Abstract
Methylphenidate (MPD), commonly known as Ritalin, is the most frequently prescribed drug to treat children and adults with attention deficit hyperactivity disorder (ADHD). Adolescence is a period of development involving numerous neuroplasticities throughout the central nervous system (CNS). Exposure to a psychostimulant such as MPD during this crucial period of neurodevelopment may cause transient or permanent changes in the CNS. Genetic variability may also influence these differences. Thus, the objective of the present study was to determine whether acute and chronic administration of MPD (0.6, 2.5, or 10.0 mg/kg, i.p.) elicit effects among adolescent WKY, SHR, and SD rats and to compare whether there were strain differences. An automated, computerized, open-field activity monitoring system was used to study the dose response characteristics of acute and repeated MPD administration throughout the 11-day experimental protocol. Results showed that all three adolescent rat groups exhibited dose-response characteristics following acute and chronic MPD administration, as well as strain differences. These strain differences depended on the MPD dose and locomotor index. Chronic treatment of MPD in these animals did not elicit behavioral sensitization, a phenomenon described in adult rats that is characterized by the progressive augmentation of the locomotor response to repeated administration of the drug. These results suggest that the animal’s age at time of drug treatment and strain/genetic variability play a crucial role in the acute and chronic effect of MPD and in the development of behavioral sensitization.
INTRODUCTION
Methylphenidate hydrochloride (MPD) is one of the most prescribed drugs to children and adults for the treatment of attention deficit hyperactivity disorder [1,32,40,69]. Attention deficit hyperactivity disorder (ADHD) is a developmental disorder that affects as much as 5 – 15% of school-aged children in the United States [4,27]. MPD is a stimulant of the central nervous system (CNS) with a neuropharmacological profile similar to psychostimulants such as amphetamine and cocaine [29,42]. Cocaine, amphetamine, and MPD are known as indirect dopamine agonists [11,24,42,61]. There are anectodal reports that catecholerminergic agonists affect adolescent rats differently as compared to adult rats [5,31,57,63].
Studies on behavioral sensitization in animals resulted from chronic amphetamine and cocaine treatment have yielded conflicting data depending upon the age of the test subject, the drug dosage, and the intervals between repetitive drug injections [6,31]. Some investigators reported that younger animals treated chronically with stimulants rarely exhibited behavioral sensitization [3,8], while others reported the presence of sensitization to the locomotor effects of cocaine [31]. Since each of the above reports used different rat strains and different drug regimens of cocaine and amphetamine but none involved MPD, the present study used three different rat strains of the same age and the same protocol with three different MPD concentrations for a dose-response assessment and strain comparison.
MATERIALS AND METHODS
ANIMALS
Male spontaneously hyperactive/hypertensive rats (SHR), Wistar-Kyoto (WKY), and Sprague-Dawley (SD) rats (Total N = 109), 34 to 41 days old, were used for this experiment. Animals were housed in the experimental room in groups of 4 per cage for adaptation. The ambient temperature of the room was 21 ± ºC with relative humidity of 37–42%. Animals were maintained on a 12:12 h light/dark (05:30–17:30 h light on) with food and water given ad libitum. Animals were kept 5 – 7 days for acclimation. One day prior to the initial recording, they were randomly divided into groups and individually placed in their testing cage (see Table 1), which became their home cage for the duration of the experiment. Briefly, each rat strain consisted of 4 groups (each N = 8, unless indicated otherwise). Group I was treated with saline. Groups II, III, and IV were treated with 0.6, 2.5, or 10.0 mg/kg MPD, i.p., respectively. This experimental protocol was adapted from previous dose-response experiments of MPD and amphetamine [19,21,22,65–68].
Table 1.
Treatment protocol involving adolescent male rats during the 11 experimental days.
| Experimental Day | Day 1 | Days 2–7 | Days 8–10 | Day 11 | |
|---|---|---|---|---|---|
| WKY | N = 8 | Saline | Saline | Washout | Saline |
| SHR | N = 8 | “ | “ | “ | “ |
| SD | N = 8 | “ | “ | “ | “ |
| WKY | N = 8 | “ | 0.6 mg/kg MPD | “ | 0.6 mg/kg MPD |
| N = 13 | “ | 2.5 mg/kg MPD | “ | 2.5 mg/kg MPD | |
| N = 12 | “ | 10.0 mg/kg MPD | “ | 10.0 mg/kg MPD | |
| SHR | N = 8 | “ | 0.6 mg/kg MPD | “ | 0.6 mg/kg MPD |
| N = 12 | “ | 2.5 mg/kg MPD | “ | 2.5 mg/kg MPD | |
| N = 8 | “ | 10.0 mg/kg MPD | “ | 10.0 mg/kg MPD | |
| SD | N = 8 | “ | 0.6 mg/kg MPD | “ | 0.6 mg/kg MPD |
| N = 8 | “ | 2.5 mg/kg MPD | “ | 2.5 mg/kg MPD | |
| N = 8 | “ | 10.0 mg/kg MPD | “ | 10.0 mg/kg MPD | |
APPARATUS
The locomotor activity testing chambers consisted of a clear, acrylic, open-field box (40.5 x 40.5 x 31.5 cm) fitted with two arrays of 16 infrared motion sensors, and each located 6 and 12.5 cm above the floor of the box. This system has been previously described in detail [16,20,21,67]. In short, the activity monitoring system checked each of the sensor beams at a frequency of 100 Hz to determine whether beams were interrupted. The interruption of any beam was recorded as an activity score. Interruption of two or more consecutive beams separated by at least 1 sec was recorded as a movement score. Repeated interruptions of the same beam(s) were recorded as stereotypic activity. Cumulative counts were compiled and down loaded every 10 min into the OASIS data collection software that recognized and differentiated these counts into various locomotor activities indices.
