the survival of ingested bacteria of approximately 53 and 46%, respectively (Figure 1B). The simultaneous use of both antagonists failed to exert additive effects on bactericidal activity. We also examined the ability of those antagonists to block the effects of exogenous PGE2. Suppression of bacterial killing by PGE2 was partially abrogated when AMs were pretreated with either EP2 or EP4 antagonists, and completely abrogated when both were used in combination. Of note, assessing bacterial ingestion in these same experimental plates confirmed our previous finding that ligation of EP2, but not EP4, suppresses phagocytosis, because the addition of EP2, but not EP4, antagonist increased K. pneumoniae phagocytosis by about 35% (Figure 1B, inset). Genetic deletion of EP2 resulted in increased bacterial killing when compared to wild-type murine AMs. Moreover, inhibition of bacterial killing by PGE2 was only partially abrogated in EP2-deficient AMs, which suggested a role for EP4 in this phenomenon (Figure 1C).
To confirm the importance of EP2 and EP4 in AM bacterial killing, we also employed specific agonists for these receptors in doses previously described (12, 30, 33). As observed in Figure 1D, the specific EP2 (butaprost free acid) and EP4 (Ono-AE1-329) agonists suppressed bacterial killing to a similar degree. Each agonist suppressed bactericidal activity as much as PGE2 (approximately 140% survival, meaning that bacteria proliferated within the macrophage after phagocytosis was allowed to occur). However, we did not observe any additional effects when both EP2 and EP4 agonists were used together (data not shown).
EP2 and EP4 Expression in Rat AMs
Previously, we reported that rat AMs express predominantly EP2 and EP3, but not EP4 and EP1, proteins by Western blot analysis (12); however, the sensitivity of EP antibodies is open to question. As our present results suggested that EP4 ligation modulated AM bacterial killing, we further explored EP receptor expression by semiquantitative RT-PCR, and the expression level of the gene of interest was normalized to β-actin. We observed similar levels of EP2 and EP4 mRNA expression in resting cells (1.5 ± 0.009 and 1.5 ± 0.01, respectively; data not shown). In this instance, then, mRNA expression of EP4 correlated better with pharmacologic data than did protein expression by Western analysis. The same profile for both mRNA (by real-time PCR) and protein expression (by Western immunoblot) was observed in the rat AM cell line, NR8383 (data not shown).
cAMP Production Mediates Suppression of AM Bacterial Killing by PGE2
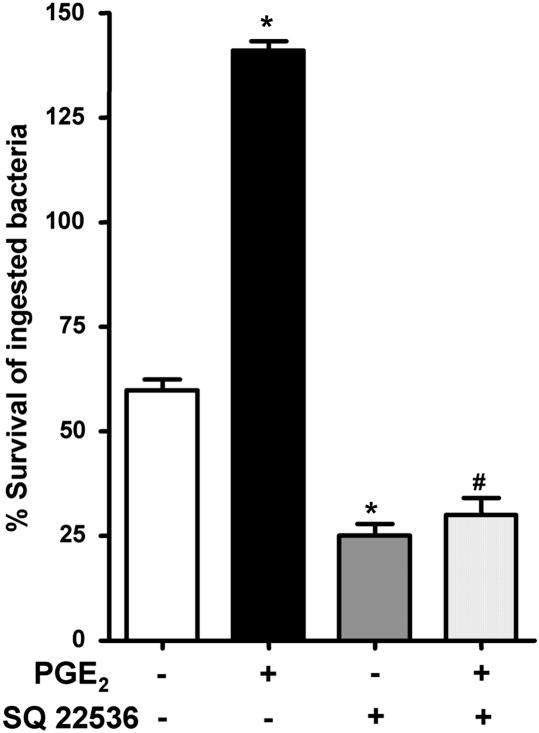
The degree of inhibition of FcR-mediated phagocytosis by PGE2 was previously found to correlate with the increase in cAMP levels (12). In addition, stable, phosphodiesterase-resistant analogs of cAMP were found to block the ability of AMs to kill phagocytosed K. pneumoniae (13). However, the ability of endogenously produced cAMP to suppress K. pneumoniae killing has not previously been examined. We found that AC inhibition using SQ 22536 increased baseline AM bacterial killing and abolished the ability of PGE2 to impair killing (Figure 2). Thus, our data suggest that, during K. pneumoniae infection, endogenously produced cAMP functions as a brake on AM bactericidal activity.
Figure 2.
Suppression of AM bacterial killing by PGE2 is dependent on adenylyl cyclase (AC) activity. Rat AMs were pretreated with vehicle or the AC inhibitor, SQ 22536 (10 μM) 30 minutes before addition of 50:1 opsonized K. pneumoniae. At 30 minutes after infection, the inhibitor was added back, with or without 1 μM PGE2. Microbicidal activity was assessed as described in Materials and Methods. Data are expressed as the mean (± SE) percentage survival of ingested bacteria from three independent experiments, each performed in triplicate. *P < 0.05 compared with control; #P < 0.05 versus SQ 22536 by ANOVA.
EP2 and EP4 Activation of Downstream cAMP Effectors in AMs
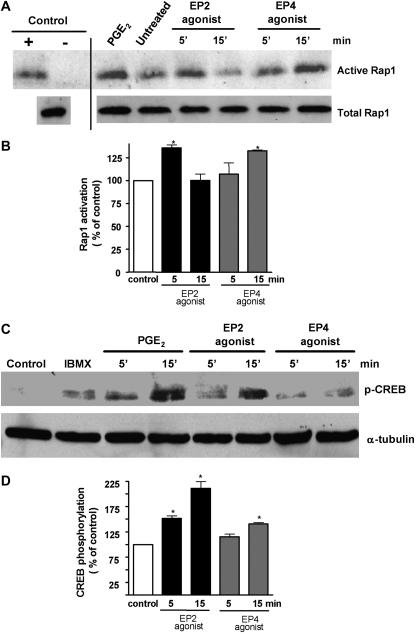
Previously, we demonstrated that PGE2 decreased FcR-mediated phagocytosis exclusively by activating the EP2 receptor, whereas the data shown in Figures 1B and 1D indicate that ligation of either EP2 or EP4 receptors can suppress bacterial killing. As we have also previously demonstrated that exogenous PGE2 suppressed AM bacterial killing via both PKA- and Epac-1–dependent pathways, we asked whether EP2 and EP4 might differentially activate these two cAMP effectors. Using NR8383 cells, we assessed PKA activation by determining phosphorylation of CREB by immunoblot analysis, and we assessed Epac-1 activation with the GTP-Rap1 pulldown assay to measure GTP-bound Rap1. PGE2 induced Rap1 activation, consistent with its activation of upstream Epac-1. Both a selective EP2 agonist (butaprost free acid) and an EP4 agonist (Ono-AE1-329) also activated Rap1, but the effect of the former peaked at 5 minutes and that of the latter at 15 minutes of treatment (Figures 3A and 3B). PGE2 induced CREB phosphorylation as early as 5 minutes, and this effect was more pronounced at 15 minutes. The same kinetics of CREB phosphorylation were observed with the EP2 agonist. The ability of the EP4 agonist to induce CREB phosphorylation at either time point was less pronounced than that of either the EP2 agonist or of PGE2 itself (Figures 3C and 3D). These data suggest differences in the extent and rapidity of PKA and Epac-1 activation after ligation of EP2 and EP4.
Figure 3.
Importance of EP2 and EP4 receptors in Epac-1 and PKA activation. (A) Approximately 3 × 106 NR8383 rat AMs were plated in 6-well dishes and serum starved overnight. Cells were stimulated with the EP2 agonist, butaprost free acid (1 μM), and the EP4 agonist, Ono-AE1-329 (1 μM), for 5 and 15 minutes. Positive control cells were treated with PGE2 (1 μM) for 15 minutes. After treatment, the macrophages were lysed on ice and assayed for the active GTPase Rap1 as described in Materials and Methods. Before the pull-down assay, positive control lysates were incubated with GTPγS, whereas negative control lysates were incubated with GDP for 30 minutes. Total levels of Rap1 in cell lysates are shown (bottom blot). (B) Relative phosphorylation was determined by densitometric analysis of immunoblots from three different experiments and expressed as percent of control. *P < 0.05 versus control. (C) Approximately 3 × 106 NR8383 cells were plated in 6-well dishes and serum starved overnight. Cells were stimulated with the EP2 agonist, butaprost free acid (1 μM), and the EP4 agonist, Ono-AE1-329 (1 μM), for 5 and 15 minutes, the proteins were subjected to SDS-PAGE as described in Materials and Methods, and the membranes were probed for phosphorylated cAMP-responsive element binding protein (CREB) (1:500). Subsequently, the membrane was stripped and probed for β-tubulin (1:100). Results are representative of three experiments. (D) Relative phosphorylation was determined by densitometric analysis of immunoblots from two different experiments and expressed as a percentage of control. *P < 0.05 versus control.