DATA ANALYSIS
All locomotor parameters were evaluated to test for the drug effects during the initial 2 h post-injection. The acute effect of MPD was calculated as the difference of experimental day 2 from that of experimental day 1. The chronic effect of MPD was determined by comparing experimental days 3 – 7 and 11 to experimental day 2 (e.g., sensitization or tolerance). Two calculations were used: (1) the 2 h data was summed into one value (Fig. 1 and 2) and (2) the data was summed to 10 min bins where 12 bins were analyzed, i.e., 120 min (see Fig. 3 temporal graph). These data points were analyzed with repeated ANOVA and Fischer’s LSD post-hoc test for differences between doses and time effect. Differences in the time course of the effect for doses were qualitatively described using the 10 min bins data to establish the maximum effect, time to maximum effect, and duration of the effect for each dose and locomotor index. Results were analyzed with within-group repeated measures ANOVA (two levels: time post-injection and experimental day of injection). Post-hoc analysis was conducted with Fischer’s LSD test at the 0.05 significance level.
Figure 1.
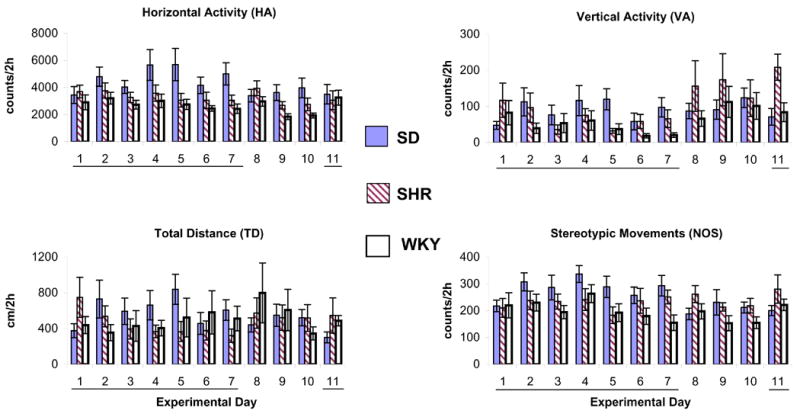
summarizes the horizontal activity, total distance traveled, vertical activity, and number of stereotypic movements for the initial 2 h post saline injection in the morning and showed, for all rat strains (adolescent SD, SHR, and WKY), that saline injection did not modulate the locomotor activity over time. Some minor fluctuations from day to day within strains were observed. Values are presented as the mean ± S.E.M. The days of saline administration are underlined.
Figure 2.
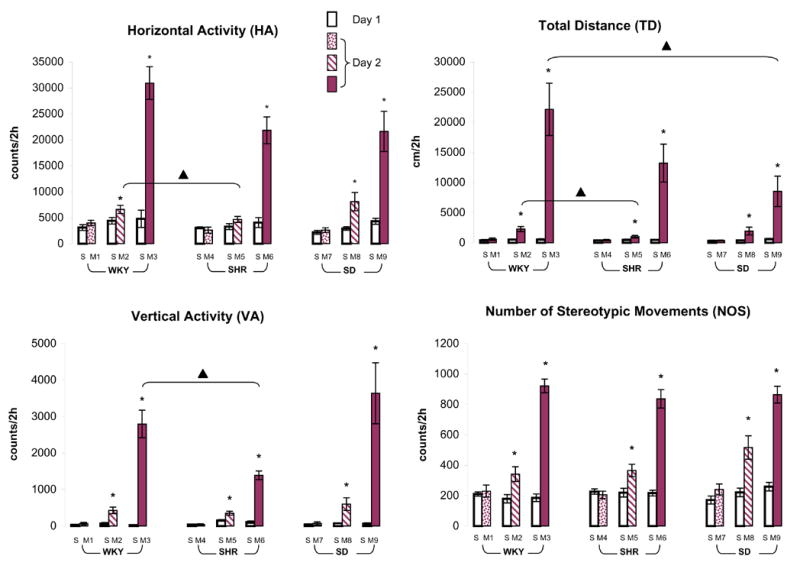
summarizes the acute dose response of 0.6, 2.5, or 10.0 mg/kg MPD as measured by horizontal activity, total distance, vertical activity, and number of stereotypic movements obtained from adolescent WKY, SHR, and SD rats. On experimental day 1, these rats received saline (S), while they were injected with 0.6 mg/kg MPD (groups M1, M4, and M7), 2.5 mg/kg MPD (groups M2, M5, and M7), or 10.0 mg/kg MPD (groups M3, M6, and M9) on experimental day 2. Values are presented as the mean ± S.E.M. The symbol * indicates significant differences at the level of P < 0.05 when compared to baseline activity on day 1. The ▴ represents significant differences at the level P < 0.05 in the comparison between rat strains on experimental day 2.
Figure 3.