EP2 and EP4 Receptor Activation Suppresses NADPH Oxidase Activation during K. pneumoniae Infection
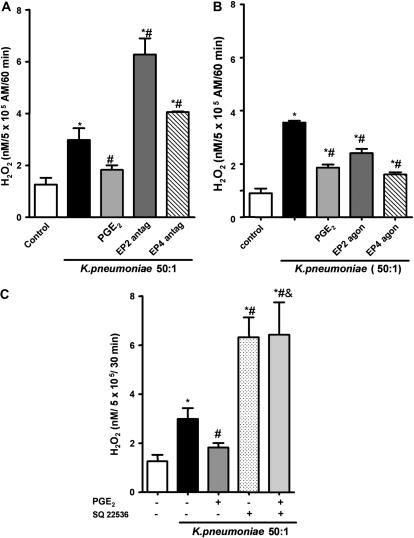
ROI generation by NADPHox represents an important bactericidal mechanism after FcR-mediated phagocytosis, and ROIs are involved in the control of K. pneumoniae infection (21). Previously, we demonstrated that exogenous PGE2 inhibited AM H2O2 production during K. pneumoniae challenge (13). However, we did not evaluate the importance of endogenous PGE2 or the role of specific EP receptors in regulating H2O2 release. The impact of endogenously produced PGE2 on cellular H2O2 production in response to K pneumoniae infection was explored using the EP2 and EP4 antagonists. As shown in Figure 4A, K pneumoniae infection induced H2O2 production by rat AMs, and EP2 antagonism increased this by approximately 100%, whereas the EP4 antagonist increased H2O2 generation by approximately 50% when compared with untreated and infected AMs. In addition, both EP2 and EP4 stimulation suppressed H2O2 during K. pneumoniae infection to the same degree as PGE2 itself (Figure 4B). We did not observe any effect of EP2 and EP4 antagonists or agonists on H2O2 production in uninfected AMs (data not shown). Endogenous cAMP formation downregulated NADPHox activation, as shown by the increase in H2O2 release in AMs treated with the AC inhibitor, SQ 22536. Furthermore, the inhibition of AC abrogated the effect of PGE2 on H2O2 release (Figure 4C). Taken together, our data suggest that endogenous PGE2 inhibits NADPHox-mediated release of H2O2 during K. pneumoniae infection via ligation of both Gs-coupled EP2 and EP4 receptors and subsequent cAMP formation.
Figure 4.
EP2 and EP4 receptor activation by PGE2 suppresses H2O2 production in AMs through a cAMP-dependent process. Rat AMs (5 × 105) were plated and preincubated with: (A) EP2 receptor antagonist, AH-6809 (100 μM), or the EP4 receptor antagonist, Ono-AE3-208 (1 μM), for 20 minutes; (B) EP2 receptor agonist, butaprost free acid (1 μM), or the EP4 receptor agonist, Ono-AE1-329, for 5 minutes; or (C) AC inhibitor, SQ 22536 (10 μM), for 20 minutes, with or without PGE2, followed by infection with 50:1 opsonized K. pneumoniae for 1 hour. The H2O2 concentration in medium was determined as described in Materials and Methods. Results represent the mean (± SE) of quadruplicate values from one of three representative experiments. *P < 0.05 versus control; #P < 0.05 versus K. pneumoniae group by ANOVA.
EP2 and EP4 Receptor Signaling Inhibits p47phox Phosphorylation during K. pneumoniae Infection
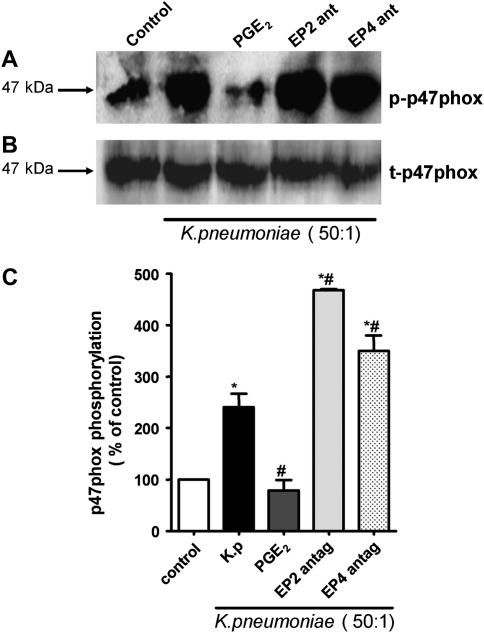
To characterize the mechanisms by which PGE2 inhibits H2O2 generation, we evaluated the phosphorylation of the cytosolic p47phox subunit of NADPHox—a requisite step in activation of this complex. AMs were pretreated with PGE2 and/or the EP2 and EP4 antagonists followed by infection with K. pneumoniae. Phosphorylation of p47phox was determined by its immunoprecipitation followed by immunoblotting for phosphoserine. Infection of AMs with opsonized K. pneumoniae increased serine phosphorylation of p47phox when compared with untreated cells (Figures 5A and 5B). EP2 and EP4 antagonists both increased the degree of p47phox phosphorylation in K. pneumoniae–infected AMs. Consistent with the magnitude of actions of EP2 and EP4 receptor activation on H2O2 production (Figure 4A), the antagonism of EP2 induced higher levels of p47phox phosphorylation than did EP4 antagonism (Figures 5A and 5B). The total amount of cytosolic p47phox did not change in any condition tested (Figure 5A).
Figure 5.
Effect of PGE2 and EP receptors on p47phox phosphorylation during K. pneumoniae infection. (A) Rat AMs (4 × 106/well) were pretreated for 20 minutes with the EP2 antagonist, AH-6809 (100 μM), the EP4 antagonist, Ono-AE3-208 (1 μM), or vehicle, or exposed for 5 minutes to PGE2 (1 μM) before the addition of 50:1 opsonized K. pneumoniae for an additional 5 minutes. Cells were then harvested and fractionated as described in Materials and Methods. The cytosolic fraction was subjected to immunoprecipitation for p47phox, followed by immunoblot analysis using an anti-phosphoserine antibody (1:1,000). Subsequently, the membrane was stripped and probed for total p47phox (1:500). Results are representative of three experiments. (B) Relative phosphorylation was determined by densitometric analysis of immunoblots from three different experiments and expressed as percent of control. *P < 0.05 versus control; #P < 0.05 versus K. pneumoniae group by ANOVA.
Exogenous PGE2 Blocks p47phox Translocation to Plasma and Phagosomal Membranes
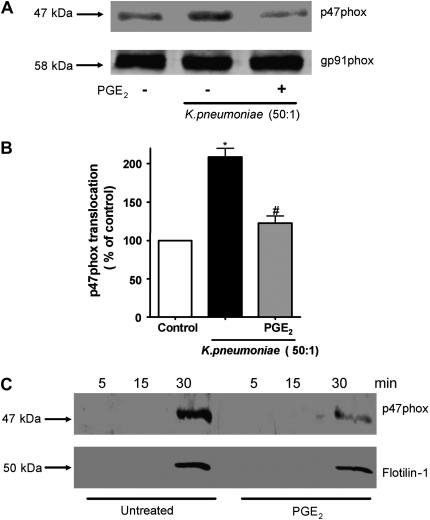
The phosphorylation of p47phox accompanies its membrane translocation. cAMP has been reported to inhibit p47phox phosphorylation and translocation in neutrophils. Thus, we investigated whether PGE2 modulated p47phox membrane translocation in AMs. In K. pneumoniae–infected AMs, pretreatment with 1 μM PGE2 for 5 minutes decreased p47phox translocation to the total membrane fraction (Figures 6A and 6B). The membrane-associated NADPHox component, gp91phox, was used as a loading control (Figure 6A).