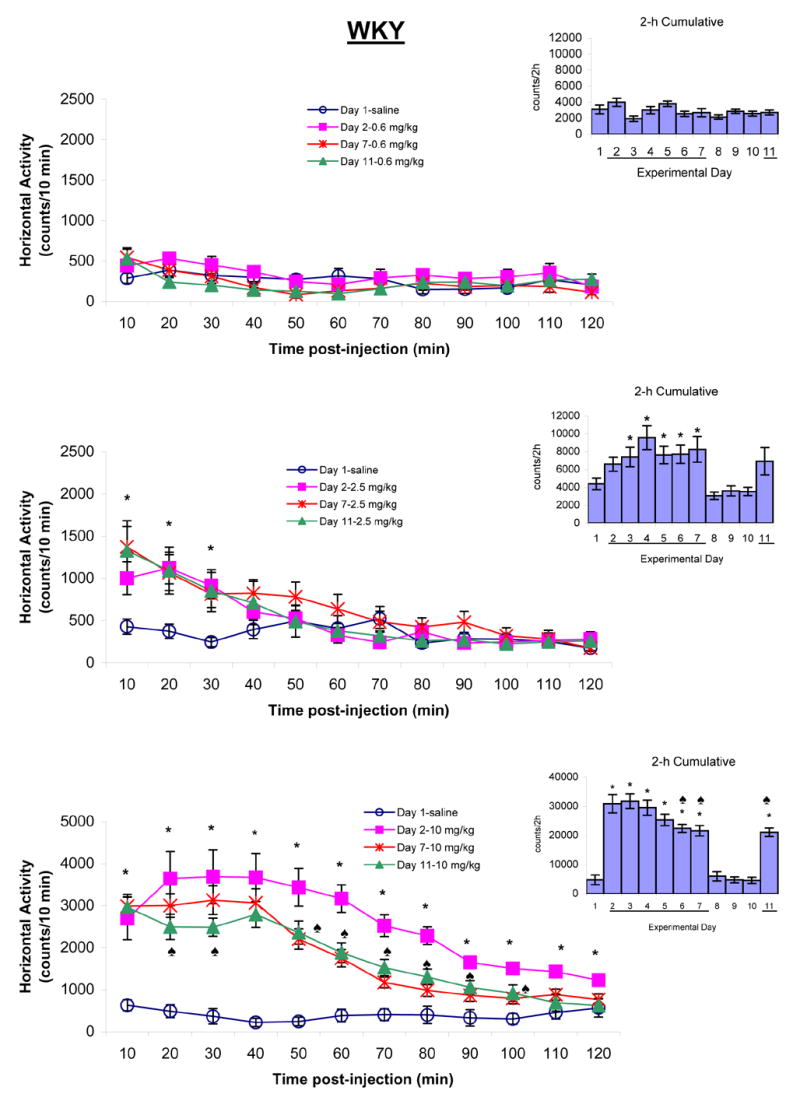
summarizes the horizontal activity of adolescent WKY rats following saline and chronic MPD treatment (0.6, 2.5, and 10.0 mg/kg MPD, i.p.). The line graphs show the temporal response of 10 min samples during the 120 min following post-injection of saline on experimental day 1 or MPD on experimental days 2, 7, and 11. The bar graphs indicate the 2 h cumulative activity on each experimental day after saline or MPD injection. The line “─” indicates days of MPD injection. The values are presented as the mean ± S.E.M. The symbol * shows significant difference at the level of P < 0.05 when experimental days 2, 7, and 11 were compared to experimental day 1 baseline. The ♠ is indicative of P < 0.05 when experimental days 7 and 11 were compared to experimental day 2.
RESULTS
CONTROL
Twenty-four rats were used for saline control groups (N = 8, each strain). Eleven consecutive locomotor recordings for 23h/day were obtained but only activities of the initial 2 h post-injection were evaluated. Saline was injected on experimental days 1 to 7 and 11. Data was collected in 10 min bins and summed into hours. Figure 1 summarizes four locomotor indices for the initial 2 h post saline injection and showed, for all rat strains (SD, SHR, and WKY), that saline injection resulted in similar locomotor activity with minor fluctuations from day to day within strains, i.e., all adolescent rats from the three strains exhibited similar baseline activity during the day time. Tests of between-subjects effects on the baseline horizontal activity, total distance, vertical activity, and number of stereotypic movements showed no significant difference among strains (ANOVA: strain x day). Therefore, data from the initial day following saline injection in the drug treated groups (experimental day 1) was used as the control for animal handling and volume of injection. Any significant deviation from this recording was considered as the drug effect.
MPD ACUTE EFFECT
Three different doses of MPD were used. The first dose of 0.6 mg/kg, i.p., MPD failed to modulate the four locomotor indices from all of the rat groups. As the MPD doses increased, differences between the groups were observed (Fig. 2). For example, the 2.5 mg/kg MPD treatment modulated the horizontal activity of the WKY [F1,25 = 4.69, *P < 0.05] and SD [F1,15 = 6.96, *P < 0.05] rats. Such increase in horizontal activity of WKY is significantly greater than that of SHR [F2,32 = 2.48, ▴P < 0.05] when compared among the strains. This same dose also significantly increased the total distance [F1,25 = 15.47, *P < 0.05; F1,23 = 4.93, *P < 0.05; F1,15 = 4.26, *P < 0.05], vertical activity [F1,25 = 14.52, *P < 0.05; F1,23 = 11.63, *P < 0.05; F1,15 = 5.56, *P < 0.05], and number of stereotypic movements [F1,25 = 8.46, *P < 0.05; F1,23 = 8.52, *P < 0.05; F1,15 = 11.11, *P < 0.05] of WKY, SHR, and SD rats, respectively. When compared among strains, the WKY rats exhibited a significantly greater total distance than SHR [F2,32 = 6.30; ▴P < 0.05]. Similarly, the 10 mg/kg MPD increased the four locomotor indices of all three strains (*P < 0.05). However, the intensity of this increase was different among the rat groups (Fig. 2).
The total distance and vertical activity of WKY rats were significantly greater than that of SD rats [F2,27 = 3.51; ▴P < 0.05] and SHR [F2,27 = 4.44; ▴P < 0.05], respectively.
CHRONIC EFFECT OF MPD
The dose response data following 0.6, 2.5, and 10.0 mg/kg, i.p., MPD for the 11 experimental days for adolescent WKY, SHR, and SD rats are summarized in Figures 3, 4, and 5. The left part of each figure shows the temporal data every 10 min for 2 h post-injection, while the right part of the figure represents activity summed under the curve from the temporal graph into a single value for each day, i.e., total horizontal activity during the initial 2 h post injection for all of the 11 experimental days.
Figure 4.