Figure 6.
Effect of PGE2 on p47phox membrane translocation during K. pneumoniae infection. (A) Rat AMs (4 × 106/well) were pretreated with PGE2 for 5 minutes before addition of 50:1 opsonized K. pneumoniae for another 5 minutes, then harvested and fractionated as described in Materials and Methods. Immunoblotting of membrane fractions was performed using anti-p47phox (1:500). To ensure equal protein loading, blots were stripped and reprobed for gp91phox. Results are representative of those from three experiments. (B) Protein amounts were determined by densitometric analysis of immunoblots from three different experiments, and the results are expressed as percent of control. *P < 0.05 versus control; #P < 0.05 versus K. pneumoniae group by ANOVA. (C) Rat AMs (4 × 106/well) were pretreated with PGE2 for 5 minutes before addition of 10:1 IgG-opsonized beads at different time points and phagosomal membranes were isolated as described in Materials and Methods. As a late phagosome marker, the membranes were probed for flotillin-1 (1:1,000) or p47phox (1:500). Results are representative of those from two experiments.
The phagosome is a specialized membrane that envelops ingested microbes and in which ROIs are generated in order to kill them. We next sought to investigate the effects of PGE2 on p47phox translocation specifically to the phagosomal membrane. We used a well established model of phagosome isolation using IgG-opsonized magnetic beads, harvesting the beads at different time points after IgG-bead challenge, in order to harvest early (5 minutes), intermediate (15 minutes), and late (30 minutes) phagosomes (34). As a marker for the phagosome fraction, we used flotillin-1 (24, 25); we detected no plasma membrane protein CD45 in this fraction, verifying its purity. Interestingly, p47phox was observed only in the late phagosome preparation. PGE2 pretreatment clearly impaired p47phox translocation to the phagosome membrane (Figure 6C).
DISCUSSION
This study demonstrates the importance of endogenous PGE2 in downregulating phagocyte bactericidal capacity and newly characterizes the molecular mechanisms involved. Our results help to explain previous reports that overproduction of PGE2 impairs (14), whereas inhibitors of PGE2 synthesis augment (13), innate immune responses and bacterial clearance in various animal models of infection. Specifically, we showed that: (1) this effect of PGE2 on bacterial killing involves a reduction in bactericidal ROI generation; (2) such inhibition is mediated by its ability to block the phosphorylation and translocation of p47phox to FcR-derived phagosomes; and (3) PGE2 effects are mediated by cAMP generated after ligation of both EP2 and EP4 receptors, with subsequent activation of downstream cAMP effectors, PKA and Epac-1.
Because both EP2 and EP4 receptors are coupled to Gs proteins, we anticipated that cAMP would be an important proximal signaling molecule in the suppression of intracellular killing by PGE2. Indeed, it has previously been shown that increases in intracellular cAMP suppress microbicidal activity in leukocytes, such as neutrophils and peritoneal macrophages infected with Legionella pneumophilia and Pseudomonas aeruginosa (14, 35–37), respectively. Interestingly, we know that, during K. pneumoniae infection, PGE2 is generated by AMs (12), and this likely results in enhanced cAMP production, which might provide a mechanism to explain the effects of AC inhibition. However, they also demonstrate for the first time that basal cAMP levels act as a brake in the microbicidal pathway.