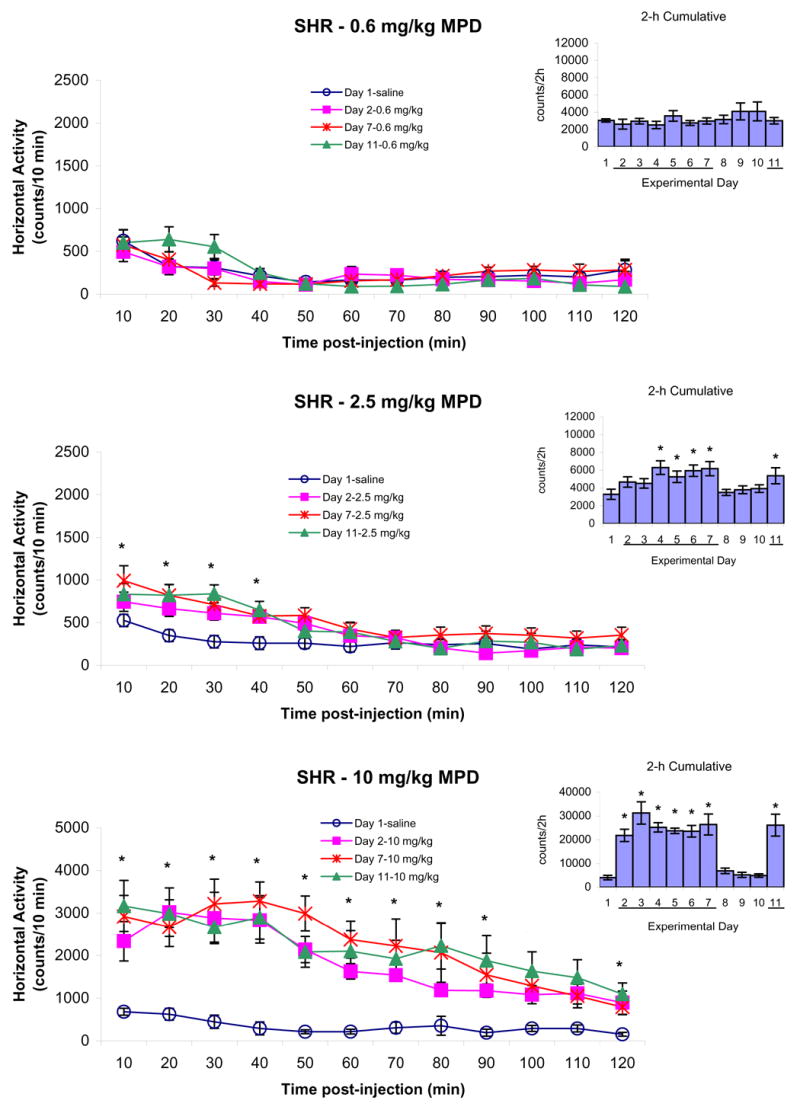
summarizes the horizontal activity of adolescent SHR rats following saline on experimental day 1 and 0.6, 2.5, and 10.0 mg/kg MPD, i.p., on experimental days 2, 7, and 11. The line graphs represent the temporal response of 10 min samples during the 120 min following post-injection of saline or MPD. The bar graphs indicate the 2 h cumulative activity on each day after saline or MPD injection. The line “─” indicates days of MPD injection. The values are presented as the mean ± S.E.M. The * indicates P < 0.05 when experimental days 2, 7, and 11 were compared to experimental day 1 baseline.
Figure 5.
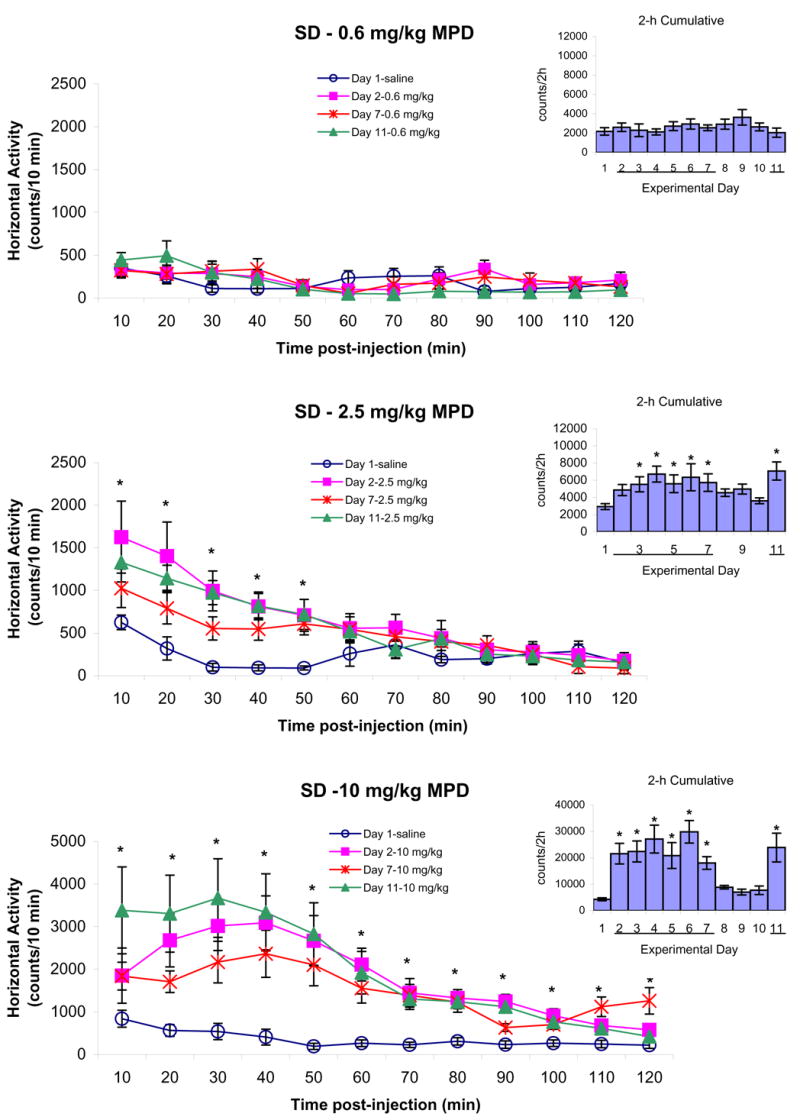
summarizes the horizontal activity of adolescent SD rats following saline on experimental day 1 and 0.6, 2.5, and 10.0 mg/kg MPD, i.p., on experimental days 2, 7, and 11. The line graphs represent the temporal response of 10 min samples during the 120 min following post-injection of saline or MPD. The bar graphs indicate the 2 h cumulative activity on each day after saline or MPD injection. The line “─” indicates days of MPD injection. The values are presented as the mean ± S.E.M. The * shows P < 0.05 when experimental days 2, 7, and 11 were compared to experimental day 1 baseline.