Our previous work had indicated that PGE2 inhibited AM phagocytosis through EP2, but not EP4, and this correlated with the greater ability of the former receptor to increase intracellular levels of cAMP (13), Interestingly, Sadikot and colleagues (37) demonstrated that COX-2– or EP2–deficient mice exhibited increased survival after lung infection with P. aeruginosa and endogenous or exogenous PGE2 modulated superoxide anion release during P. aeruginosa infection in bone marrow–derived macrophages. It was therefore somewhat surprising that our current results clearly demonstrate that EP4 can impair bacterial killing in these cells. As there is little known about activation of cAMP effectors in macrophages in general, and AMs in particular, we sought to examine activation of both PKA and Epac-1. We found that EP4 ligation was indeed able to robustly stimulate activation of the downstream Epac-1 target, Rap1. As Epac-1 activation has been shown to inhibit ROI generation and bacterial killing in AMs (13), this intracellular signaling event elicited by EP4 ligation would seem to explain its ability to impair killing in the present study. However, Epac-1 activation has also been shown to inhibit AM phagocytosis, raising the question of why EP4 ligation was unable to impact ingestion in our previous study. We speculate that its inability to do so may reflect the fact that the EP4 effects on Epac-1 occurred too slowly to impact this initial macrophage response to the microbe. The delayed activation of Epac-1 by EP4 ligation, however, is able to attenuate the subsequent killing of those bacteria that had been previously ingested. Because killing, but not phagocytosis, has also been shown (13) to be downregulated by activation of PKA, the greater capacity of EP2 antagonism than that of EP4 antagonism to enhance ROI generation and p47phox phosphorylation may be related to the more robust activation of PKA by EP2 ligation. Thus, EP2 mediates more potent and diverse immunosuppressive actions of PGE2 in AMs than does EP4, because it alone activates both of these cAMP effector systems. Further insights into the kinetics and compartmentalization of activation of PKA versus Epac-1 and how they modulate AM effector functions must await additional investigation.
The intracellular killing of K. pneumoniae is likely mediated by several microbicidal molecules, including antimicrobial peptides, reactive nitrogen intermediates, and ROIs (21, 38–41). However the individual contributions of such molecules are unknown. We previously reported that the inhibition of either nitric oxide synthase or NADPHox decreased K. pneumoniae killing by AMs (21). In addition, in vivo treatments of mice with the nitric oxide synthase inhibitor, L-NAME, impaired host defense against K. pneumoniae (41). Interestingly, Hickman-Davis and colleagues (40) demonstrated (in a cell-free system) that, although neither ROIs nor nitric oxide alone were toxic to K. pneumoniae, potent bacterial killing could be observed when a combination of nitric oxide and superoxide was present (at a pH of 5.0), suggesting that optimal bactericidal activity can be achieved when peroxynitrite is formed in an acidic milieu. Our data support this result, as we find requirements for both nitric oxide and NADPHox activation in macrophage killing of K. pneumoniae. Although we have now documented regulatory effects of PGE2 on the latter system, our study leaves open the possibility that PGE2 might also impair nitric oxide (and peroxynitrite) generation.
Within the cell after particle engulfment, cytosolic NADPHox components, such as p47phox, translocate to the forming phagosome. To assess phagosome maturation, we challenged AM with IgG beads and isolated phagosomes at early, intermediate, and late time points. Our data show for the first time that PGE2 inhibits p47phox translocation to the mature phagosome. Interestingly, we observed less flotillin-1 in the late phagosomes of PGE2-treated cells. Whether this merely reflects the consequence of a reduction in overall phagocytosis by PGE2 or if PGE2 directly interferes with phagosome formation and/or maturation is a question currently being explored. In addition, the effects of flotillin-1 (which is also enriched in lipid rafts [42, 43]) in phagocyte effector functions await further study.
In summary, this study identifies an important effect of endogenously generated PGE2 acting through the Gs-coupled receptors, EP2 and EP4, and downstream cAMP effectors to limit AM microbicidal activity against a relevant bacterial pathogen. This effect involves inhibition of NADPHox assembly and activation on the phagosome. These data provide new insights into the regulation of host innate immune cell function by endogenous mediators, and suggest potential strategies for immunostimulant therapeutics.
Acknowledgments
The authors acknowledge Teresa Marshall for technical contributions, Thomas G. Brock, Nicolas Flamand, and Steven Huang for helpful discussions, and the excellent secretarial support of Patricia Urban.