Figure 3 summarizes the horizontal activity of adolescent WKY rats following 0.6, 2.5, and 10.0 mg/kg MPD, i.p., and demonstrates that the lowest MPD dose (0.6 mg/kg) and saline expressed similar activity level after the initial MPD injection and following five consecutive daily injection, as well as an injection of the same MPD dose on experimental day 11 after three days of washout. The 2.5 mg/kg, i.p., MPD on experimental days 2, 7, and 11 elicited a significant increase in horizontal activity for the initial 30 min post-injection [10 min: F3,51 = 3.87, *P < 0.05; 20 min: F2,51 = 3.64, *P < 0.05; 30 min: F3,51 = 3.80, *P < 0.05] after which the activity returned to similar baseline level on experimental day 1. Behavioral sensitization was not observed (Fig. 3 – temporal graph). When the horizontal activity of 2 h post-injection of 2.5 mg/kg dose was summed into one value (Fig. 3 – right histograms), the effect of this MPD dose was observed compared to baseline activity on experimental day 1 (F10,142 = 4.83; *P < 0.05). Furthermore, the effect of the drug on experimental days 3 to 7 and 11 exhibited similar increase in activity as the initial MPD dose. The 10.0 mg/kg MPD elicited a robust increase in horizontal activity for longer duration than the 2.5 mg/kg MPD dose (F10,131 = 33.24; *P < 0.05). The increase in horizontal activity after the initial injection (experimental day 2; *P < 0.05) was higher and for longer duration time (120 min) compared to experimental days 7 and 11. On experimental days 7 and 11, the MPD effect was shorter in duration (100 min compared to 120 min) and lower in intensity (Fig. 3 – temporal and bar graphs; ♠P < 0.05). Similar observations were obtained in the other locomotor indices.
Figure 4 summarizes the horizontal activity of the adolescent SHR following 0.6, 2.5, and 10.0 mg/kg, i.p., MPD and shows that handling of the animals elicited the same increase in activity for about 10 min whether it was saline or 0.6 mg/kg MPD. There was not any difference obtained between the activity after saline injection or 0.6 mg/kg MPD during the 2 h post-injection (Fig. 4 – upper histogram). The middle dose of MPD (2.5 mg/kg) significantly elevated the horizontal activity for about 40 min post-injection on experimental days 2, 7, and 11 (Fig. 4 – left temporal graph; *P < 0.05). When the horizontal activity for the total 120 min post-injection was summed into one value for each experimental day, the effect of the drug was observed in experimental days 4 to 7 (Fig. 4 – right histogram, F10,131 = 3.00; *P < 0.05). The 10.0 mg/kg, i.p., MPD, which was the highest dose used in this experiment, elicited a robust increase in horizontal activity for the 120 min post-injection with the most increase in activity observed in the first 90 min post-injection (Fig. 4 – 10 mg/kg; *P < 0.05). This augmentation was clearly evident in the bar graph (F10,87 = 14.27; *P < 0.05). Similar observations were obtained in the other locomotor analyses.
Figure 5 summarizes the horizontal activity recorded from adolescent SD groups following 0.6, 2.5, and 10.0 mg/kg, i.p., MPD. The 0.6 mg/kg MPD did not produce any effect on horizontal activity, while the 2.5 mg/kg, i.p., MPD increased the horizontal activity significantly (*P < 0.05) on experimental days 2, 7, and 11 for the initial 50 min. No differences were obtained when the 2.5 mg/kg MPD was injected to naïve animals (experimental day 2) or to the same animals injected repeatedly with the drug on experimental day 7 or 11 (Fig. 5 – temporal graph, 2.5 mg/kg). When the 120 min activity post injection was summed into one value (total number of activity under the curve of the 120 min), it shows that this dose of MPD had similar effect on experimental days 3 to 7 and 11 as compared to experimental day 2 but significantly greater than experimental day 1 (F10,87 = 2.6; *P < 0.05). The 10.0 mg/kg, i.p., MPD elicited a robust increase in all locomotor indices of SD rats for 90 min with significant increases in horizontal activity remained for 120 min post-injection (*P < 0.05). This robust horizontal activity was similar on experimental day 2, 7, and 11 with some non-significant fluctuations. In the bar graph, the drug effect of 10.0 mg/kg MPD was evident when compared to experimental day 1 (F10,87 = 6.21; *P < 0.05). Similar observations were found in the other locomotor indices (data not shown).
Figure 6 compares the dose-response effect of 0.6, 2.5, or 10.0 mg/kg, i.p., MPD for the adolescent WKY, SHR, and SD rats as indicated by total distance, vertical activity, and number of stereotypic movements. In general, the comparison shows some differences among the three strains but without any specific pattern. For example, the total distance of WKY rats following 2.5 and 10.0 mg/kg MPD on experimental day 2 (drug given to MPD-naïve adolescent animals) was significantly different when compared to that of SHR and SD rats [F2,32 = 6.30, ▴P < 0.05], while the same dose of MPD exerted similar effects in vertical activity and number of stereotypic movements in all three rat strains (Fig. 6). The 0.6 mg/kg MPD on experimental day 11 [F2,23 = 2.72, ▴P < 0.05] and the 10.0 mg/kg MPD on experimental day 2 [F2,27 = 4.44, ▴P < 0.05] exhibited different vertical activity level among the rat strains (Fig. 6 – middle histogram), while the number of stereotypic movements elicited by the three MPD doses was similar among the strains.
Figure 6.
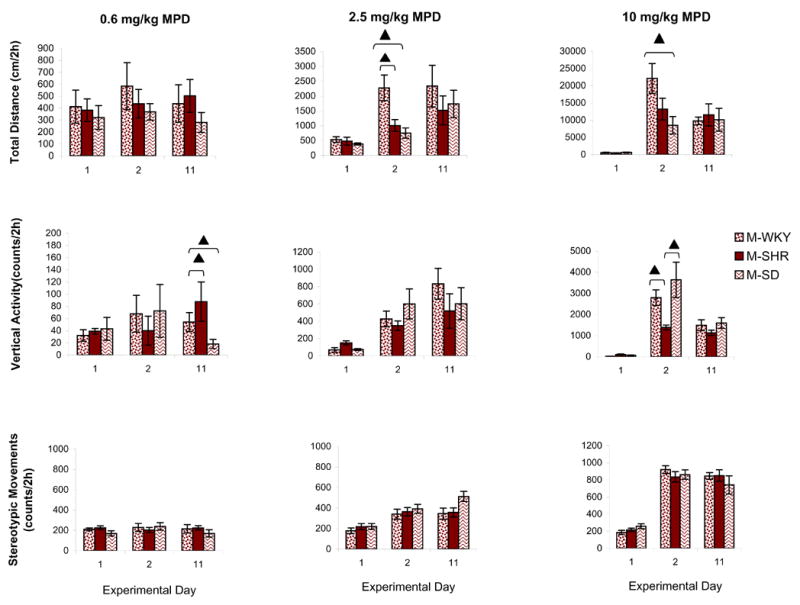
compares the dose-response effect of 0.6, 2.5, or 10.0 mg/kg, i.p., MPD for the adolescent WKY, SHR, and SD rats as indicated by total distance, vertical activity, and number of stereotypic movements. Values are presented as the mean ± S.E.M. with ▴P < 0.05 when compared between strains.
DISCUSSION
The main findings of the present study are that (1) adolescent WKY, SHR, and SD rats exhibited similar baseline activity during the day time and throughout the 11 experimental days; (2) the dose-response characteristics to the acute effects of 0.6, 2.5, or 10.0 mg/kg MPD exhibited incremental increase in locomotor activity, with the intensity of this increase being different among the rat strains and locomotor indices; (3) similar dose-response characteristics were observed following chronic administration of 0.6, 2.5, or 10.0 mg/kg MPD; (4) chronic administration of all three doses of MPD failed to elicit behavioral sensitization or tolerance in adolescent WKY, SHR, and SD rats; and (5) strain differences were observed following chronic treatment of MPD as indicated by the total distance traveled and vertical activity of these animals.
Two data evaluations were performed: (1) temporal profile of the drug effects for every 10 min bins over the 2 h post-injection and (2) the total activity under the curve of 2 h post injection. The latter evaluation failed to show that the 2.5 mg/kg dose elicited any effect on locomotion in all three rat strains. However, the temporal evaluation showed that the 2.5 mg/kg MPD exerted significant effects for 30, 40, and 50 min post-injection for the WKY, SHR, and SD rats, respectively. Besides the difference in the duration of the drug effect, there was also difference in the intensity of the 2.5 mg/kg drug effects between the three adolescent groups of rats. In contrast, the 10.0 mg/kg MPD elicited similar robust effects on locomotion in all three strains with some differences in the intensity and time duration of the drug effects. This observation suggests that dose response protocol and temporal data evaluation is essential in order to determine whether differences in the response to MPD exist among the rat strains.
The three rat strains used in this study were SD, SHR, and WKY. Each strain of rats comprised of a different gene pool which could lead to differences in the susceptibility to psychostimulants and their chronic effects such as sensitization [26,30,47,55]. Because MPD is the drug most often used for treating adolescents with ADHD, adolescent animal models that exhibit the ADHD syndrome are one of the most desired choices for studying the effects of MPD [54].
Many animal models for ADHD exist [53,54], including rats selected from a general population [44], rats reared in social isolation [46], rats exposed to environmental pollutants [28,56], rats that have undergone neonatal anoxia [13], rats that have undergone hippocampal x-irradiation in infancy [14], rats that have undergone neurotoxic brain lesions [2], Naples High/Low excitability rats [50], and knock-out mice [25]. There are also genetic models, including the SHR, which was bred from progenitor WKY rats [35,37,39,41,51,64]. The SHR strain is a genetic mutant of WKY, which has led many researchers to use the SHR strain as the animal model for ADHD and the WKY rats as their control strain. Moreover, the SHR is the only animal strain hyperactive in a variety of behavioral paradigms and has behavioral characteristics that are comparable to the behavioral disturbances of children with ADHD and showing many behavioral characteristics consistent with ADHD, including motor and cognitive impulsiveness, impaired sustained attention, hyperactivity, and reduced dopamine (DA) function [49,54,51,59,60].
It seems that the SHR is one of the “best” animal models to study the effects of acute and chronic MPD treatments, and this rat strain is most frequently used as ADHD model [54,35]. Thus, many investigators are using the SHR strain with the WKY strain as the control in their investigation of ADHD/MPD studies [39,52,53,54]. Since we used MPD as the psychostimulant to elicit sensitization in the present study, the SHR and WKY strains would provide a good comparison with other studies. Pharmacogenetic research using genetically deficient rodent strains has provided information about the contribution of genetic factors to drug related behaviors. There are few reports on genetic/strain differences in determining vulnerability to different drugs [9,23,33,34,38,43]. Therefore, it is important to investigate and compare the effects of MPD on another rat strain often used in drug research. In the recent Medline study of 200 random papers using psychostimulants, it was found that the SD rat strain was used in 52% of the papers. Thus, we selected the SD rats as an additional genetic/strain for this study. Moreover, in previous experiments, we studied dose response characteristics of the acute and chronic effects of MPD and other drugs on locomotor activity of adult SD rats [19,20,58,67,68]. Therefore, we used SD, WKY, and SHR in this study.
None of our experimental groups exhibited behavioral sensitization, while there were some reports [3,6,8,31] that showed that adolescent rats exhibited sensitization to the locomotor activating effects of cocaine. In these experiments, the drug injection and the recording were performed in test cages, while in the present study drug treatment and recordings were performed in the rats’ home cages. An additional difference between our finding and the above observation is the drug. We studied the effects of MPD on adolescent rats; whereas, they studied a different psychostimulant. Moreover, the definition of adolescent rats varies among the different published reports. Based on the papers that used rats of different ages and correlated their ages to that of humans [5,7,10,12,15,17,18,31,36,45,48,57,62], we made the following determination:
| Juvenile | Periadolescent | Adolescent | Young Adult |
|---|---|---|---|
| P-21 to P30 | P-31 to P-39 | P-40 to P-50 | P-60 to P-75 |
P indicates the post-natal day.