This work was supported by National Institutes of Health grants HL078727 and HL058897 (M.P.-G.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0153OC on June 21, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 2001;70:163–170. [PubMed] [Google Scholar]
- 2.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 2001;41:661–690. [DOI] [PubMed] [Google Scholar]
- 3.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 2001;108:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashida M, Bito T, Budiyanto A, Ichihashi M, Ueda M. Involvement of EGF receptor activation in the induction of cyclooxygenase-2 in HaCaT keratinocytes after UVB. Exp Dermatol 2003;12:445–452. [DOI] [PubMed] [Google Scholar]
- 5.Dremier S, Kopperud R, Doskeland SO, Dumont JE, Maenhaut C. Search for new cyclic AMP–binding proteins. FEBS Lett 2003;546:103–107. [DOI] [PubMed] [Google Scholar]
- 6.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway: differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 2000;5:D678–D693. [DOI] [PubMed] [Google Scholar]
- 7.Henning SW, Cantrell DA. GTPases in antigen receptor signalling. Curr Opin Immunol 1998;10:322–329. [DOI] [PubMed] [Google Scholar]
- 8.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol 2001;2:369–377. [DOI] [PubMed] [Google Scholar]
- 9.Self AJ, Caron E, Paterson HF, Hall A. Analysis of R-Ras signalling pathways. J Cell Sci 2001;114:1357–1366. [DOI] [PubMed] [Google Scholar]
- 10.Makranz C, Cohen G, Reichert F, Kodama T, Rotshenker S. cAMP cascade (PKA, Epac, adenylyl cyclase, Gi, and phosphodiesterases) regulates myelin phagocytosis mediated by complement receptor-3 and scavenger receptor-AI/II in microglia and macrophages. Glia 2006;53:441–448. [DOI] [PubMed] [Google Scholar]
- 11.Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol 2006;176:7361–7370. [DOI] [PubMed] [Google Scholar]
- 12.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor–mediated increase in intracellular cyclic AMP. J Immunol 2004;173:559–565. [DOI] [PubMed] [Google Scholar]
- 13.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 2005;174:595–599. [DOI] [PubMed] [Google Scholar]
- 14.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Olkiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol 2006;177:5499–5508. [DOI] [PubMed] [Google Scholar]
- 15.Nokta MA, Pollard RB. Human immunodeficiency virus replication: modulation by cellular levels of cAMP. AIDS Res Hum Retroviruses 1992;8:1255–1261. [DOI] [PubMed] [Google Scholar]
- 16.Azzam R, Kedzierska K, Leeansyah E, Chan H, Doischer D, Gorry PR, Cunningham AL, Crowe SM, Jaworowski A. Impaired complement-mediated phagocytosis by HIV type-1–infected human monocyte–derived macrophages involves a cAMP-dependent mechanism. AIDS Res Hum Retroviruses 2006;22:619–629. [DOI] [PubMed] [Google Scholar]
- 17.Crawford MA, Aylott CV, Bourdeau RW, Bokoch GM. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J Immunol 2006;176:7557–7565. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuyama T, Takeshige K, Minakami S. Cyclic AMP inhibits the respiratory burst of electropermeabilized human neutrophils at a downstream site of protein kinase C. Biochim Biophys Acta 1993;1177:167–173. [DOI] [PubMed] [Google Scholar]
- 19.Hwang TL, Yeh SH, Leu YL, Chern CY, Hsu HC. Inhibition of superoxide anion and elastase release in human neutrophils by 3′-isopropoxychalcone via a cAMP-dependent pathway. Br J Pharmacol 2006;148:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol 1996;157:5221–5224. [PubMed] [Google Scholar]
- 21.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 2005;106:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods 1985;82:131–140. [DOI] [PubMed] [Google Scholar]
- 23.Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J 1997;16:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz DA, Chen XM, McLaughlin BJ. Isolation of the phagocytic compartment from macrophages using a paramagnetic, particulate ligand. Anal Biochem 1993;214:205–211. [DOI] [PubMed] [Google Scholar]
- 25.Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. The phagosome proteome: insight into phagosome functions. J Cell Biol 2001;152:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng Yan Hing JD, Desjardins M, Descoteaux A. Proteomic analysis reveals a role for protein kinase C-α in phagosome maturation. Biochem Biophys Res Commun 2004;319:810–816. [DOI] [PubMed] [Google Scholar]