A previous experiment using adult rats of the same three strains and similar protocol reported that chronic 2.5 mg/kg MPD elicited locomotor sensitization of male WKY and SD rats and tolerance to the 10.0 mg/kg MPD to all three rat strains [68]. This suggests that the chronic response to MPD between adult and adolescent rats are different, implying that the ontogeny of the effects of psychostimulants on the CNS/behavior during the time of neuronal pruning and adulthood warrants further investigation.
In conclusion, the present study demonstrated that the age at the time of drug treatment and pharmacokinetic differences in the absorption, distribution and/or metabolism of the drug as well as strain/genetic variability could significantly influence both the acute and chronic effect of psychostimulants (e.g., MPD) and the development of behavioral sensitization. Furthermore, strain/genetic comparisons, such as those performed in the present study, are crucial since in animal models could simulate the heterogeneity of populations in clinical studies involving patients.
Acknowledgments
The authors wish to thank Mallinckrodt, Inc., for its gift of MPD. This research was supported in part by the Pat Rutherford Chair in Psychiatry (A.C.S.) and the National Research Service Award from the National Institutes of Health (P.B.Y. – Grant #F31-DA14441).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Accardo P, Blondis TA. What's all the fuss about Ritalin? J Pediatr. 2001;138:6–9. doi: 10.1067/mpd.2001.111505. [DOI] [PubMed] [Google Scholar]
- 2.Archer T. Neurotoxin-induced cognitive and motor activity modifications: a catecholamine connection. In: Sagvolden T, Archer T, editors. Attention deficit disorder: clinical and basic research. Hillsdale: Lawrence Erlbaum; 1998. pp. 287–322. [Google Scholar]
- 3.Barr GA, Wang S. Behavioral effects of chronic cocaine treatment in the week-old rat pup. Eur J Pharmacol. 1993;233:143–149. doi: 10.1016/0014-2999(93)90360-t. [DOI] [PubMed] [Google Scholar]
- 4.Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reducer risk for substance use disorder. Pediatric. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 5.Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 6.Bowman BP, Kuhn CM. Age-related differences in the chronic and acute response to cocaine in the rat. Dev Psychobiol. 1996;29:597–611. doi: 10.1002/(SICI)1098-2302(199611)29:7<597::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacol. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes JJ, Pritchard GA, Koff JM, Miller LG. Prenatal cocaine exposure: Decreased sensitization to cocaine and decreased striatal dopamine transporter binding in offspring. Neuropharmacology. 1993;32:721–723. doi: 10.1016/0028-3908(93)90087-j. [DOI] [PubMed] [Google Scholar]
- 9.Cailhol S, Mormède P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842:200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- 10.Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Psychiatry and Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 11.Challman TD, Lipsky JJ. Methylphenidate: Its pharmacology and uses. Mayo Clin Proc. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- 12.Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increase in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Developmental Brain Res. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Dell'Anna ME, Luthman J, Landqvist E, Olson L. Development of monoamine systems after neonatal anoxia in rats. Brain Res Bull. 1993;32:159–170. doi: 10.1016/0361-9230(93)90070-r. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Granados JL, Greene PL, Amsel A. Selective activity enhancement and persistence in weanling rats after hippocampal X-irradiation in infancy: possible relevance for ADHD. Behav Neural Biol. 1994;61:251–259. doi: 10.1016/s0163-1047(05)80008-1. [DOI] [PubMed] [Google Scholar]
- 15.Döhler KD, Wuttke W. Changes with age in level serum gonadotrophine, prolactin, and gonadal steroid in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty PM, Dong WQ, Faillace LA, Dafny N. Trans-cranial electrical stimulation attenuates abrupt morphine withdrawal in rats assayed by remote computerized quantification of multiple motor behavior indices. Eur J Pharmacol. 1990;175:187–195. doi: 10.1016/0014-2999(90)90229-y. [DOI] [PubMed] [Google Scholar]
- 17.Durmans TL, Varlinskaya EI, Spears LP. Age related differences in elevated plus maze behaviors between adolescent and adult rats. Ann NY Acad Sci. 2004;1021:427–430. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara Y, Kazahaya Y, Nakashima M, Sato M, Otsuki S. Behavioral sensitization to methamphetamine in the rat: an ontogenic study. Psychopharmacology. 1987;91:316–319. doi: 10.1007/BF00518183. [DOI] [PubMed] [Google Scholar]
- 19.Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Dose response characteristics of methylphenidate on different indices of rats' locomotor activity at the beginning of the dark cycle. Brain Res. 1996;727:13–21. doi: 10.1016/0006-8993(96)00296-x. [DOI] [PubMed] [Google Scholar]
- 20.Gaytan O, Al-Rahim S, Swann A, Dafny N. Sensitization to locomotor effects of methylphenidate in the rat. Life Sci. 1997;61:PL101–PL107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- 21.Gaytan O, Swann A, Dafny N. Diurnal differences in rat's motor response to amphetamine. Eur J Pharmacol. 1998;345:119–128. doi: 10.1016/s0014-2999(97)01558-6. [DOI] [PubMed] [Google Scholar]
- 22.Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- 23.George FR, Porrino LJ, Ritz MC, Goldberg SR. Inbred rat strain comparisons indicate different sites of action for cocaine and amphetamine locomotor stimulant effects. Psychopharmacol. 1991;104:457–462. doi: 10.1007/BF02245649. [DOI] [PubMed] [Google Scholar]
- 24.Gerasimov MD, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;296:51–57. [PubMed] [Google Scholar]
- 25.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 26.Glick SD, Hinds PA. Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol. 1984;99:119–121. doi: 10.1016/0014-2999(84)90442-4. [DOI] [PubMed] [Google Scholar]
- 27.Goldman L, Genel M, Bezman R, Slanetz P. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 28.Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94:213–224. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- 29.Kallman WM, Isaac W. The effects of age and illumination on the dose-response curve for three stimulants. Psychopharmacologia (Berl) 1975;40:313–318. doi: 10.1007/BF00421469. [DOI] [PubMed] [Google Scholar]
- 30.LaHoste GJ, Mormède P, Rivet JM, LeMoal M. New evidence for distinct patterns of brain organization in rats differentiated on the basis of inherent laterality. Brain Res. 1988;474:296–308. doi: 10.1016/0006-8993(88)90443-x. [DOI] [PubMed] [Google Scholar]