- 27.Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, Moore BB, Peebles RS, Faccioli LH, Peters-Golden M. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol 2007;178:1628–1634. [DOI] [PubMed] [Google Scholar]
- 28.Chen BC, Liao CC, Hsu MJ, Liao YT, Lin CC, Sheu JR, Lin CH. Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I κ B kinase, and NF-κ B. J Immunol 2006;177:681–693. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki K, Noguchi K, Endo H, Kondo H, Ishikawa I. Prostaglandin E2 downregulates interleukin-12 production through EP4 receptors in human monocytes stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans and interferon-γ. Oral Microbiol Immunol 2003;18:150–155. [DOI] [PubMed] [Google Scholar]
- 30.Katsuyama M, Ikegami R, Karahashi H, Amano F, Sugimoto Y, Ichikawa A. Characterization of the LPS-stimulated expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem Biophys Res Commun 1998;251:727–731. [DOI] [PubMed] [Google Scholar]
- 31.Rosch S, Ramer R, Brune K, Hinz B. Prostaglandin E2 induces cyclooxygenase-2 expression in human non-pigmented ciliary epithelial cells through activation of p38 and p42/44 mitogen-activated protein kinases. Biochem Biophys Res Commun 2005;338:1171–1178. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Huang Y, Porta R, Yanagisawa K, Gonzalez A, Segi E, Johnson DH, Narumiya S, Carbone DP. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res 2006;66:9665–9672. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi A, Shimamoto C, Katsu K, Ito S, Imai Y, Nakahari T. EP1 and EP4 receptors mediate exocytosis evoked by prostaglandin E(2) in Guinea-pig antral mucous cells. Exp Physiol 2001;86:451–460. [DOI] [PubMed] [Google Scholar]
- 34.Morrissette NS, Gold ES, Guo J, Hamerman JA, Ozinsky A, Bedian V, Aderem AA. Isolation and characterization of monoclonal antibodies directed against novel components of macrophage phagosomes. J Cell Sci 1999;112:4705–4713. [DOI] [PubMed] [Google Scholar]
- 35.Egawa K, Klein TW, Yamamoto Y, Newton CA, Friedman H. Cyclic AMP inhibition of lipopolysaccharide-induced restriction of Legionella pneumophila growth in macrophage cultures. Infect Immun 1992;60:1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjornson AB, Knippenberg RW, Bjornson HS. Bactericidal defect of neutrophils in a Guinea pig model of thermal injury is related to elevation of intracellular cyclic-3′,5′-adenosine monophosphate. J Immunol 1989;143:2609–2616. [PubMed] [Google Scholar]
- 37.Sadikot RT, Zeng H, Azim AC, Joo M, Dey SK, Breyer RM, Peebles RS, Blackwell TS, Christman JW. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur J Immunol 2007;37:1001–1009. [DOI] [PubMed] [Google Scholar]
- 38.Kohashi O, Ono T, Ohki K, Soejima T, Moriya T, Umeda A, Meno Y, Amako K, Funakosi S, Masuda M, et al. Bactericidal activities of rat defensins and synthetic rabbit defensins on Staphylococci, Klebsiella pneumoniae (Chedid, 277, and 8N3), Pseudomonas aeruginosa (mucoid and nonmucoid strains), Salmonella typhimurium (Ra, Rc, Rd, and Re of LPS mutants) and Escherichia coli. Microbiol Immunol 1992;36:369–380. [DOI] [PubMed] [Google Scholar]
- 39.Sahly H, Schubert S, Harder J, Kleine M, Sandvang D, Ullmann U, Schroder JM, Podschun R. Activity of human β-defensins 2 and 3 against ESBL-producing Klebsiella strains. J Antimicrob Chemother 2006;57:562–565. [DOI] [PubMed] [Google Scholar]
- 40.Hickman-Davis JM, O'Reilly P, Davis IC, Peti-Peterdi J, Davis G, Young KR, Devlin RB, Matalon S. Killing of Klebsiella pneumoniae by human alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 2002;282:L944–L956. [DOI] [PubMed] [Google Scholar]
- 41.Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, Chen GH, Standiford TJ. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun 1997;65:1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett 2007;581:2098–2104. [DOI] [PubMed] [Google Scholar]
- 43.Grzybek M, Kozubek A, Dubielecka P, Sikorski AF. Rafts: the current picture. Folia Histochem Cytobiol 2005;43:3–10. [PubMed] [Google Scholar]