- 31.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. The Journal of Pharmacology and Experimental Therapeutics. 1995;275:345–357. [PubMed] [Google Scholar]
- 32.Levin FR, Kleber HD. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Rubaleava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacol. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 34.Lucki A, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacol. 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 35.McCarty R, Kopin IJ. Patterns of behavioral development in spontaneously hypertensive rats and Wistar-Kyoto normotensive controls. Dev Psychobiol. 1979;12:239–243. doi: 10.1002/dev.420120307. [DOI] [PubMed] [Google Scholar]
- 36.McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: Sensitization to both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- 37.Mook DM, Jeffrey J, Neuringer A. Spontaneously hypertensive rats (SHR) readily learn to vary but not repeat instrumental responses. Behav Neural Biol. 1993;59:126–135. doi: 10.1016/0163-1047(93)90847-b. [DOI] [PubMed] [Google Scholar]
- 38.Morse AC, Erwin G, Jones BC. Pharmacogenetics of cocaine: a critical review. Pharmacogenetics. 1995;5:183–192. doi: 10.1097/00008571-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Myers MM, Musty RE, Hendley ED. Attenuation of hyperactivity in the spontaneously hypertensive rat by amphetamine. Behav Neural Biol. 1982;34:42–54. doi: 10.1016/s0163-1047(82)91397-8. [DOI] [PubMed] [Google Scholar]
- 40.Nolan EE, Gadow KD, Sprafkin J. Stimulant medication withdrawal during long-term therapy in children with comorbid attention-defiict hyperactivity disorder and chronic multiple Tic disorder. Pediatric. 1999;103:730–737. doi: 10.1542/peds.103.4.730. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 42.Patrick KS, Markowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers, and penoline in attention deficit/hyperactivity disorder. Human Psychopharmacol. 1997;12:527–546. [Google Scholar]
- 43.Phillips TJ. Behavior genetics of drug sensitization. Crit Rev Neurobiol. 1997;11:21–33. doi: 10.1615/critrevneurobiol.v11.i1.20. [DOI] [PubMed] [Google Scholar]
- 44.Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Sirviö J. Behavioral and pharmacological studies on the validation of new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- 45.Rezvoni BA, Levin ED. Adolescent and adult rats respond differently to nicotine and alcohol motor activity and body temperature. Int J Devel Neurosci. 2004;22:309–354. doi: 10.1016/j.ijdevneu.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Robbins TW, Jones GH, Sahakian BJ. Central stimulants, transmitters and attentional disorder: a perspective from animal studies. In: Sagvolden T, Archer T, editors. Attention deficit disorder: clinical basic research. Hillsdale: Lawrence Erlbaum; 1989. pp. 199–222. [Google Scholar]
- 47.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 48.Roffman JL, Raskin LA. Stereotyped behavior: effects of d-amphetamine and methylphenidate in the young rat. Pharmacol Biochem Behav. 1997;58:1095–1102. doi: 10.1016/s0091-3057(97)00321-3. [DOI] [PubMed] [Google Scholar]
- 49.Russell VA. The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat (SHR) as studied in vitro by the superfusion slice technique. Neurosci Biobehav Rev. 2000;24:133–136. doi: 10.1016/s0149-7634(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 50.Sadile AG, Pellicano MP, Sagvolden T, Sergeant JA. NMDA and non-NMDA sensitive [L-3H]glutamate receptor binding in the brain of the Naples high- and low-excitability rats: an autoradiographic study. Behav Brain Res. 1996;78:163–174. doi: 10.1016/0166-4328(95)00244-8. [DOI] [PubMed] [Google Scholar]
- 51.Sagvolden T, Metzger MA, Schiørbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- 52.Sagvolden T, Pettersen MB, Larsen MC. Spontaneously hypertensive rats (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol Behav. 1993;54:1047–1055. doi: 10.1016/0031-9384(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 53.Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder-from brain dysfunctions to behaviour. Behav Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- 54.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 55.Segal DS, Kuczenski RR. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- 56.Silbergeld EK, Goldberg AM. Lead-induced behavioral dysfunction: an animal model of hyperactivity. Exp Neurol. 1974;42:146–157. doi: 10.1016/0014-4886(74)90013-2. [DOI] [PubMed] [Google Scholar]
- 57.Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 58.Sripada S, Gaytan O, Al-Rahim S, Swann A, Dafny N. Dose-related effects of MK-801 on acute and chronic methylphenidate administration. Brain Res. 1998;814:78–85. doi: 10.1016/s0006-8993(98)01035-x. [DOI] [PubMed] [Google Scholar]
- 59.Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS, Cantwell DP. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429–433. [PubMed] [Google Scholar]
- 60.Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding YS, Hitzemann R, Pappas N. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:PL7–PL12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 62.Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Shelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (p41–50) exhibit increased susceptibility to d-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (p21–30 or p31–40) or adult rats (p51–60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 63.White PJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 64.Wultz B, Sagvolden T, Moser EI, Moser MB. The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: effects of methylphenidate on exploratory behavior. Behav Neural Biol. 1990;53:88–102. doi: 10.1016/0163-1047(90)90848-z. [DOI] [PubMed] [Google Scholar]
- 65.Yang P, Beasley A, Swann A, Dafny N. Valproate modulates the expression of methylphenidate (Ritalin) sensitization. Brain Res. 2000a;874:216–220. doi: 10.1016/s0006-8993(00)02500-2. [DOI] [PubMed] [Google Scholar]
- 66.Yang P, Swann A, Dafny N. NMDA receptor antagonist disrupts acute and chronic effects of methylphenidate. Physiol Behav. 2000b;71:133–145. doi: 10.1016/s0031-9384(00)00318-8. [DOI] [PubMed] [Google Scholar]
- 67.Yang P, Singhal N, Modi G, Swann A, Dafny N. Effects of lithium chloride on induction and expression of methylphenidate sensitization. Europ J Pharmacol. 2001;426:65–72. doi: 10.1016/s0014-2999(01)01213-4. [DOI] [PubMed] [Google Scholar]
- 68.Yang PB, Behrang A, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- 69.Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. Amer Med Assoc. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]


